Abstract
Atherosclerosis is a widespread disease caused by the deposition of lipids on arterial walls. Such lipid plaques in coronary arteries can be fatal. Although many factors related to diet, life-style, etc. contribute to the worsening of the ailment, the primary cause, the lipids in the circulatory system, come from a series of low-density lipoproteins. These lipoproteins are necessary for the transport of lipids to and from different organs. It would be valuable to medicine and the field of drug design if a more detailed understanding of the organization of lipid and protein in these molecules were available. Unfortunately because of heterogeneity in their size and lipid composition, all classes of the low-density serum lipoproteins appear to be not amenable to the most widely used method for obtaining detailed atomic data—X-ray crystallography. However there appears to be a recently identified homolog that is relatively homogeneous, and crystal structures have been obtained. Used as a molecular model, the homolog serves as a source of conformational information that might help to unravel the processes involved in the lipid loading of the low-density lipoproteins. The review attempts to give a brief summary of the structural biology of the serum low-density lipoproteins relative to the molecular model of lipovitellin.
Introduction
Although many factors play a role in atherosclerosis and other forms of heart disease, the serum lipid carriers function as the principal source of the problematic lipid depositions. The serum lipid carriers consist of a series of lipoproteins that vary greatly in both protein and lipid content. The assembly and secretion of the serum lipoproteins also differ significantly depending on their type. Because of the severity of arterial plaque formation, a great deal of attention has been focused on these macromolecular complexes or particles. The results of scientific studies from the last 50 years or so have identified numerous factors contributing to their elevated levels and, therefore, to atherosclerosis. Contributory elements include dietary factors, ethnicity, sex, mutations, other illnesses, etc. Nonetheless, the release of lipids from the serum low-density lipoproteins is a primary issue in the growth of arterial plaques. Therefore understanding the molecular structure of the serum lipoproteins remains a central factor in controlling this form of heart disease.
During the many years encompassing investigations of the human serum lipoproteins, the number of review articles has kept pace with the number of studies. For those who might want to examine the more recent biochemical aspects of the human serum lipoproteins and their assembly, reviews from 2000 onward are listed in Citation1–8. In the text, which follows, an attempt will be made to focus only on structural data related to the assembly and secretion of very low-density lipoproteins and chylomicrons. The following abbreviations are widely used in studies of the human serum lipoproteins: LDLs or low-density lipoproteins, vLDLs or very-low-density lipoproteins and chylomicrons. Conformational aspects of the vLDLs and chylomicrons is also a topic covered extensively in one of the more recent reviews Citation6. Having such great medical significance, one could ask why more is not known about the conformation of these macromolecular complexes or particles. The answer is linked closely to their overall heterogeneity in size and lipid composition, and the likely mobility of the lipid components. All forms of the low-density particles contain a heterogeneous accruement of lipids, and such variations make it unlikely that high-resolution structural studies can be carried out; such studies depend on homogeneity and the ability to form highly ordered crystalline or paracrystalline specimens. Although crystallizations of LDL have been reported Citation9, the crystals produced only relatively unusable diffraction data. A detailed molecular structure of any form of apoB-100 and/or the low-density serum particles will be very difficult for the reasons mentioned above.
Key messages
The similarity amongst the microsomal triglyceride transfer protein, lipovitellin, and the NH2-terminal region of apolipoprotein B was reported as including intron locations (exon boundaries) and some resemblance in their amino acid sequences.
With the NH2-terminal segment of apoB entering into the endoplasmic reticulum first, it is likely that this segment initiates the assembly process by forming some yet undemonstrated transient assembly with a complex formed between the microsomal triglyceride transfer protein and protein disulfide isomerase. These proposed micellar structures named lipid microdomains would have an external surface of relatively randomly oriented hydrocarbon chains with certain regions stabilized by the headgroups of phospholipids.
Table I. The biochemistry of apoB-containing lipoproteins: A: Abbreviations; and B: Review referrals—topics.
also contains a list of the lipoprotein abbreviations that will be used throughout this discussion as well as a few editorial notes on the collection of recent reviews. Note that the proteins listed in Part A of are interrelated via the fact that they are assembled in the endoplasmic reticulum (ER) system. High-density lipoproteins (HDLs) principally contain the apolipoprotein A1 Citation10. HDLs are not assembled in cellular ER systems but rather by transfer processes occurring in the serum itself. Chylomicrons, very-low-density lipoproteins (vLDL), and LDL contain a higher percentage of lipid and a protein labeled apoB or truncated forms thereof (apoB-100 ∼550kD Mr or 4563 amino acids; apoB-48 in chylomicrons ∼260kD Mr or 2190 amino acids). Much of the text that follows may well apply to the assembly of any apoB-containing particle. As noted above, they have in common the observation that they contain apoB or truncated segments thereof and a varying mixture of phospholipids, cholesterol, cholesterol esters, and triacylglycerol. Chylomicrons, for example, are generated by enterocytes in the small intestine using the truncated version of apoB (apoB-48) Citation10. vLDLs are formed in and secreted by hepatocytes and contain the entire apoB polypeptide chain.
Because of the lipid heterogeneity mentioned above, it is necessary to look elsewhere for representative systems upon which to try to build a working model of the vLDL. In the yolk part of eggs from oviparous animals, there is a lipoprotein (vitellogenin/lipovitellin), which serves as the principal source of nutrients for the developing embryo. Like the vLDLs, it is secreted by the liver through the ER system, taken up through a specific receptor system by the ovaries, and accumulated into the yolk segment of the maturing oocytes Citation11. As will be described in a later section, the ER assembly and secretion coupled by receptor-mediated uptake closely resembles the biochemistry of the apoB-containing particles. Lipovitellin (LV) on the other hand, is a very homogeneous lipoprotein that has been crystallized and its molecular structure determined by X-ray crystallography Citation12–14. In fact the yolk system of many eggs is composed of microcrystals of lipovitellin. For a variety of properties including analyses of the gene structure, the LV system may be used as a model for the human low-density lipoproteins. The similarity between the LV protein and the apoB-containing lipoproteins will be described in more detail in a later section. This ‘biological’ homology has been used by workers to deduce molecular models for the NH2-terminal region of apoB and by simple extension to the nascent apoB-containing particles. The focus of this review is to present the structure of lipovitellin, review any models of apoB, and offer additional commentary on lipoprotein structure and assembly based on the homology and other biophysical data.
The genes of LV, apoB, and MTP—evidence for homology
The similarity amongst the microsomal triglyceride transfer protein (MTP), LV and the NH2-terminal region of apoB were reported as including intron locations (exon boundaries) and some resemblance in their amino acid sequences Citation15, Citation16. However, the evidence for the presence of a lipoprotein family including the apoB-containing lipoproteins has since been accumulating to include a variety of other biochemical factors. They now take into account the correspondence between the lipoprotein receptors and the pathway of removal from the serum Citation17.
Recent discoveries also demonstrate that gene products found in the liver and associated with the ER secretory processes are necessary for the production of both vLDL and LV Citation18. From the preliminary MW[, the ER protein for LV assembly is probably MTP. In the case of the vLDL, these proteins consist of a complex between MTP and protein disulfide isomerase (PDI) Citation19, Citation20. Therefore, the formation of LV in the liver of egg-laying animals follows a pathway similar to the conduit or secretory process that is shown in and used for the production of apoB-containing lipoproteins in higher organisms Citation18. The oviparous animal forms vitellogenin in the liver with the aid of proteins like the mammalian MTP:PDI complex. Vitellogenin is then secreted through the ER:Golgi apparatus into the bloodstream. This precursor protein finds receptors on early oocytes in the ovaries. The receptor is homologous to the LDL receptors in mammals Citation11. One difference from the apoB-containing lipoproteins is that vitellogenin is proteolytically cleaved during the uptake process to form lipovitellin, LV. Proteolysis affects the solubility, and LV aggregates into microcrystals or small yolk aggregates. The similarity in the assembly of the two lipoproteins, apoB-containing lipoproteins, and LVs is most likely due to the need for lipid loading prior to secretion. A second requirement may be related to the folding process itself, particularly the formation of the correct disulfide bonds. Last of all, the transit through the ER:Golgi will include the addition of any covalently linked carbohydrate.
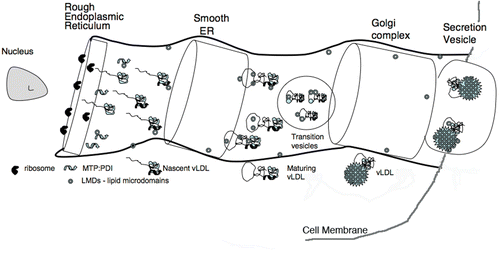
Figure 1. Secretion of apoB-containing lipoproteins. The schematic drawing illustrates the cellular components involved in the secretion of apoB-containing lipoproteins into the serum. The apoB polypeptide chain either apoB-100 or apoB-48 is synthesized by ribosomes attached to the rough ER and cotranslationally moved into this secretion system. The NH2-terminal segment of undefined length forms a complex with the heterodimeric MTP:PDI complex. MTP and nascent apoB begin loading with lipid. As the nascent lipoproteins increase their lipid content and apoB continues its folding process, more mature form(s) of appear and are transferred to the Golgi where secretion vesicles are formed and released from the cell. LMDs are defined as some yet undefined lipid microdomains produced by MTP or the MTP:PDI complex. A single LMD is metastable and would contain only a small portion of the overall lipid load. Formed by fragmentation of lipid droplets or membrane segments, LMDs may contain phospholipids, triacylglycerols, and various forms of cholesterol. Their unique property would be a covering having both hydrophobic and polar surfaces. Although not explicitly shown in the schematic drawing, a sorting system removes misfolded proteins from the secretory process. This hypothetical assembly route is similar to that proposed by Olofsson et al. Citation62.
Properties of serum apoB-containing lipoproteins
The products of the assembly/secretion system are a series of molecules whose buoyant densities are far removed from simple proteins/enzymes. In fact, the operational definition of both HDLs and LDLs is related to their buoyant densities. Chylomicrons have a density of less than 0.95 g/mL and contain the truncated form of apoB, apoB-48. They contain a high percentage of triacylglycerols and are formed in the enterocytes of the small intestines Citation21. vLDLs belong in the 0.95–1.006 g/mL range, while intermediate-density lipoproteins (IDL) belong to the buoyant density range of 1.006–1.019 g/mL. IDLs are often included with vLDLs, expanding their range from 0.95 g/mL to 1.019 g/mL. LDLs have a density in the range of 1.019–1.063 g/mL Citation10. For comparison, most lipid-free proteins have a buoyant density greater than 1.21 g/mL. All of the aforementioned forms of the serum lipoproteins contain some form of the polypeptide chain called apoB. Because of the packing of methylene carbons belonging to lipids, the buoyant density is inversely related to the lipid content. For example, vLDLs may contain as much as 90% w/w of lipid.
While their hydrodynamic density remains the most useful and identifiable property of the apoB-containing lipoproteins, information about their overall shape and size has also come from other approaches. Scattering of X-rays or neutrons from lipoproteins in solution give the most accurate measurement of the radius of gyration (Rg). The Rg is related to a spherically averaged equivalent molecule because of the rapid tumbling of any molecule during the scattering measurement. Fine structure in the scattering pattern may give some information about the overall shape and density distribution. For example, a neutron scattering study suggested a bow-shaped model for apoB alone Citation22. Since apoB is insoluble in aqueous buffers, these measurements were made in the presence of a detergent, and the effect of these additives on the conformation of the apolipoprotein is unknown.
As opposed to the apolipoprotein, a variety of solution scattering techniques have suggested that the intact LDL has a roughly spherical shape. So for the LDL fractions, the particles can be thought of as either roughly spherical or spherical with Rg ranging upwards from 90 Å Citation23, Citation24. As opposed to the spherical models, some imaging studies have suggested that the LDLs really are cylindrical in overall form Citation25, Citation26. Much of the early small-angle X-ray studies were done in the late 1970s and led to values for the Rg of 90–120 Å Citation27–29. The neutron-scattering data from apoB in detergent solution and mentioned in the previous paragraph has been used to derive a hypothetical model of an LDL molecule Citation22. It is based on a more or less overall spherical shape. This rudimentary model envisions the apoB present as an arc-shaped protein on a sphere-like low-density collection of lipid molecules Citation22. The most striking feature of Johs’ model is the arc-like conformation of the protein portion, apoB. Spherical, cylindrical, or irregular, the crude shape is only the first step in defining the overall structure and organization of these molecules.
Structural studies of apoB-containing lipoproteins using electron microscopy
Direct visualization of biological macromolecules, such as the serum lipoproteins, is always possible using electron microscopy (EM). However, EM observations are limited by two factors: 1) the contrast between the particle and the substrate supporting it in the electron beam, and 2) specimen damage due to the electron beam. Nonetheless, studies of the basic shape and, by labeling methods, the distribution of protein/lipid have produced structural insights into the apoB-containing lipoproteins. Such views, while not yielding atomic level models, provide shape and size information and boundary limits for other methods.
EM images fall into two categories: negatively stained or images of frozen specimens. Both methods have been used to study the structure of the LDLs, and the results lead to information about the overall shape but little about the protein:lipid organization Citation26, Citation30–34. Furthermore, no matter how the contrast between molecule and substrate is produced, resolution in an electron microscope is generally limited to 25 Å or less. At such resolution, domain structure would generally not be apparent. Based on the images that have been recorded for LDLs, the EM projections all appear to suggest either a near-spherical molecule or disc-like structure with a diameter ranging between 200 and 320 Å Citation35. In lesser number, some of the LDLs may have an elliptical shape with an aspect ratio of about 1.5. The detergent-solubilized apoB resembled a ‘rope’ or an extended polypeptide chain Citation36. In addition, electron micrographs have been obtained of dimyristoyl phosphatidyl choline-solubilized apoB leading to disc-like structure forming stacks or rouleaux Citation37. In both cases, the results are difficult to extrapolate to a molecular model of apoB either alone or within a lipid assembly Citation36, Citation37. Occasionally, a bump might appear on an LDL molecule but this observation appears to be a relatively rare event. The EM studies at the highest resolution and with a tilting stage appear to support a near-spherical structure with somewhat higher electron density (probably protein) on the outermost edges Citation35.
It should be possible to fit together the small-angle X-ray and neutron scattering in solution mentioned in the previous section with the EM results above. Models based on solution scattering are spherically averaged and generally the closer the molecule is to a sphere, the more accurate will be the small angle scattering model. Rouleaux formation from disc-like particles would be incompatible with small-angle X-ray or neutron scattering; any form of aggregate always dominates solution scattering. The spherical EM particles have a relatively broad distribution of sizes ranging from radii of 125 to 250 Å Citation35; the former value agrees well with small-angle scattering results.
If one were to combine the results of both the early and more recent biophysical studies such as EM and X-ray or neutron scattering, a consensus model of LDLs would be described as a spherical or nearly spherical particle with protein (apoB) on the surface. (Because of the commonality of apoB in the low-density lipoproteins, it seems reasonable to assume they have common features in the overall architecture of vLDL, LDL, IDL, and even chylomicrons.) Within this sphere would be at least one layer of phospho- or polar lipids and cholesterol. Near the center would be a core of cholesterol esters and triacylglycerol. But this arrangement of neutral and polar lipids is largely speculative. In LV, the lipid domain is partly reversed. The only visible lipid segments in the electron density map were neutral methylene chains fitting the nooks and crannies of a hydrophobic surface in a protein cavity. Some of the polar headgroups of phospholipids are probably on the surface away from visible protein, but, nonetheless, the lipid domain was not layered as proposed for the LDLs. Given the similarities between MTP and LV, it seems likely that lipid systems or microdomains generated by the former may not have the ‘classical’ structure with polar lipids solely on the external side. This discrepancy may also apply to attributes that might provide insight into the mechanism of lipid loss as must occur in atherogenesis.
In trying to envision the end point of the assembly process and the overall organization of lipid and apoB protein, it will be necessary to obtain higher resolution models for the apoB-containing lipoproteins. But there can be steps along the way and approaches for redefining and improving the rough models mentioned above. The previously described homology of the NH2-terminal segment of apoB with LV and MTP represents a potential starting point. Since the homology region is located at the NH2-terminal, it is available for either apoB-48- or apoB-100-containing lipoproteins. Indeed, the initial starting structure for vLDL and chylomicron assembly is likely to be the same. Supplementary questions then involve the protein and/or membrane complexes that are formed between MTP:PDI and the apoBs, and how the large lipid-protein complex is transported to the Golgi apparatus in COPII-coated vesicles Citation38.
Hypothetical models for the apoB-containing lipoproteins
Many of the current thoughts on the conformation and folding of apoB, on the addition of lipid, and on any intervening protein complexes on the way to lipoprotein production, stem from the homology mentioned earlier. They are derived from the purported conformational similarity of LV, MTP, and the NH2-terminal region of apoB Citation15, Citation39. To reiterate, the combined evidence for similar structures included intron/exon boundaries, sequence homology, similarity in the positioning of the disulfide bonds, and the overall cell biology of the three proteins. Based on the aforementioned resemblance, models have been built of the NH2-terminal region of apoB Citation40 and the entire MTP protein Citation41. This is called homology modeling. In regions where the amino acid sequences are similar, the corresponding model provides new insights into the apoB structure. In regions where the sequences are dissimilar and contain notable insertions or deletions, the hypothetical model becomes much less reliable.
With the biophysical studies leading to only the most elementary representation, a number of investigators have turned to developing lipoprotein models based on other criteria—some are based solely on homology rules; others use predictive methods for estimating the positions of β-strands and α-helical segments Citation40, Citation41. Both approaches produce models at a new level of detail. To understand the significance of these derived models, it is necessary to first study the molecular structure of LV derived from the crystallographic coordinates. It is this conformation, which forms the basis of most of the current NH2-terminal of apoB-containing lipoproteins and MTP homologs. A cartoon image of the crystallographic structure of lamprey LV is shown in Citation12–14. Some differences between LV and the apoB-containing lipoproteins must be kept in mind. The protein component of LV is smaller than apoB, ranging upwards to only 2000 amino acids. In addition, LV is proteolytically cleaved during uptake from the serum resulting in multiple polypeptide chains; for the lamprey crystal structure of LV there are four in total. Although several known segments, including a highly phosphorylated portion, were not visible in the electron density maps, the 2-fold symmetrical dimer appeared to be defined by three protein domains. These are labeled a, b, and c in —colored green, red, and blue respectively Citation14.
Figure 2. The dimer of lipovitellin. The cartoon model depicts the crystal structure of lamprey lipovitellin. It is thought to be a homolog of the NH2-terminal of apoB and the intact MTP proteins. The LV dimer has 2-fold rotational symmetry, with the position of the 2-fold axis indicated by the elliptical black mark in the center of the diagram. Each subunit, labeled 1 and 2, is composed of multiple domains illustrated by the colors red, blue, and green (or shades of gray) and the letters a, b, c. The green domains labeled 1a and 2a include residues from the NH2-terminal region. The domains shown in red (1b and 2b) are comprised of a double layer of α-helices. The blue domains, labeled 1c and 2c, consist of mainly of β-strands forming several sheets that surround the lipid-binding cavity.
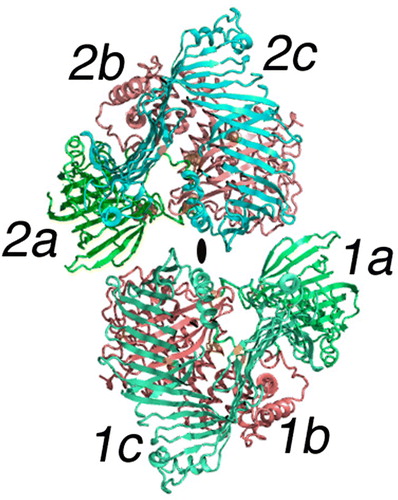
In the crystalline complex, which is the blueprint for the discussion of the structures of MTP and the NH2-terminal region of apoB and therefore low-density lipoproteins, the dimeric structure of LV contains a lipid-binding domain within each subunit. Stated another way, the lipoprotein LV has no common intersection of the lipid domains. Essentially all of the lipovitellins from a variety of oviparous species that have been studied are dimeric Citation42–47. Like nearly all homodimeric proteins, the subunits are arranged in a symmetrical fashion. The elliptically shaped marker near the center of indicates the 2-fold rotation axis governing the steric relationship of one subunit to its mate in the dimer. Could the various forms of the LDLs like LV contain two copies of apoB? Current analytical data says no, but for such very large molecules, establishing stoichiometry is often difficult. Current methods depend on lipid removal and immunoprecipitation followed by nephelometry Citation48. Although suitable for high-throughput clinical studies, the nephelometric approach may be somewhat crude for determining molecular stoichiometry.
As part of the current working hypothesis, amino acid sequences of the NH2-terminal domain of apoB Citation8 and the entire MTP molecule were used to construct hypothetical models from X-ray crystallographic coordinates of a monomer of LV. Hence the model building did not include both subunits of LV. This brings to the forefront the question of whether or not the data used to identify homology lead to similarity in the quaternary structure. And the answer is that in many cases it does. Therefore the model in may be incomplete for MTP and possibly the conformation of the NH2-terminal of apoB as it begins the maturation process in the ER/Golgi. One can predict that both MTP and the NH2-terminal of apoB might behave as a dimer. In this dimer, the subunit interface of LV puts the edge of the helical domain (2b, red in ) in opposition to NH2-terminal domain (1a, green in ) of the opposing subunit. Each subunit of LV contains 30–40 molecules of lipid per monomer, but there is no connection between the two lipid-binding domains Citation12. As mentioned earlier during the assembly and secretion process, LV is also believed to require an MTP-like protein for maturation, strengthening the argument that the latter is also a dimer.Colored versions of figures are found in the online version.
Although more difficult to see in , the four disulfide bonds observed in the LV crystal structure are included and colored yellow; they are: 1) C156-C182; 2) C198-C201; 3) C443-C449; and C1408-C1429. ApoB and any resulting LDLs also contain a number of disulfide bonds that must be formed during the assembly process. For apoB, 16 of the 25 cysteines exist in the form of disulfide bonds Citation49. With but two exceptions, the disulfides that are present occur between cysteines that are adjacent and separated by relatively short segments in the amino acid sequence. This might be taken to mean that the NH2-terminal domain of both LV and apoB could be folded without much regard for segments more toward the COOH-terminal Citation50. Stated in another way, the NH2-terminal region of apoB may be a globular unit before translation is completed.
From the crystals of LV and the diffraction studies, little information about the organization of the lipid was initially obtainable Citation12. However, a limited number of partial acyl chains could be placed in the electron density maps at higher resolution Citation14. Taken as a whole, the LVs contain approximately 15% w/w of lipid; overall, between 30 and 40 molecules of lipid are present in the lamprey LV subunit shown in . The location of the lipid and subunit mates may be very important in understanding the assembly of the apoB homolog. Neutron scattering studies combined with scattering density variation indicated that the lipid is divided into two separate cavities in LV. Each is within one subunit of the molecule Citation51. The cavity visible in is surrounded by mainly the β-strands of the blue domain (1c in ), and only some of the acyl chains were visible during the X-ray study. They are visible in black in . The molecular boundaries of the cavity consisted of the side chains from the β-strands of the penultimate domain (LV, blue).
Figure 3. Lipid molecules attached to a subunit of LV. The cartoon is color-coded the same as but shows only a single subunit of LV in an orientation where the major lipid-containing region is visible. The green portion labeled 1a is again the NH2-terminal domain; 1b in red is the helical domain; 1c is the β-sheet domain containing the lipid core. Two unique lipid-binding sites are labeled L1 and L2 and are partially hidden but visible as black spherical nets. Other lipid molecules or parts thereof observed in the crystallographic studies are evident in the lipid cavity as black bonds. L2 is a phospholipid as reported in the original study Citation14. The center of this segment of the LV molecule is labeled lipid core. Although not containing any visible electron density in the crystal structure, other experiments establish this location as that containing most of the bound lipid; for LV, 15% w/w is lipid, mostly phospholipid but neutral lipid as well.
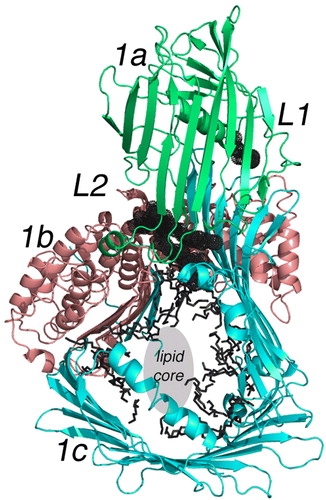
This LV cavity was lined almost entirely with the side chains from hydrophobic amino acids. Based on the known composition and a core lacking in visible electron density found during the X-ray studies, much of the bound lipid is disordered. Its location must be in the central-most part of the cavity that is labeled ‘lipid core’ in . Nonetheless in the LV X-ray structure, the lipid-binding cavity interacts mainly with the uncharged segments of any lipid molecule—more so neutral lipid or the acyl chains of phospholipids. This means that if it was released from the protein cavity, a relatively hydrophobic segment of a lipid micelle is formed. In and in text below, this lipid form is named a lipid microdomain (LMD). Released from the cavity of LV, it would contain 30–40 molecules of lipid and in part its surface would be hydrophobic.
Last of all there are two bound lipid molecules that are relatively separated from the rest of the lipid core. They are labeled ‘L1 and L2’ in Citation14, and because of their location they may have special significance to the assembly process. L1 is only visible as a fragmented acyl chain, but L2 appears as a complete phospholipid. The location of L2 in the X-ray structure of LV is such that it may serve as a cofactor in the lipidation process. It is positioned very close to a hinge-like conformation that may alter the volume of the lipid-binding cavity. As such, binding of L2 would necessarily precede the further lipid addition during the assembly process Citation14. Since the L2 site may be present in the MTP and apoB homologs, these unique binding sites may play a role in the formation of the apoB-containing lipoproteins.
Transport into and through the ER:Golgi
Since the assembly of the LDLs from apoB-100 or apoB-48 and lipid occurs in the ER:Golgi of the secreting cell, knowledge of the assembly process may add important clues to their structure. The insertion of the polypeptide chain of apoB into the secretion organelles occurs cotranslationally as is illustrated in . Although entirely schematic, the illustration contains a number of elements that are vital to the text that follows. Mentioned already and most important is that the addition of lipid and the entire maturation process is dependent on another protein complex labeled MTP:PDI (see ) Citation19. MTP is believed to be responsible for shuttling lipid from the membrane system or lipid microdroplets (LMDs in ) to nascent forms of apoB. The role of PDI is unclear although it obviously might serve as the catalyst for forming or re-forming the disulfide bonds in the growing apoB chain. The MTP dependence of the lipoprotein assembly process has been convincingly established with the discovery of individuals with mutations in the MTP gene and therefore lacking a working MTP:PDI complex Citation52. There is a phenotypic absence of chylomicrons, vLDLs, and LDLs.
With the NH2-terminal segment of apoB entering into the ER first, it is likely that this segment initiates the assembly process by forming some yet undemonstrated transient complex with MTP:PDI. In , a complex of apoB and MTP:PDI is labeled as the ‘nascent vLDL’. An interesting discovery regarding the assembly process was the accumulation of evidence that there is homology between apoB (NH2-terminal region), MTP, and LV. This provided a tool for modeling MTP and parts of the NH2-terminal region of apoB. Using the similarity between the molecular structure of LV and its homology with MTP, a model has been described for MTP and a mechanism proposed for the initial transfer of lipid to apoB Citation41. The process would involve the initial perturbation of lipids within the membrane-like structures in the rough and smooth ER. This perturbation is followed by transfer of some lipid molecules to the MTP:PDI complex and from this protein complex to the nascent vLDL molecule Citation41. This affixes the dependence of the formation of lipoprotein to the lipid transfer activity of MTP:PDI. In fact, other workers have demonstrated the ability of MTP to transfer lipids between liposomal systems Citation53–56.
The pipeline or assembly process of lipoprotein production shown in leaves unanswered the question of how the remaining segments of the nascent apoB chain are stabilized in the early stages of maturation. Some workers have found that lipoproteins are secreted so long as the apoB chain reaches 1-1544 amino acids Citation57. These experiments produced soluble lipoproteins, but it is not clear what would be the result of omitting amino acids from the NH2-terminal of apoB so long as an ER-targeting sequence was present. It is postulated that as the maturing apoB-containing lipoprotein travels through the secretory system, increasing amounts of neutral lipid are added till a mature molecule is formed. Mature lipoproteins presumably leave the cell through a vesicular budding process. The MTP:PDI complex remains in the ER system because the COOH-terminal segment of PDI contains the ER retention sequence -K-D-E-L. However, some MTP does reach the Golgi apparatus Citation58. In fact, the presence of MTP in the Golgi has led to a two-step assembly process; one in which some lipid is added in the ER, and the apoB-containing lipoprotein is further loaded with triglyceride by the MTP present in the Golgi Citation59, Citation60.
In the case of lipoprotein maturation and as apoB enters the ER system, it is possible that the NH2-terminal region of apoB begins to fold and then recognizes the MTP:PDI complex. If proper protein folding does not occur, the secretion system has mechanisms for disposing of the aberrant protein Citation61. In terms of the secretion system, it is clearly necessary for the apoB chain to be completed before processing beyond the rough ER can take place. The ER:Golgi secretion conduit could have any relatively large -COOH segment of apoB start as an unfolded polypeptide chain accompanied by molecules of lipid from the organelle's membrane. The lack of structural data on the COOH-terminal of apoB introduces a significant dilemma for the models to be described in the next section. Based on analyses of the amino acid sequence, much of the COOH-terminal segment is predicted to be β-sheet (extended polypeptide) structure Citation40. As to the transfer of lipids, there is still little evidence on how this occurs. MTP obviously can transfer lipid molecules from micelle to micelle, but this would appear to be a slow and random process. A more appealing mechanism might be to transfer quantities of lipid in multiple small steps via small lipid microdomains (LMDs) as shown in .
A model for apoB-containing lipoprotein assembly
The ER:Golgi system was referred to in an earlier section as a conduit or pipeline for lipoprotein secretion (see ). The proteins involved in the assembly within this organelle could be drug targets for reducing serum levels of blood lipids and therefore go a long way toward reducing atherogenic symptoms. With the hope that the future will bring more structural data on both apoB and MTP, the following section puts forward hypothetical steps along the way to LDL formation. With increased information about the assembly processes, any one stage could be a point of drug interdiction. In developing ideas of these processes, some liberties have been taken. However, the homologs have been proposed by other workers and are themselves speculative. The hypotheses that follow stay within similar limits and in many ways resemble the assembly process proposed by Olofsson and coworkers Citation62.
(1) The synthesis of apoB involves the cotranslocation of the nascent polypeptide chain into the ER. This protein is very large and it appears practicable that folding into a native structure begins before translation of a single apoB message is complete. Hence the NH2-terminal of apoB is likely to have its conformation before the rest of the protein is synthesized, and this could be a factor in directing subsequent steps in the assembly of apoB-containing lipoproteins. Given the homology, the prefolding of the NH2-terminal region of apoB results in a globular domain similar in conformation to LV. Furthermore, through the similarities discussed above, the resulting conformation also appears analogous to that of the ER protein called MTP. MTP is in a complex with PDI, but one of unknown stability.
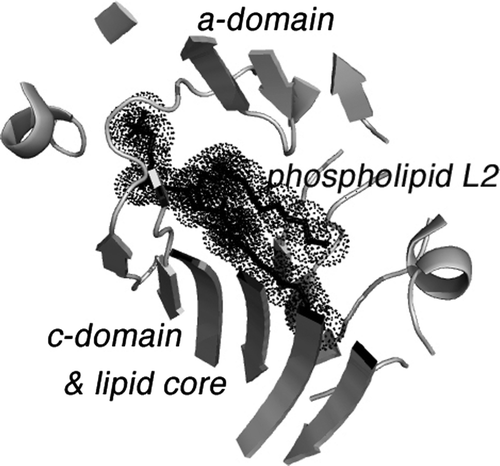
(2) This first domain of apoB binds lipid molecules at sites corresponding to L1 and L2 in LV. The binding event results in the preparation of the rest of this domain for the binding of additional lipid Citation14. The L2 site is shown in expanded form in . These unique lipid binding sites in LV should be present in its homologs, MTP and apoB. By interposing the acyl chains of a phospholipid at this site, one set of the β-sheets shielding the lipid core may move in a sliding fashion. This conformational change would expand the volume of the lipid cavity and allow for the addition of more lipid molecules Citation14. The result is a lipid microdomain (LMD) within the confines of MTP and subsequently in apoB. These proposed micellar structures would have an external surface of relatively randomly oriented hydrocarbon chains with certain regions stabilized by the headgroups of phospholipids. Because of their hydrophobicity, any unbound LMDs would have a relatively short lifetime in an aqueous environment but would be exchangeable with lipid-seeking proteins.
Figure 4. The binding site of phospholipid L2 in lipovitellin. The drawing is a cartoon representing the X-ray crystal structure of LV in a region near the bound phospholipid L2. L2 is visible as a black stick model surrounded by dot spheres. The binding site occurs between β-strands of the a- and c-domains. Note how the two methylene chains of the phospholipid are positioned directly between the two β-sheets, one from the a-domain and another from the c-domain. It is hypothesized that movement at this junction could change the volume of the lipid core, and hence the binding of L2 is a signal for further lipid loading to occur. Removal of L2 could cause contraction of the main lipid cavity and expulsion of some of the accompanying lipid.
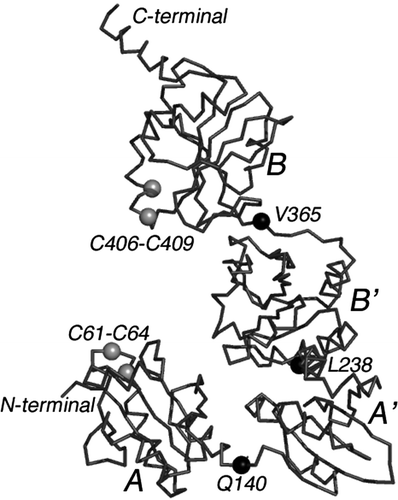
(3) The function of PDI during the assembly of apoB-containing lipoproteins remains very uncertain. PDI may be transiently involved in the formation of the disulfide bonds contained in the NH2-terminal segment of apoB but the nature of these steps in the maturation process is unclear. PDI is said to be in a stable complex with MTP, and the ER contains an excess of this disulfide isomerase. PDI has been copurified with MTP Citation19, Citation63. To date, only the molecular structure of PDI from yeast has been determined, and it is shown in Citation64. The disulfide forming redox centers occur in the A and A' domains. Each of the four domains is approximately 130 residues and is interconnected to the next by what appears to be a relatively flexible polypeptide segment. Each of the three interconnecting segments is marked with a black ball and labeled Q140, L238, and V365. Hence the four domains labeled A, B, B', and A' in may have different steric relationships in the MTP:PDI complex from that shown in . The chemical properties of PDI must depend to some extent on the domain:domain flexibilities.
Figure 5. The backbone structure of yeast PDI. The Cα model of yeast protein disulfide isomerase is shown (PDB accession—2b5e) Citation64. Both the NH2- and COOH-terminals are labeled. As noted in the text, PDI consists of approximately 130 residue domains labeled A, B, B' and A' in the accompanying drawing. Like beads on a string, the four domains are interconnected by what appears to be relatively flexible loops marked approximately in the middle by black balls (Q140, L238, and V365). Disulfide redox centers are present only in domains A and A'. They are labeled as C61-C64 and C406-C409.
(4) In the complete absence of MTP, or by certain mutations of MTP, no form of apoB-containing lipoproteins are generated Citation19, Citation65, Citation66. The MTP:PDI complex responsible for this phenomenon has a MW of 220,000 Citation63. However, the high MW combined with the homology of MTP to LV suggests that the protein complex may actually be a heterotetramer—MTP2PDI2. The chance that MTP forms dimers suggests that it may also be capable of forming heterodimers with apoB. Heterodimer or heterotetramer, this complex resides in the ER and apparently at least one function is to aid in the addition of lipid to apoB.
(5) The hydrophobic nature of the primary lipid binding domains in apoB would have a high affinity for LMDs. In the absence of apoB segments, LMDs would be taken up rapidly by any nearby MTP or membrane surface. Unlike LV and MTP, apoB has extra polypeptide chain to use for nucleating or stabilizing additional lipid. During apoB's movement through the ER:Golgi, more and more lipid is added at various hydrophobic regions along the apoB polypeptide chain. In the absence of MTP, and therefore LMDs, nascent apoB polypeptide chains remain with no lipid, and they are eventually removed from the ER:Golgi. This hypothesis differs from existing suppositions only in that the lipoprotein in its early stages adds lipid molecules at a variety of different sites along the apoB chain. Early on, it probably does not attempt to build a lipid core.
(6) As the vLDL particle continues to expand with the further acquisition of lipid in the form of LMDs, questions arise as to how the addition process stops. In the model presented here, lipid addition would cease when there was no longer any binding sites for LMDs. A two-step lipidation process has been suggested for lipidation that operationally appears to depend on the size of apoB Citation67. A second step in the assembly process might be the coalescence of LMDs to form a contiguous lipid layered core. As lipidation proceeds it is not surprising to find that some heterogeneity occurs in the size of mature lipoprotein molecules. Perhaps the number, composition, and distribution of the LMDs vary as assembly proceeds.
(7) Glycosylation of the apoB chain occurs independently of the lipid addition and is continuous during the maturation process.
(8) The secreted apoB-containing lipoprotein is a roughly spherically shaped molecule with a small protrusion containing the NH2-terminal segment of apoB with the L2 lipid and other lipids within and stabilizing the nucleating cavity. The LMDs contained in any one single molecule may not even fuse but could be stabilized independently by bound water molecules and segments of the apoB polypeptide chain. Some of the LMDs may be easily released by small changes in apoB or the environment.
(9) MTP or the MTP:PDI complex is responsible for the formation of lipid LMDs to be transferred to the maturing apoB polypeptide chain. As shown in , some of the LMDs may be bound to membrane surfaces in the ER/Golgi while some could remain free in solution. However, they are probably metastable being easily incorporated back into the lipid cavity of MTP, reincorporated into the membrane system or aggregating to larger forms of micellar structures and then back to the membrane system.
Summary
The formation of chylomicrons and vLDLs takes place in the secretory apparatus of several different cell types. Because of variation in the overall lipid content, the structure of these relatively large lipoprotein molecules is and will continue to be difficult to obtain by methods such as X-ray crystallography. Trying to add some molecular detail to the processes occurring in the secretory pathway of the ER:Golgi, investigators have discovered a similarity between the lipoprotein involved in oogenesis in oviparous animals and lipid transport in humans. The homology involves LV, MTP, and the NH2-terminal region of apoB. The apoB-containing lipoprotein assembly process is complicated by observations that MTP is believed to exist in the secretion organelle in a complex with PDI. Besides forming a complex with MTP, PDI's function is to aid in disulfide bond formation of secreted proteins including apoB. ER protein:protein complexes in the lipoprotein pathway may therefore include MTP:PDI, apoB-PDI to apoB-MTP, etc. The dimeric crystal structure of the LVs may be used to speculate about complexes that may be present between MTP and the NH2-terminal of apoB. MTP serving to shuttle lipid between ER sources and the maturing apoB-containing lipoproteins may do this by producing relatively small domains of neutral and phospholipid that attach themselves to the more hydrophobic region of apoB (LMDs). The LMDs can be pictured as resembling the lipid within the cavity of LV—having a very hydrophobic but disordered surface.
Acknowledgements
The authors are grateful to Professor C. Shoulders (MRC, Clinical Sciences Center, London, UK) and Dr. J. Thompson (Mayo Medical School, Rochester, MN, USA) for their reading of and helpful comments on early stages of the manuscript. We also recognize and are grateful to the University of Minnesota and the Minnesota Supercomputer Institute for supporting ongoing research in the lipoprotein area during the writing of this manuscript.
References
- Davidson NO, Shelness GS. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu Rev Nutr. 2000; 20: 169–93
- Hevonoja T, Pentikainen MO, Hyvonen MT, Kovanen PT, Ala-Korpela M. Structure of low density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochim Biophys Acta. 2000; 1488: 189–210
- Shelness GS, Sellers JA. Very-low-density lipoprotein assembly and secretion. Curr Opin Lipidol. 2001; 12: 151–7
- Hussain MM, Fatma S, Pan X, Iqbal J. Intestinal lipoprotein assembly. Curr Opin Lipidol. 2005; 16: 281–5
- Parhofer K, Barrett H. What we have learned about VLDL and LDL metabolism from human kinetics studies. J Lipid Res. 2006; 47: 1620–30
- Shoulders CC, Shelness GS. Current biology of MTP: implications for selective inhibition. Curr Top Med Chem. 2005; 5: 283–300
- Olofsson S, Billton P, Asp L. Intracellular assembly of VLDL Two major steps in separate cell compartments. Trends Cardiovasc Med. 2000; 10: 338–45
- Segrest JP, Jones MK, De Loof H, Dashti N. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res. 2001; 42: 1346–67
- Lunin VY, Lunina NL, Ritter S, Frey I, Berg A, Diederichs K, et al. Low-resolution data analysis for low-density lipoprotein particle. Acta Crystallogr D Biol Crystallogr. 2001; 57: 108–21
- Vance JE, Davis RA. Structure, assembly and secretion of lipoproteins. Elsevier, Amsterdam 1996
- Wahli W, Dawid I, Ryffel G, Weber R. Vitellogenesis and the Vitellogenin Gene Family. Science. 1981; 212: 298–304
- Raag R, Appelt K, Xuong NH, Banaszak L. Structure of the lamprey yolk lipid-protein complex lipovitellin-phosvitin at 2.8 A resolution. J Mol Biol. 1988; 200: 553–69
- Anderson TA, Levitt DG, Banaszak LJ. The structural basis of lipid interactions in lipovitellin, a soluble lipoprotein. Structure. 1998; 6: 895–909
- Thompson J, Banaszak L. Lipid-Protein interactions in Lipovitellin. Biochemistry. 2002; 41: 9398–409
- Mann CJ, Anderson TA, Read J, Chester SA, Harrison GB, Kochl S, et al. The Structure of Vitellogenin Provides a Molecular Model For the Assembly and Secretion of Atherogenic Lipoproteins. J Mol Biol. 1999; 285: 391–408
- Babin P, Bogerd J, Kooiman F, Van Marrewijk W, Van der Horst D. Apolipophorin II/I, apolipoprotein B, vitellogenin and microsomal triglyceride transfer protein genes are derived from a common ancestor. J Mol Evol. 1999; 49: 150–60
- Schneider W. Removal of lipoproteins from plasma. Biochemistry of Lipids, Lipoproteins and Membranes, DE Vance, JE Vance. Elsevier, Amsterdam 1996
- Sellers J, Hou L, Schoenberg D, Batistuzzo de Medeiros S, Wahli W, Shelness G. Microsomal triglyceride transfer protein promotes the secretion of Xenopus laevis Vitellogenin A1. J Biol Chem. 2005; 280: 13902–5
- Wetterau J, Zilversmit D. Purification and characterization of microsomal triglyceride and cholesteryl ester transfer protein from bovine liver microsomes. Chem Phys Lipids. 1985; 38: 205–22
- Wetterau J, Lin M, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997; 1345: 136–50
- Cartwright I, Higgins J. Direct evidence for a two-step assembly of apoB48-containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes. J Biol Chem. 2001; 276: 48048–57
- Johs A, Hammel M, Waldner I, May R, Laggner P, Prassl R. Modular structure of solubilized human apolipoprotein B-100. Low resolution model revealed by small angle neutron scattering. J Biol Chem. 2006; 281: 19732–9
- Meyer D, Nealis A, Bruckdorfer K, Perkins S. Characterization of the structure of polydisperse human low-density-lipoprotein by neutron-scattering. Biochem J. 1995; 310: 407–15
- Meyer D, Mayans M, Groot P, Suckling K, Bruckdorfer R, Perkins S. Time-course studies by neutron solution scattering and biochemical assays of the aggregation of human low-density-lipoprotein during Cu2 + -induced oxidation. Biochem J. 1995; 310: 417–26
- Witte D, Taskinen M, Perttunen-Nio H, Van Tol A, Livingstone S, Colhoun H. Study of agreement between LDL size as measured by nuclear magnetic resonance and gradient gel electrophoresis. J Lipid Res. 2004; 45: 1069–76
- Teerlink T, Scheffer P, Bakker S, Heine R. Combined data from LDL composition and size measurement are compatible with a discoid particle shape. J Lipid Res. 2004; 45: 954–66
- Luzzati V, Tardieu A, Aggerbeck L. Structure of serum low-density lipoprotein: I. A solution x-ray scattering study of a hyperlipidemic monkey low-density lipoprotein. J Mol Biol. 1979; 131: 435–73
- Baumstark M, Kreutz W, Berg A, Frey I, Keul J. Structure of human low-density lipoprotein subfractions, determined by x-ray small-angle scattering. Biochim Biophys Acta. 1990; 1037: 48–57
- Muller K, Laggner P, Glatter O, Kostner G. The structure of human-plasma low-density lipoprotein B: An x-ray small-angle scattering study. Euro J Biochem. 1978; 82: 73–90
- van Antwerpen R, Gilkey J. Cryo-Electron microscopy reveals human low density lipoprotein substructure. J Lipid Res. 1994; 35: 2223–31
- Spin J, Atkinson D. Cryoelectron microscopy of low-density-lipoprotein in vitreous ice. Biophys J. 1995; 68: 2115–23
- Orlova E, Sherman M, Chiu W, Mowri H, Smith L, Gotto A. Three-dimensional structure of low density lipoproteins by electron cryomicroscopy. PNAS. 1999; 96: 8420–5
- Sherman M, Orlova EV, Decker G, Chiu W, Pownall H. Structure of triglyceride-rich human low-density lipoproteins according to cryoelectron microscopy. Biochemistry. 2003; 42: 14988–93
- Chatterton R, Phillips M, Curtiss L, Milne R, Fruchart J, Schumaker V. Immunoelectron microscopy of low density lipoproteins yields a ribbon and bow model for the conformation of apolipoprotein B on the lipoprotein surface. J Lipid Res. 1995; 36: 2027–37
- van Antwerpen R, La Belle M, Navratilova E, Krauss R. Structural heterogeneity of apo b-containing serum lipoproteins visualized using cryo-electron microscopy. J Lipid Res. 1999; 40: 1827–36
- Gantz D, Walsh M, Small D. Morphology of sodium deoxycholate-solubilized apolipoprotein B-100 using negative stain and vitreous ice electron microscopy. J Lipid Res. 2000; 41: 1464–72
- Jiang Z, Gantz D, Bullitt E, McKnight C. Defining lipid-interacting domains in the N-terminal region of apolipoprotein B. Biochemistry. 2006; 45: 11799–808
- Jones B, Jones E, Bonney S, Patel H, Mensenkamp A, Eichenbaum-Voline S, et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003; 34: 29–31
- Bradbury P, Mann C, Kochl S, Anderson T, Chester S, Hanock J, et al. A common binding site on the Microsomal Triglyceride Transfer protein for Apolipoprotein B and Protein Disulfide Isomerase. J Biol Chem. 1999; 274: 3159–64
- Segrest J, Jones M, Dashti N. N-terminal domain of apolipoprotein B has structural homology to lipovitellin and microsomal triglyceride transfer protein: a ‘lipid pocket’ model for self-assembly of apoB-containing lipoprotein particles. J Lipid Res. 1999; 40: 1401–16
- Read J, Anderson T, Ritchie P, Vanloo B, Amey J, Levitt D, et al. A mechanism of membrane neutral lipid acquisition by the microsomal triglyceride transfer protein. J Biol Chem. 2000; 275: 30372–7
- Lange R. Lattice parameters as revealed by electron microscopy and a comparison between lipoprotein crystals from cyclostome eggs formed in vivo and in vitro. J Mol Biol. 1984; 179: 765–8
- Lange R. Highly conserved lipoprotein assembly in teleost and amphibian yolk-platelet crystals. Nature. 1981; 289: 329–30
- Lange R, Magdowski G. Lipoprotein crystals in the yolk platelet of a teleost, Pelvicachromis pulcher (cichlidae). Cell Tissue Res. 1980; 209: 511–3
- Lange R, Grodzinski Z, Kilarski W. Yolk-platelet crystals in three ancient bony fishes: Polypterus bichir (polypteri), Amia calva l., and Lepisosteus osseus (l.) (holostei). Cell Tissue Res. 1982; 222: 159–65
- Lange R, Richter H. A symmetric lipovitellin-phosvitin dimer in cyclostome yolk platelet crystals: structural and biochemical observations. J Mol Biol. 1981; 148: 487–91
- Lange R. The lipoprotein crystals of cyclostome yolk platelets (myxine glutinosal., lampetra planeri [bloch], l.fluviatilis [l.]). J Ultrastruct Res. 1982; 79: 1–17
- Murdoch SJ, Boright AP, Paterson AD, Zinman B, Steffes M, Cleary P, et al. LDL composition in E2/2 subjects and LDL distribution by Apo E genotype in type 1 diabetes. Atherosclerosis. 2007; 192: 138–47
- Yang C-Y, Kim T, Weng S-A, Lee B, Yang M, Gotto AM, Jr. Isolation and characterization of sulfhydryl and disulfide peptides of human apolipoprotein B-100. PNAS. 1990; 87: 5523–7
- Thornton J. Disulphide bridges in globular proteins. J Mol Biol. 1981; 151: 261–87
- Timmins P, Poliks B, Banaszak L. The location of bound lipid in the lipovitellin complex. Science. 1992; 257: 652–5
- Rehberg EF, Samson-Bouma ME, Kienzle B, Blinderman L, Jamil H, Wetterau JR, et al. A novel abetalipoproteinemia genotype: identification of a missense mutation in the 97 kDa subunit of the microsomal triglyceride transfer protein that prevents complex formation with protein disulfide isomerase. J Biol Chem. 1996; 271: 29945–52
- Rava P, Ojakian GK, Shelness GS, Hussain MM. Phospholipid transfer activity of microsomal triacylglycerol transfer protein is sufficient for the assembly and secretion of apolipoprotein B lipoproteins. J Biol Chem. 2006; 281: 11019–27
- Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005; 202: 529–39
- Hussain M, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apo B assembly. J Lipid Res. 2003; 44: 22–32
- Magnin DR, Biller SA, Wetterau J, Robl JA, Dickson JK, Jr, Taunk P, et al. Microsomal triglyceride transfer protein inhibitors: discovery and synthesis of alkyl phosphonates as potent MTP inhibitors and cholesterol lowering agents. Bioorg Med Chem Lett. 2003; 13: 1337–40
- Shelness GS, Hou L, Ledford AS, Parks JS, Weinberg RB. Identification of the lipoprotein initiating domain of apolipoprotein B. J Biol Chem. 2003; 278: 44702–7
- Levy E, Stan S, Delvin E, Menard D, Shoulders C, Garofalo C, et al. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. J Biol Chem. 2002; 277: 16470–7
- Innerarity T, Boren J, Yamanaka S, Olofsson S. Biosynthesis of apolipoprotein B48-containing lipoproteins: regulation by novel post-translational mechanisms. J Biol Chem. 1996; 271: 2353–6
- Swift L, Zhu M, Kakkad B, Jovanovska A, Neely M, Valyi-Nagy K, et al. Subcellular localization of microsomal triglyceride transfer protein. J Lipid Res. 2003; 44: 1841–9
- Kaufman R. Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci. 2004; 29: 152–8
- Olofsson S, Asp L, Boren J. The assembly and secretion of apolipoprotein B-containing lipoproteins. Curr Opin Lipidol. 1999; 10: 341–6
- Wetterau J, Combs K, Spinnner S, Joiner B. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem. 1990; 265: 9800–7
- Tian G, Xiang S, Noiva R, Lennarz W, Schindelin H. The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell. 2006; 124: 61–73
- Wetterau J, Combs K, McLean L, Spinner S, Aggerback L. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991; 30: 9728–35
- Shoulders C, Brett D, Narcisi T, Jarmuz A, Grantham T, Leoni P, et al. Abetalipoproteinemia is caused by defects of the gene encoding the 97 kDa subunit of a microsomal triglyceride transfer protein. Hum Mol Gen. 1993; 2: 2109–16
- Stillemark-Billton P, Beck C, Boren J, Olofsson S. Relation of the size and intracellular sorting of apoB to the formation of VLDL 1 and VLDL 2. J Lipid Res. 2005; 46: 104–14
