Abstract
Staphylococcal infections are one of the main causes of complications in patients with implanted foreign prosthetic material. Implants are associated with a significant reduction of the threshold at which contaminating Gram-positive bacteria, particularly Staphylococcus epidermidis, become infectious and develop a biofilm with phenotypic resistance to almost all antibiotics. A 1000-fold increase in minimal bactericidal levels against most antibiotics except rifampin has been repeatedly observed. Since only removal of the foreign material reverses these phenomena, the clinical challenge consists in finding approaches to cure the infection without removal of the implanted device. Rifampin combinations with other antibiotics, administration of exceedingly high antibiotic concentrations in situ, and early therapy before biofilm development are efficacious. Although these strategies have dramatically improved the outcome of foreign body infections, an improved understanding of biofilm-grown S. epidermidis is necessary to develop new antibacterial agents. Here, we review the pathogenesis, prevention, and treatment of implant infections due to S. epidermidis and highlight some new compounds with already promising in vitro results.
Introduction
Implanted medical devices such as central venous catheters (CVC) or joint prostheses are among the most remarkable advances in medicine. The current shift in the demographics of developed countries towards an increasingly aged population has resulted also in the significantly increased use of this medical technology Citation1. In the USA, approximately 400,000 total hip and knee replacements are carried out annually at present Citation1, Citation2. Although the infection rate has been reduced to <1% for hip and <2% for knee orthopaedic implants Citation3, the overall number of patients with infected prostheses has increased due to the increasing number of replacements required Citation2. In the case of infected hip or knee prostheses, treatment costs are 5.3–7.2-fold higher than the initial operations Citation4. Similarly, 250,000–500,000 primary blood-stream infections result each year from the 150 million intravascular devices implanted in the USA Citation5. For CVC-related blood-stream infection, extra costs for each case range from US$4,000 (2560 euro) to US$56,000 (35,840 euro) Citation5. To the best of our knowledge, cost analysis for Europe as a whole or Asia are not available.
Biofilm-associated infection, often caused by coagulase-negative staphylococci (CoNS), in particular Staphylococcus epidermidis, is of increasing importance and related to the high use of implanted devices Citation6, Citation7. This review focuses on S. epidermidis infections of joint prostheses and CVC as the most frequent examples of implant-associated infections.
Methods
We conducted a search of the PubMed database to identify articles in English, French, and German published before 15 September 2007 using the following terms: ‘foreign body’, ‘biofilm’, ‘prosthesis’, ‘implant’, ‘catheter infection’, ‘antibiotic lock technique’, and paired in a second step with ‘Staphylococcus epidermidis’ or ‘coagulase-negative staphylococci’. Among 1738 different publications retrieved, only a minority focused on foreign body infections due to S. epidermidis in the aforementioned search languages and were considered as relevant. Bibliographies of these articles were also hand-searched for additional references. Finally, 165 articles have been consulted and retained for this review.
Key messages
In biofilms, a 1000-fold increase in minimal bactericidal levels against most antibiotics except rifampin has been repeatedly observed.
Rifampin combinations with other antibiotics are efficacious against implant-related infections due to S. epidermidis.
Abbreviations
Epidemiology
Joint replacement
Staphylococcus aureus and CoNS cause approximately two-thirds of joint replacement infections Citation1, Citation3. Infections associated with prosthetic joints can be classified as early (those that develop less than 3 months after surgery), subacute (3–24 months after surgery), or late (more than 24 months after surgery, predominantly acquired by haematogenous seeding). Early infections typically manifest as an acute onset of joint pain, effusion, erythema at the implant site, and fever, and are commonly caused by S. aureus. Patients with subacute infection usually present with subtle signs and symptoms such as implant loosening, persistent joint pain, or both, which may be difficult to distinguish from S. epidermidis Citation3. Of approximately 600 prosthetic joint infections treated over a 5-year period at the Mayo Clinic, Rochester, USA, 30% were due to S. epidermidis Citation8. Among 112 patients with prosthetic joint infections, CoNS were the most frequently isolated pathogens in Oxford, United Kingdom Citation9.
Central venous catheters
On average, microbiologically documented, device-related blood-stream infections occur in 4–5/1000 CVCs with approximately two episodes per 1000 CVC days Citation5. However, these rates are likely underestimated since many episodes of catheter-related sepsis can be culture-negative Citation10, Citation11. Data from the National Nosocomial Infections Surveillance System (NNIS) in the USA from 1990 to 1999 showed that CoNS are the most commonly isolated pathogens (37%) from blood-stream infections in intensive care unit patients Citation12. Other reports confirmed this predominance of CoNS in at least one-third of cases Citation13, Citation14, especially in neonates Citation15. Overall, S. epidermidis accounts for at least 60% of CVC infections with CoNS Citation10. Over the past two decades, the incidence of CoNS primary bacteraemia has increased Citation16, probably due to an improved awareness among clinicians, changes in blood culture techniques, and an increase in the numbers of blood cultures performed and the use of intravascular devices Citation17, Citation18. CVC-related blood-stream infections due to S. epidermidis can reveal a significant mortality, although higher rates are cited in older Citation19 rather than in recent publications Citation18. According to a meta-analysis published in 1998, mortality associated with primary CoNS bacteraemia varies between 5% and 28% Citation17.
Pathogenesis
S. epidermidis is a skin commensal Citation6, Citation7. Most surgical site infections are believed to be acquired at the time of surgery and are caused by endogenous flora Citation20, Citation21. Arguments to support this hypothesis are the efficacy of peroperative antibiotic prophylaxis, and laminar air-flow conditions in the operating room, together with the similarity of skin flora and pathogens. Studies have demonstrated a significant association between distal catheter tip colonization and CVC-related bacteraemia Citation10. Although bacterial colonization may be present at an early stage of infection, it does not necessarily indicate infection. The ultimate proof of implant-associated infection requires the presence of clinical manifestations, infection signs adjacent to the implant, and the growth of S. epidermidis in several microbiological samples Citation1.
Staphylococcus epidermidis
S. epidermidis frequently shows multiresistance to many antibiotics Citation22 acquired through common mechanisms such as efflux pumps, modifying enzymes, target mutations Citation23, and staphylococcal cassette chromosome mec (SCCmec) islands conferring the genomic mec element for methicillin resistance Citation24. According to the 1999 NNIS report, 80% of CoNS had become resistant to methicillin during the previous decade. Resistance to gentamicin has risen to 60%–70%, whereas resistance to rifampin has remained at 10% Citation12. In 1996, the first episode of blood-stream infection in the USA caused by a vancomycin-intermediate S. epidermidis isolate (minimal inhibitory concentration (MIC) 8–6 µg/mL) was reported in the USA Citation25. Methicillin-resistant S. epidermidis (MRSE) is now the most commonly encountered variant of S. epidermidis in many health care institutions Citation12, Citation26, Citation27. As an example, shows the resistance pattern of S. epidermidis at the University of Geneva Hospitals in 2006. Due to the emergence of resistant S. epidermidis isolates through the high use of beta-lactam antibiotics, physicians are obliged to rely on an armamentarium which is increasingly restricted to glycopeptides.
Table I. Antibiotic resistance among all Staphylococcus epidermidis isolates at the University of Geneva Hospitals, January–December 2006.
Apart from antibiotic resistance, a polyclonal expansion of joint Citation28, prosthetic valve Citation29, or CVC-related bacteraemia with S. epidermidis Citation30, has potential implications for the choice of antibiotic regimens. This polyclonality could be one of the explanations for the clinical failure of treatment for foreign body infections or bacteraemias as laboratories do not always perform antibiotic susceptibility testing for all isolates. Thus, a S. epidermidis isolate may be present, but not covered by the antibiotic due to the absence of antibiogram data Citation30. In addition, small-colony variants of S. epidermidis, a phenomenon well known in S. aureus Citation31, can emerge during glycopeptide therapy Citation32. Small colony variants constitute a subpopulation of bacteria and exhibit a slow growth rate, atypical colony morphology, and unusual biochemical characteristics, thus making them a challenge for clinical microbiologists to identify. Clinically, small colony variants are better able to persist in mammalian cells and are less susceptible to antibiotics than their wild-type counterparts, thus more prone to be responsible for recurrent infections Citation33.
S. epidermidis undergoes undefined and complex metabolic changes which, in combination with biofilm formation, allow it to persist on the foreign body and become less susceptible not only to the immune system but also to antibiotics Citation34, Citation35. It is probable that only a small part of these metabolic changes have been explored so far.
Biofilm
Upon introduction into the human body, foreign bodies become rapidly coated by host components. Pathogen attachment is the second step Citation36. Virulence factors such as adhesive proteins, enzymes, and toxins play a role Citation35, Citation37. Interactions involve non-specific physicochemical forces such as van der Waal's forces, hydrophobic interactions, and polarity Citation37, Citation38. Intercellular adhesion requires the synthesis of the polysaccharide intercellular adhesin (PIA) under the control of an intercellular adhesion (ica) operon Citation38, considered as one of the main genetic determinants involved in the accumulation phase during biofilm formation, even if ica or PIA-negative biofilm-forming S. epidermidis infections occur Citation39. In the case of S. epidermidis, exposure to foreign bodies in vitro and in vivo induces a sharp increase in ica expression Citation40 which is significantly correlated with the ability for biofilm formation in contrast to ica-negative isolates Citation39.
Finally, the cells that attach irreversibly to surfaces form microcolonies and produce extracellular polymers that define a biofilm (). Its structure is heterogeneous, both in space and over time, with channels that allow the transport of nutrients and oxygen Citation37. S. epidermidis produces large-size biofilms more frequently than S. aureus Citation41. However, not all S. epidermidis isolates form biofilms Citation42.
Figure 1. A: Intraluminal biofilm on a central venous catheter (with detachment). Scanning electron microscopy (magnification×180). B: Biofilm of Staphylococcus epidermidis on a catheter surface with embedded staphylococcal cells within the matrix. The arrow shows dividing cocci. Scanning electron microscopy (magnification×15,000). 1µ=1µm=0.001 mm.
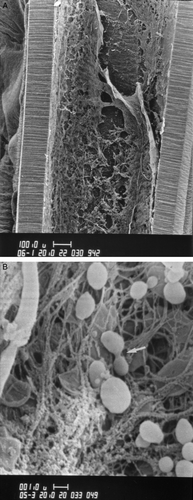
The staphylococcal biofilm has potent immunomodulatory properties. Chemotactic responsiveness is diminished and degranulation of specific granule content is increased. Additionally, the biofilm inhibits the genesis of mononuclear cells, T and B lymphocytes, thus adversely acting both on cytotoxic and humoral defence responses Citation41. Vandecasteele and colleagues observed an impressive decrease in bacterial metabolism and protein synthesis in vitro Citation43. The authors measured directly the amount of 16S rRNA by quantitative polymerase chain reaction (PCR) as a surrogate marker of the actual metabolic activity of S. epidermidis. The initial 16S rRNA content was similar to that observed during the in vitro exponential growth phase. However, an extensive decrease in the 16S rRNA content was reported already beginning only 2 hours after. The ica operon was mainly transcribed during early, but not late infection Citation43.
Biofilms provide significant resistance to antibiotics and innate host defences. Common mechanisms such as drug-modifying enzymes, mutations, and efflux pumps are not involved. Antibiotics penetrate poorly into the thick, acidic matrix. Bacteria in deep layers are metabolically inactive and have an inherent lack of susceptibility to antibiotics Citation44, whereas planktonic cultures of the same organism do not Citation45. This resistance is lost once the biofilm-attached bacteria revert to planktonic growth Citation44. To date, no standardized antimicrobial susceptibility tests are available to evaluate drug activity on adherent bacteria Citation45. Minimal inhibitory concentration and minimal bactericidal concentration evaluate only drug efficacy on planktonic bacteria in the logarithmic phases of growth Citation46. For cell wall active antibiotics to be effective in biofilms, 100 to 1000 times the standard concentration is often required Citation45.
Group behaviour is an important intercellular communication mechanism in bacteria. Small signalling molecules are released in the natural environment and trigger specific responses in a co-ordinated manner in neighbouring bacteria of the same species. This is known as ‘quorum sensing’ and plays an important role in biofilm formation in S. epidermidis Citation47, Citation48.
Neutrophil defects
The interaction of neutrophils with the foreign body can induce a neutrophil defect, enhancing the susceptibility to infection Citation49, Citation50. The inoculum of staphylococci required to induce infection is reduced by several orders of magnitude to as few as 100 colony-forming units Citation49. Ultra-high molecular weight polyethylene wear particles released by prosthetic material may further compromise neutrophil antibacterial activity Citation51.
Prevention
Prevention of prostheses infection: antibiotic prophylaxis
Established risk factors for surgical site infections have been described Citation52. First- and second-generation cephalosporins are generally used in surgery because of their good intrinsic activity against staphylococci, few side-effects, and lower costs Citation53. On the other hand, nosocomial S. epidermidis isolates are often resistant to methicillin and thus to all cephalosporins Citation18, Citation22, Citation26, Citation54, Citation55 (). To our knowledge, there are only two studies, both using cefuroxime as prophylaxis, which evaluated the impact of cephalosporins on the prevention of implant infections due to CoNS. The first study reported that 40% of all positive tissue cultures removed before hip replacement in operating rooms grew MRSE. In the follow-up period, 25% of patients revealed MRSE skin colonization on admission Citation26. The second study reported that 24% of tissue samples in arthroplasty patients were MRSE-positive Citation55. Strikingly, patients with MRSE infections demonstrated lower survival rates than patients with methicillin-sensitive infections. These findings led the authors to introduce a preoperative screening for MRSE carriage in addition to methicillin-resistant S. aureus (MRSA) Citation55. So far, it remains unknown at what moment patients become colonized with MRSE upon hospital admission. Probably colonization occurs very fast. In a Swedish study, the majority of patients on an orthopaedic ward were colonized with methicillin-resistant CoNS at day 14 of admission Citation27. In a prospective survey among surgical patients in Switzerland, only 20% of CoNS sampled from the future surgical site were methicillin-resistant upon admission, but 47% were immediately after elective surgery on the same site Citation56.
Despite these two studies, neither routine MRSE screening nor systematic vancomycin prophylaxis, even for medical implants, should be warranted Citation57 for the following reasons. First, the above-mentioned studies Citation26, Citation55 were retrospective and non-randomized. Second, a large proportion of CoNS isolates may still be susceptible to cephalosporins (). Third, there is an emergence of vancomycin-resistance among enterococci and CoNS Citation12. An increase in the MIC of S. epidermidis against glycopeptide antibiotics has been also observed in vivo Citation58, Citation59. Fourth, vancomycin needs slow infusion to avoid excessive histamine liberation (known as the ‘red man’-syndrome) which makes its use more difficult in a setting where the accurate timing of administration is of the utmost importance Citation60. Fifth, the incidence of prosthetic joint infections due to S. epidermidis is usually low Citation8, Citation9. Sixth, a routine glycopeptide prophylaxis is not a guarantee for decreased infection rates due to methicillin-resistant staphylococci Citation61. A review of four randomized trials comparing prophylactic teicoplanin versus a prophylactic cephalosporin in settings with a high MRSE prevalence identified the same infection rates in both groups Citation61. A recent systematic review and economic model of switching from non-glycopeptide to glycopeptide antibiotic prophylaxis for surgery in endemic MRSA settings failed to show increased efficacy in preventing surgical sites infection due to methicillin-resistant staphylococci Citation62. Even for MRSA, there is insufficient evidence to determine whether there is a threshold prevalence to justify a switch to general glycopeptides prophylaxis Citation62.
Prevention of catheter infection
Implementation of specific guide-lines is known to reduce catheter infection rates Citation63. Prevention strategies Citation10 with a sustained impact over several years have been reported Citation64. Large-scale interventions, including a continuing quality improvement programme and using a multimodal approach, have proven to be effective Citation10, Citation65. According to a meta-analysis, antibiotic and chlorhexidine-silver sulfadiazine coatings are effective to decrease catheter colonization and blood-stream infections for short (∼1 week) insertion durations. In contrast, there is no evidence for a positive effect of silver-impregnated collagen cuffs for either short- or long-term catheter insertion times Citation66. Importantly, no antibiotic prophylaxis for CVC insertion is required Citation63
Treatment
Treatment of infected prostheses due to S. epidermidis
There are no established guide-lines for the treatment of S. epidermidis prosthetic infections due to the absence of randomized trials. Normally, antibiotics should be initially administered parenterally for 2 weeks and followed by an oral therapy for a total treatment duration of 3 months in patients with hip prostheses and 6 months in those with knee prostheses Citation1, Citation3, Citation67. In North America, débridement with device retention and a one-stage exchange (in which the infected prosthesis is removed and a new one implanted in the same procedure) is performed less frequently than in Europe. The interval between resection and reimplantation of the prosthesis in a two-stage exchange is typically 6 weeks Citation1, Citation3, Citation67. Débridement with retention is a reasonable option for patients with an early postoperative or acute haematogenous infection if the duration of clinical signs and symptoms is less than 3 weeks, the implant is stable, the soft tissue is in good condition, and an agent with activity against biofilm micro-organisms is available Citation3. The activity of antibiotic-containing bone cement against S. epidermidis has been proven in vitro Citation68, but no data from large human studies exist. In practice, many centres use gentamicin-containing cement already at primary prosthetic surgery. Resistance to gentamicin, the most widely used compound in cements, which is frequent among S. epidermidis isolates, represents an additional issue Citation12 ().
Ideally, the antimicrobial agent should have bactericidal activity against slow-growing and biofilm-producing bacteria and reveal good bone penetration and oral bioavailability. Rifampin fulfils most of these criteria and can penetrate phagocytes and kill intracellular bacteria Citation69, but may lead to the rapid emergence of rifampin-resistant S. epidermidis during monotherapy. For this reason, rifampin should always be used in combination Citation46, Citation70 and early in the infection process Citation43 before the biofilm has been established. Monzon and colleagues reported a dramatically decreased effect of the vancomycin-rifampin combination for S. epidermidis in biofilms older than 6 hours Citation70.
The only blinded, randomized study hitherto with staphylococcal implant infections (including S. epidermidis) used rifampin in combination with ciprofloxacin. The clinical cure rate was 100% in the ciprofloxacin-rifampin group compared with 58% in the ciprofloxacin-placebo group (P = 0.02) Citation71. Widmer et al. conducted a prospective study of 11 patients with orthopaedic implant infections in whom the device could not be removed, and used rifampin in combination with a beta-lactam antibiotic or ciprofloxacin. The treatment was successful in 9 (82%) of 11 patients Citation72. Drancourt et al. achieved an overall success rate of 74% using a combination of rifampin and ofloxacin for treatment of staphylococcal infections of orthopaedic implants Citation73. Taken together, considering the ease of administration, peroral bioavailability, side-effects, and costs, rifampin administered in combination was revealed to be the most potent choice in vivo ().
Table II. Treatment of foreign body infections due to Staphylococcus epidermidis, 1987–2006, selected reports with references.
A panel of different antibiotics have been used in combination with rifampin, such as cotrimoxazole Citation57, Citation74, fusidic acid Citation57, Citation73, Citation75, tigecycline Citation57, Citation76, daptomycin Citation46, Citation57, Citation77, linezolid Citation22, Citation75, Citation78, quinupristin/dalfopristin Citation22, Citation57, Citation79, dalbavancin Citation57, minocycline Citation74, Citation80, ofloxacin Citation81, ciprofloxacin Citation71, Citation72, and levofloxacin Citation82, Citation83. The efficacy of most of these possible combinations is not evidence-based in humans. For instance, the combination of rifampin with lincosamides (clindamycin, macrolides) or moxifloxacin is still uncertain in humans Citation83. summarizes the activity of new antibiotics in the treatment of foreign body infections due to S. epidermidis.
Table III. Key elements of more recently developed antibiotics in foreign body infection due to Staphylococcus epidermidis, selected reports with references.
Treatment of central venous catheter infection due to S. epidermidis
No prospective, randomized studies have been performed to evaluate the optimal therapeutic option for CVC infections. Catheter removal is mandatory in situations of septic shock, persistent fever, endocarditis, distant metastatic infections, or bacteraemia due to virulent and difficult-to-treat pathogens such as S. aureus, Pseudomonas aeruginosa, or fungi Citation10, Citation63, Citation84. For S. epidermidis, some authors do not recommend any antibiotic treatment after catheter removal. Others, including the authors of this review, prefer to treat CoNS catheter-related infections with intravenous antibiotics for 5–7 days Citation10 because of substantial morbidity and mortality Citation16.
Antibiotic lock technique
For S. epidermidis-related catheter infections, catheter removal can be postponed if the patient's status quickly improves during antibiotic therapy or a new intravascular access is difficult to obtain, such as surgically implanted central venous ports. For this situation, the antibiotic lock technique may be particularly helpful. Presently, there is no evidence-based recommendation for the optimal concentration of antibiotic and/or heparin, or on optimal intraluminal dwell duration Citation63. In practice, an antibiotic solution of 2–5 mg/mL is instilled into the lumen and allowed to dwell for several hours or days Citation85, Citation86, thus enabling the in vitro eradication of S. epidermidis biofilms within 5 days Citation87. Most antibiotics lack anticoagulant characteristics and need to be combined with heparin Citation86. The lock technique can also be performed with ethanol or taurolidine Citation84, Citation86 and should not be used as prophylaxis.
Guide-lines published by the Infectious Diseases Society of America Citation63 advocate daily catheter locking for 14 days in addition to 7–14 days of systemic administration of antibiotics via the infected lumen for uncomplicated catheter infections caused by CoNS, even if this duration is not evidence-based. For S. epidermidis, cure rates are sufficiently high Citation63 and can even reach 92% Citation88. While combination locking seems to be more effective in vitro against CoNS compared to monotherapy, similar outcomes are not evidence-based in vivo yet Citation84.
Perspectives
Despite significant progress in the understanding of foreign body infections due to S. epidermidis, improved knowledge of metabolic properties of biofilm-grown bacteria is still needed. The high genetic variability of the S. epidermidis genome and the discovery of specific genomic elements promoting its invasiveness Citation89 may offer the possibility to develop new drug targets. For example, Balaban and colleagues proposed to prevent S. epidermidis foreign body infections in rats by using a quorum-sensing inhibitor Citation90. Additional prospective trials need to be performed before this concept and other innovative approaches such as urokinase Citation91, bacteriophages Citation92, ultrasonically enhanced antibiotic activity Citation93, or electric current treatment of biofilm Citation94 may prove to be superior to established combined antibiotic therapies.
Acknowledgements
We are indebted to Rosemary Sudan for editorial assistance and to Dr P. Rohner and Professor J. Schrenzel from the Central Laboratory of Bacteriology of the University of Geneva Hospitals for the information provided in .
Declaration of interest: The authors report no conflicts of interest: no financial support, grants, financial interests or consultancy that could lead to a conflict of interest. The authors alone are responsible for the content and writing of the paper.
All authors state that they have read and approved the manuscript. It has not been published elsewhere, nor is it under consideration for publication elsewhere. Parts of this review have been presented on 11 October 2006 as an abstract and oral presentation at the Third International Symposium on Resistant Gram-Positive Infections, Niagara-on-the Lake, Canada.
References
- Darouiche RO. Treatment of infections associated with surgical implants. New Engl J Med. 2004; 350: 1422–9
- Widmer AF. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis 2001; 33(suppl 2)S94–106
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. New Engl J Med. 2004; 351: 1645–54
- Bengtson S. Prosthetic osteomyelitis with special reference to the knee: risks, treatment and costs. Ann Med. 1993; 25: 523–9
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81: 1159–71
- Rupp ME, Archer GL. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–43; quiz 44–5.
- Kloos WE, Bannerman TL. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994; 7: 117–40
- Steckelberg JM, Osmon DR. Prosthetic joint infections. In: Bisno AL, Waldvogel FA. Infections associated with indwelling medical devices. 3rd ed. Washington, DC: American Society for Microbiology Press; 2000. p. 173–209.
- Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect. 2007; 55: 1–7
- Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000; 355: 1864–8
- Hugonnet S, Sax H, Eggimann P, Chevrolet JC, Pittet D. Nosocomial bloodstream infection and clinical sepsis. Emerg Infect Dis. 2004; 10: 76–81
- National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990–May 1999, issued June 1999. Am J Infect Control. 1999;27:520–32.
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999; 27: 887–92
- Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999; 29: 239–44
- Hira V, Sluijter M, Estevao S, Horst-Kreft D, Ott A, de Groot R, et al. Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates. Pediatr Infect Dis J. 2007; 26: 607–12
- Thylefors JD, Harbarth S, Pittet D. Increasing bacteremia due to coagulase-negative staphylococci: fiction or reality?. Infect Control Hosp Epidemiol. 1998; 19: 581–9
- Eggimann P, Pittet D. Overview of catheter-related infections with special emphasis on prevention based on educational programs. Clin Microbiol Infect. 2002; 8: 295–309
- Falcone M, Micozzi A, Pompeo ME, Baiocchi P, Fabi F, Penni A, et al. Methicillin-resistant staphylococcal bacteremia in patients with hematologic malignancies: clinical and microbiological retrospective comparative analysis of S. haemolyticus, S. epidermidis and S. aureus. J Chemother. 2004; 16: 540–8
- Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol. 1999; 20: 396–401
- Haas JP, Evans AM, Preston KE, Larson EL. Risk factors for surgical site infection after cardiac surgery: the role of endogenous flora. Heart Lung. 2005; 34: 108–14
- Whitehouse JD, Sexton D. Epidemiology and pathogenesis of and risk factors for surgical site infection. UpToDate Online. 2007;16:1 ( www.ufdol.com).
- Raad I, Alrahwan A, Rolston K. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin Infect Dis. 1998; 26: 1182–7
- Petrelli D, Zampaloni C, D'Ercole S, Prenna M, Ballarini P, Ripa S, et al. Analysis of different genetic traits and their association with biofilm formation in Staphylococcus epidermidis isolates from central venous catheter infections. Eur J Clin Microbiol Infect Dis. 2006; 25: 773–81
- Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005; 187: 2426–38
- Garrett DO, Jochimsen E, Murfitt K, Hill B, McAllister S, Nelson P, et al. The emergence of decreased susceptibility to vancomycin in Staphylococcus epidermidis. Infect Control Hosp Epidemiol. 1999; 20: 167–70
- Mohanty SS, Kay PR. Infection in total joint replacements. Why we screen MRSA when MRSE is the problem?. J Bone Joint Surg. 2004; 86: 266–8
- Thore M, Kuhn I, Lofdahl S, Burman LG. Drug-resistant coagulase-negative skin staphylococci. Evaluation of four marker systems and epidemiology in an orthopaedic ward. Epidemiol Infect. 1990; 105: 95–105
- Galdbart JO, Morvan A, Desplaces N, el Solh N. Phenotypic and genomic variation among Staphylococcus epidermidis strains infecting joint prostheses. J Clin Microbiol. 1999; 37: 1306–12
- Van Wijngaerden E, Peetermans WE, Van Lierde S, Van Eldere J. Polyclonal staphylococcus endocarditis. Clin Infect Dis. 1997; 25: 69–71
- Rijnders BJ, Van Wijngaerden E, Van Eldere J, Peetermans WE. Polyclonal Staphylococcus epidermidis intravascular catheter-related infections. Clin Microbiol Infect. 2001; 7: 388–91
- Vaudaux P, Kelley WL, Lew DP. Staphylococcus aureus small colony variants: difficult to diagnose and difficult to treat. Clin Infect Dis. 2006; 43: 968–70
- Adler H, Widmer A, Frei R. Emergence of a teicoplanin-resistant small colony variant of Staphylococcus epidermidis during vancomycin therapy. Eur J Clin Microbiol Infect Dis. 2003; 22: 746–8
- Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nature Rev. 2006; 4: 295–305
- Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes Infect. 2002; 4: 481–9
- Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003; 112: 1466–77
- Peters G. Pathogenesis of S epidermidis foreign body infections. Brit J Clin Prac. 1988; 57: 62–5
- Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001; 33: 1387–92
- von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002; 2: 677–85
- Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001; 39: 2151–6
- Vandecasteele SJ, Van Eldere J, Merckx R, Peetermans WE. The effect of systemic antibiotics on the microbiological diagnosis of experimental foreign body infections caused by Staphylococcus epidermidis. Diag Microbiol Infect Dis. 2004; 48: 89–95
- Lew D, Schrenzel J, Francois P, Vaudaux P. Pathogenesis, prevention, and therapy of staphylococcal prosthetic infections. Curr Clin Top Infect Dis. 2001; 21: 252–70
- Rohde H, Burandt EC, Siemssen N, Frommelt L, Burdelski C, Wurster S, et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials. 2007; 28: 1711–20
- Vandecasteele SJ, Peetermans WE, Carbonez A, Van Eldere J. Metabolic activity of Staphylococcus epidermidis is high during initial and low during late experimental foreign-body infection. J Bacteriol. 2004; 186: 2236–9
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scot HM. Microbial biofilms. Ann Rev Microbiol. 1995; 49: 711–45
- Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999; 37: 1771–6
- Widmer AF, Frei R, Rajacic Z, Zimmerli W. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis. 1990; 162: 96–102
- Vadyvaloo V, Otto M. Molecular genetics of Staphylococcus epidermidis biofilms on indwelling medical devices. Int J Artif Organs. 2005; 28: 1069–78
- Mack D, Davies AP, Harris LG, Rohde H, Horstkotte MA, Knobloch JK. Microbial interactions in Staphylococcus epidermidis biofilms. Anal Bioanal Chem. 2007; 387: 399–408
- Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984; 73: 1191–200
- Johnson GM, Lee DA, Regelmann WE, Gray ED, Peters G, Quie PG. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986; 54: 13–20
- Bernard L, Vaudaux P, Huggler E, Stern R, Frehel C, Francois P, et al. Inactivation of a subpopulation of human neutrophils by exposure to ultrahigh-molecular-weight polyethylene wear debris. FEMS Immunol Med Microbiol. 2007; 49: 425–32
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132; quiz 3–4; discussion 96.
- Kaiser AB, Petracek MR, Lea JW, Kernodle DS, Roach AC, Alford WC, Jr, et al. Efficacy of cefazolin, cefamandole, and gentamicin as prophylactic agents in cardiac surgery. Results of a prospective, randomized, double-blind trial in 1030 patients. Ann Surg. 1987; 206: 791–7
- Widerstrom M, Monsen T, Karlsson C, Wistrom J. Molecular epidemiology of meticillin-resistant coagulase-negative staphylococci in a Swedish county hospital: evidence of intra- and interhospital clonal spread. J Hosp Infect. 2006; 64: 177–83
- James PJ, Butcher IA, Gardner ER, Hamblen DL. Methicillin-resistant Staphylococcus epidermidis in infection of hip arthroplasties. J Bone Joint Surg. 1994; 76: 725–7
- Zingg W, Demartines N, Ruef C. Reduced susceptibility of coagulase-negative staphylococci in surgical patients after surgical procedures. 45th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 16–19 December, 2005. Abstract no. K-1378; 2005.
- Trampuz A, Zimmerli W. Antimicrobial agents in orthopaedic surgery: prophylaxis and treatment. Drugs. 2006; 66: 1089–105
- Vedel G, Leruez M, Lemann F, Hraoui E, Ratovohery D. Prevalence of Staphylococcus aureus and coagulase-negative staphylococci with decreased sensitivity to glycopeptides as assessed by determination of MICs. Eur J Clin Microbiol Infect Dis. 1990; 9: 820–2
- Sloos JH, van de Klundert JA, Dijkshoorn L, van Boven CP. Changing susceptibilities of coagulase-negative staphylococci to teicoplanin in a teaching hospital. J Antimicrob Chemother. 1998; 42: 787–91
- Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. New Engl J Med. 1992; 30: 281–6
- Mini E, Nobili S, Periti P. Methicillin-resistant staphylococci in clean surgery. Is there a role for prophylaxis? Drugs. 54 suppl 1997; 6: 39–52
- Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, , et al. A systematic review and economic model of switching from non-glycopeptide to glycopeptide antibiotic prophylaxis for surgery. Health Technol Assess. 2008;12: iii–iv, xi–xii, 1–147.
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001; 32: 1249–72
- Eggimann P, Hugonnet S, Sax H, Harbarth S, Chevrolet JC, Pittet D. Long-term reduction of vascular access-associated bloodstream infection. Ann Intern Med. 2005; 142: 875–6
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. New Engl J Med. 2006; 355: 2725–32
- Walder B, Pittet D, Tramer MR. Prevention of bloodstream infections with central venous catheters treated with anti-infective agents depends on catheter type and insertion time: evidence from a meta-analysis. Infect Control Hosp Epidemiol. 2002; 23: 748–56
- Bernard L, Hoffmeyer P, Assal M, Vaudaux P, Schrenzel J, Lew D. Trends in the treatment of orthopaedic prosthetic infections. J Antimicrob Chemother. 2004; 53: 127–9
- Anagnostakos K, Kelm J, Regitz T, Schmitt E, Jung W. In vitro evaluation of antibiotic release from and bacteria growth inhibition by antibiotic-loaded acrylic bone cement spacers. J Biomed Mater Res B Appl Biomater. 2005; 72: 373–8
- Mandell GL. The antimicrobial activity of rifampin: emphasis on the relation to phagocytes. Rev Infect Dis 1983; 5(suppl 3)S463–7
- Monzon M, Oteiza C, Leiva J, Amorena B. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J Antimicrob Chemother. 2001; 48: 793–801
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998; 279: 1537–41
- Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin Infect Dis. 1992; 14: 1251–3
- Drancourt M, Stein A, Argenson JN, Roiron R, Groulier P, Raoult D. Oral treatment of Staphylococcus spp. infected orthopaedic implants with fusidic acid or ofloxacin in combination with rifampicin. J Antimicrob Chemother. 1997; 39: 235–40
- Archer GL, Johnston JL, Vazquez GJ, Haywood HB3rd. Efficacy of antibiotic combinations including rifampin against methicillin-resistant Staphylococcus epidermidis: in vitro and in vivo studies. Rev Infect Dis. 1983; 5(suppl 3)S538–42
- Saginur R, Stdenis M, Ferris W, Aaron SD, Chan F, Lee C, et al. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006; 50: 55–61
- Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob Agents Chemother. 2007; 51: 1556–8
- LaPlante KL, Mermel LA. In vitro activity of daptomycin and vancomycin lock solutions on staphylococcal biofilms in a central venous catheter model. Nephrol Dial Transplant. 2007; 22: 2239–46
- Mogenet I, Raetz-Dillon S, Canonge JM, Archambaud M, Bonnet E. Successful treatment of Staphylococcus epidermidis hip prosthesis infection with oral linezolid. Ann Pharmacother. 2004; 38: 986–8
- Saleh-Mghir A, Ameur N, Muller-Serieys C, Ismael F, Lemaitre F, Massias L, et al. Combination of quinupristin-dalfopristin (Synercid) and rifampin is highly synergistic in experimental Staphylococcus aureus joint prosthesis infection. Antimicrob Agents Chemother. 2002; 46: 1122–4
- Isiklar ZU, Darouiche RO, Landon GC, Beck T. Efficacy of antibiotics alone for orthopaedic device related infections. Clin Orthop Relat Res. 1996; 332: 184–9
- Gagnon RF, Richards GK, Kostiner GB. Time-kill efficacy of antibiotics in combination with rifampin against Staphylococcus epidermidis biofilms. Adv Perit Dial. 1994; 10: 189–92
- Soriano A, Jurado A, Marco F, Almela M, Ortega M, Mensa J. In vitro activity of linezolid, moxifloxacin, levofloxacin, clindamycin and rifampin, alone and in combination, against Staphylococcus aureus and Staphylococcus epidermidis]. Rev Esp Quimioter. 2005; 18: 168–72
- Lemmen SW, Hafner H, Klik S, Lutticken R, Zolldann D. Comparison of the bactericidal activity of moxifloxacin and levofloxacin against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli and Klebsiella pneumoniae. Chemotherapy. 2003; 49: 33–5
- Simon A, Bode U, Beutel K. Diagnosis and treatment of catheter-related infections in paediatric oncology: an update. Clin Microbiol Infect. 2006; 12: 606–20
- Sherertz RJ, Boger MS, Collins CA, Mason L, Raad II. Comparative in vitro efficacies of various catheter lock solutions. Antimicrob Agents Chemother. 2006; 50: 1865–8
- Vercaigne LM, Sitar DS, Penner SB, Bernstein K, Wang GQ, Burczynski FJ. Antibiotic-heparin lock: in vitro antibiotic stability combined with heparin in a central venous catheter. Pharmacotherapy. 2000; 20: 394–9
- Lee JY, Ko KS, Peck KR, Oh WS, Song JH. In vitro evaluation of the antibiotic lock technique (ALT) for the treatment of catheter-related infections caused by staphylococci. J Antimicrob Chemother. 2006; 57: 1110–5
- Rubin LG, Shih S, Shende A, Karayalçin G, Lanzkowsky P. Cure of implantable venous port-associated bloodstream infections in pediatric hematology-oncology patients without catheter removal. Clin Infect Dis. 1999; 29: 102–5
- Yao Y, Sturdevant DE, Villaruz A, Xu L, Gao Q, Otto M. Factors characterizing Staphylococcus epidermidis invasiveness determined by comparative genomics. Infect Immun. 2005; 73: 1856–60
- Balaban N, Giacometti A, Cirioni O, Gov Y, Ghiselli R, Mocchegiani F, et al. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J Infect Dis. 2003; 187: 625–30
- Nakamoto DA, Rosenfield ML, Haaga JR, Merritt K, Sachs PB, Hutton MC, et al. In vivo treatment of infected prosthetic graft material with urokinase: an animal model. J Vasc Interv Radiol. 1994; 5: 549–52
- Cerca N, Oliveira R, Azeredo J. Susceptibility of Staphylococcus epidermidis planktonic cells and biofilms to the lytic action of staphylococcus bacteriophage K. Lett Appl Microbiol. 2007; 45: 313–7
- Carmen JC, Roeder BL, Nelson JL, Beckstead BL, Runyan CM, Schaalje GB, et al. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. J Biomater Appl. 2004; 18: 237–45
- van der Borden AJ, van der Mei HC, Busscher HJ. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials. 2005; 26: 6731–5
- Wayne, PA. Performance standards for antimicrobial susceptibility testing. CLSI. 2007 ( www.clsi.org).
- Evans RC, Holmes CJ. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob Agents Chemother. 1987; 31: 889–94
- Pascual A, Ramirez de Arellano E, Perea EJ. Activity of glycopeptides in combination with amikacin or rifampin against Staphylococcus epidermidis biofilms on plastic catheters. Eur J Clin Microbiol Infect Dis. 1994; 13: 515–7
- Peck KR, Kim SW, Jung SI, Kim YS, Oh WS, Lee JY, et al. Antimicrobials as potential adjunctive agents in the treatment of biofilm infection with Staphylococcus epidermidis. Chemotherapy. 2003; 49: 189–93