Abstract
The development of secondary sexual characters, the petasma, and thelycum growth were studied in Xiphopenaeus kroyeri. In adult females, the thelycum is a single plate and its anterolateral portion is characterized by a reduced hood. The aperture resembles a transverse ridge. In immature stages, the ridge has a space between the plates, which becomes narrower as it reaches the end of development. The female gonopore is ‘comma’ shaped. In adult males, the endopods of the petasma are linked at the dorsomedial margin by a large quantity of cincinnuli. In juveniles, cincinnuli gradually increase in number until they join both endopods. At the end of development the petasma is T-shaped. The male gonopore is C-shaped. The relative growth of the petasma total length versus juvenile body length showed a highly positive allometry, whereas in adults the growth was isometric. For the relationship carapace length versus thelycum width, the juvenile phase of females is characterized by an isometry and the adult phase by a negative allometry.
Introduction
Penaeoidean shrimps are gonochoristic with external fertilization (Malecha and Hedgecock Citation1989). These crustaceans in natural populations are sexually dimorphic, and females are larger than males in most of the known species, for instance in Rimapenaeus constrictus (Stimpson 1874) studied by Costa and Fransozo (Citation2004), and Xiphopenaeus kroyeri (Heller 1862) studied by Nakagaki and Negreiros-Fransozo (Citation1998) and Castro et al. (Citation2005).
The secondary sexual characters of penaeoid shrimp include the thelycum in females, and the petasma and appendix masculina in males. The morphology of such structures is characteristic of each species, and a useful tool in the taxonomy of this group (Dall et al. Citation1990; Pérez-Farfante and Kensley Citation1997).
According to Bauer (Citation1986), the thelycum of penaeoids is any external modification of female's posterior (somites 13–14) thoracic sternites and/or coxae that are related to sperm transfer and storage. In closed-thelycum species, the external plates are modified to form the seminal receptacles, internal invaginations of the cuticle (cuticular bags or pouches within the body cavity; Bauer Citation1991). In open-thelycum species, the seminal receptacles are absent (Pérez-Farfante and Kensley Citation1997).
The petasma is a complex structure formed from the joined endopods of the first pleopods in male penaeoid shrimps (Bauer Citation1991). The shape of the petasma generally varies among species (Pérez-Farfante and Kensley Citation1997); however, in most cases, the type of attachment of the first pleopod pair is the same (Tuma Citation1967; Tirmizi Citation1968; Tirmizi and Javed Citation1976; Hassan Citation1981). The union of the endopod of the first pleopod is assured by means of very small hooks, known as cincinnuli, which are found on the medial face of each endopod.
The petasma type is related to the degree at which the ventral face of the endopod reaches the other face. In the open petasma, the lateral lobes are completely flexible, partially flexible or totally expanded, with the ventral face directed, or not, to the ventral region. In the semi-open petasma, the lateral lobes are flexible but not folded, with the ventral face distinctly directed ventromesially, delimiting a relatively large space which extends from the proximal to the distal portion. In the semi-closed petasma, the lateral lobes are not very flexible, markedly folded, supported by strong ribs, with the ventral face more joined, delimiting a large space in the distal region, where they usually overlap the well-developed projections in the dorsomedial region. In the closed petasma, the lateral lobes are strongly sclerotized, sometimes rigid, with the ventral face situated ventromesially, delimiting a small space; the lateral lobe usually shows lateral expansions like horns (Pérez-Farfante and Kensley Citation1997).
The petasma may function for insemination, but there is no firm evidence that it actually has such a role (Bauer Citation1996). In fact, there are only ideas and hypotheses in the literature (Burkenroad Citation1934; Pérez-Farfante and Kensley Citation1997) about the role of the petasma. The only one paper found in the literature that tested the usual hypothesis of closed-petasma function is that of Bauer (Citation1996) in a study of Sicyonia dorsalis. Bauer (Citation1996) did not find any evidence that the petasma injects sperm into the female seminal receptacles. Instead, he proposed other hypotheses, e.g. a female-stimulating (courtship) device, or a mechanism to attach the male so that one of his gonopores is near to the female while the male's genital papilla injected sperm into the female seminal receptacle.
Xiphopenaeus kroyeri (Heller 1862) has a closed thelycum with a single smooth broad plate of sternite XIV, and its anterolateral hood is extremely reduced. The proximal anterior sternal invagination is as broad as a sternite, forming a spacious pocket extending to the posterior thoracic ridge. The median protuberance of sternite XIII is also broad but considerably shorter (Pérez-Farfante and Kensley Citation1997). The seminal receptacle is a bilobed pair of invaginated sac-like sperm receptacles, whose apertures are hidden by the thelycum, and the main lobes may extend back into the first pleonic somite (Burkenroad Citation1934).
Xiphopenaeus kroyeri has a semi-closed petasma. It is symmetrical, containing large lateral lobes like horns and distolateral projections (Pérez-Farfante and Kensley Citation1997).
In closed-thelycum shrimps, mating occurs between males in the intermoult period and recently moulted females (Misamore and Browdy Citation1996), whereas in open-thelycum species, mating occurs when both sexes are in the intermoult period (Yano et al. Citation1988; Dall et al. Citation1990).
The analysis of secondary sexual characters in Crustacea began with Hartnoll (Citation1974), who assessed the relative growth of certain body structures for different brachyuran species. This author verified that there are some differences in the growth of certain body parts of individuals within a population, between sexes and among developmental phases for each sex within the same species. The growth rate in crustacean decapods generally changes during their ontogeny and the growth includes a series of phases in which the growth rate is constant. Allometric analysis often shows a shift in reproductive morphology after the puberty moult. A graphic representation of the species relative growth can evidence the coincidence of the line inflexion with the change from juvenile to adult phase. However, in some cases, the relationship is evidenced by a point overlap in some size classes that corresponds to a transition period between juvenile and adult phase of the species.
The lack of knowledge on secondary sexual differentiation and its importance in the reproductive biology of X. kroyeri, as well as good parameters in rational exploitation of natural stocks, preventing commercial capture of specimens smaller than the maturation size justifies this analysis. In this sense, this study provides information on the secondary sexual characters of X. kroyeri, including the thelycum morphology, described for males and females, but only in adult shrimps (Pérez-Farfante and Kensley Citation1997). Scanning electron microscopy (SEM) was used in the study of the development of the secondary sexual characters of this species. In addition, the petasma and thelycum morphometry of X. kroyeri were analysed using an allometric technique.
Material and methods
Xiphopenaeus kroyeri specimens were collected by trawling in Ubatuba Bay (23°25′S; 45°03′W), Ubatuba, São Paulo, Brazil, and transported alive to the laboratory in buckets filled with seawater. The seabob shrimp is easily identified among the other shrimp species from the littoral of São Paulo because the size of its rostrum is the largest among all species. It has five unequal teeth and 4th and 5th well-developed pereopods.
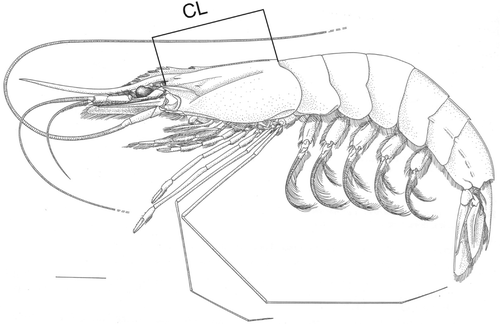
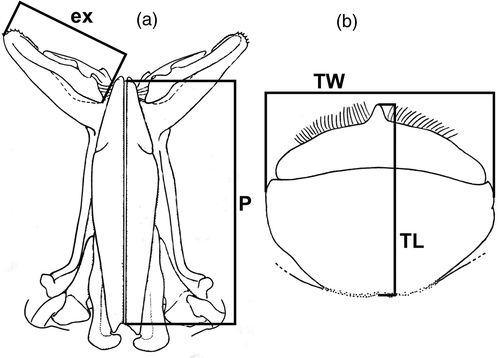
The size of the shrimps was measured as carapace length (CL), which is the linear distance between the postero-orbital margin and the median notch of the posterior margin of the carapace ().
In the laboratory, 15 females (from 6.0 to 28.0 mm CL) and 10 males (from 5.0 to 26.0 mm CL) were anesthetized by cold and each thelycum and petasma were dissected for SEM preparations.
Scanning electron microscopy
For SEM, samples were fixed in 2.5% glutaraldehyde solution in 0.1 M phosphate buffer (pH 7.3) from 24 to 48 h. Then, they were postfixed in 1% osmium tetroxide solution in 0.1 M phosphate buffer (pH 7.3) for 2 h, dehydrated through a graded series of acetone, critical-point-dried with liquid CO2, and sputtered-coated with gold. Samples were analysed and photographed under Quanta 200 SEM from the FEI Company. The setal descriptions were based on Garm (Citation2004).
Measurement procedures and calculation of the petasma and thelycum relative growth
A sub-sample of shrimp specimens was measured for CL. In males, the length of the endopod of the first pleopod (=petasma) was measured; P means pleopod without terminal expansion; P + ex means pleopod including the terminal expansion size (). In females, the thelycum length (TL) and thelycum width (TW) () were measured.
Figure 2. P, petasma length; ex, petasma terminal expansion; TW, thelycum width; TL, thelycum length. Scale bar = 10 mm. (Modified from Pérez-Farfante and Kensley Citation1997.).
CL dimensions were obtained with a vernier caliper and appendage sizes were measured under a stereomicroscope containing an ocular rule provided with an image system. The morphological condition of the petasma, i.e., linked or separated endopods, was also verified and recorded for each specimen.
The data were plotted in graphs, the point dispersion was analysed and the power function was adjusted to the empiric points. CL was used as the independent variable and petasma or thelycum size as the dependent variable. The growth analysis was performed separately for each group of specimens, i.e. with linked and separated endopods or juveniles and adult specimens. The size in which petasma or thelycum growth differs between the juvenile and the adult phases, i.e. the size of the sexual morphological maturity, was determined using ‘K-means clustering’. This procedure is based on the establishment of pre-determined groups, attributing each specimen to one of the groups by means of an iterative process that minimizes the variances within groups and maximizes the variances between groups. Then, a discriminate analysis was performed, allowing a new classification of such groups, which isolated them in distinct categories: juveniles and adults. Then, the power function was transformed to the logarithm form (ln Y = ln a + bln X).
When size overlap does not occur between the juvenile and adult groups, the size of sexual morphological maturity can be obtained by the intersection value of the group equations. When size overlap occurs between these groups, the size of sexual morphological maturity can be obtained by the size at which 50% of individuals (males or females) of the population are mature (CL50). In this procedure, the relative frequencies of mature individuals are associated with size class and then the logistic function Y = 1/(1 + e − r ( X − CL50)) can be applied, where ‘CL50’ is the size of the sexual morphological maturity and ‘r’ is the slope. The adjustments of the parameters are performed by the minimum square method (Vazzoler Citation1996).
In the allometric analysis, the constant ‘b’ (slope of the line) means the allometric degree of the body part studied (petasma or thelycum) and ‘a’ is the ‘y-intercept’. Positive allometry is characterized by b > 1, negative allometry by b < 1, and isometry by b = 1 (Huxley Citation1950). The ‘b’ value found in each relationship was tested by a Student's t-test with the 5% significance level. A covariance analysis (ANCOVA, α = 5%) was used to test the slopes and, when necessary, intercepts of each studied relationship (Zar Citation1996).
This statistical procedure followed those carried out by Sampedro et al. (Citation1999) and Corgos and Freire (Citation2006).
Results
Analysis of the secondary sexual characters
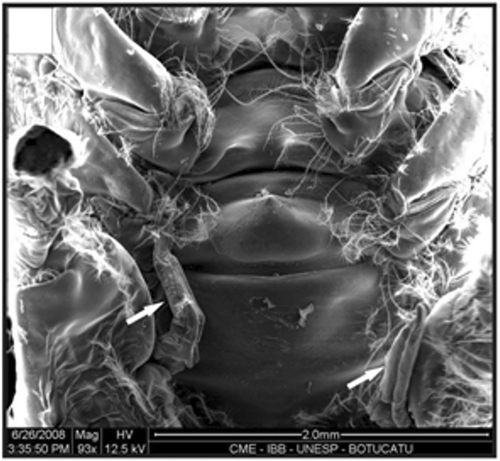
In adult females (>10 mm CL), the thelycum is a single smooth broad plate originating in the region of sternites 13 and 14. The anterolateral portion of this structure is characterized by an extremely reduced hood (Figures ). The thelycum aperture resembles a transverse ridge that extends from the right to the left (Figures ).
Figure 3. Thelycum drawing of X. kroyeri. Transversal ridge (arrow); gonopore (G); hood (*). Scale bar = 1 mm. (Modified from Pérez-Farfante and Kensley Citation1997).
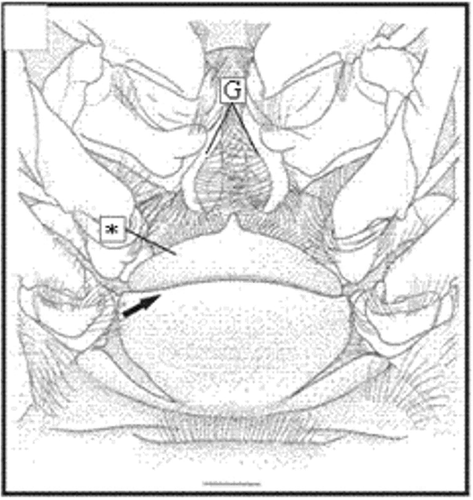
Figure 4. Macroscopy of the thelycum of one female (11.2 mm CL). Transversal ridge (arrow); gonopore (G); hood (*). Scale bar = 1.0 mm.
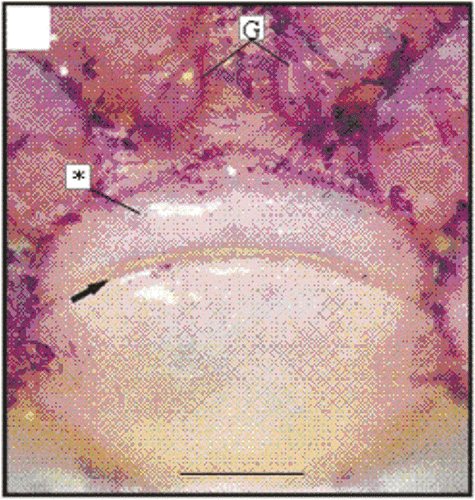
Figure 5. SEM of the thelycum of a female (28.5 mm CL). Transversal ridge (arrow); gonopore (G); hood (*). Scale bar = 2.0 mm.
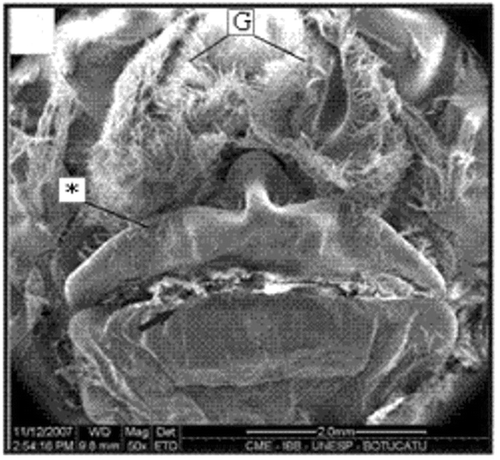
Figure 6. Thelycum of a specimen with 15.3 mm CL. Transversal ridge (arrow); gonopore (G); hood (*). Scale bar = 2 mm.
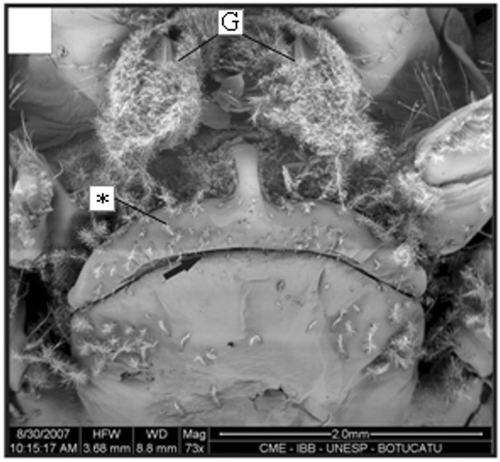
In immature stages, when the thelycum begins its development, the ridge has a wide space between the plates (), which becomes narrower as the final development is reached ( and ). The posterior plate grows gradually, covering part of the anterolateral plate. Both plates form two pockets underneath. There is slight variation in the setation pattern during the thelycum ontogeny. Also, during the thelycum development, the gonopores change from a round shape ( and ) to a curved with a significant increase in the number of papoose setae (). The morphological features of an adult petasma can be observed in males larger than 11 mm CL (Figures ).
Figure 7. Thelycum of a specimen with 7.5 mm CL. Transversal ridge (arrow); gonopore (G); hood (*). Scale bar = 1 mm.
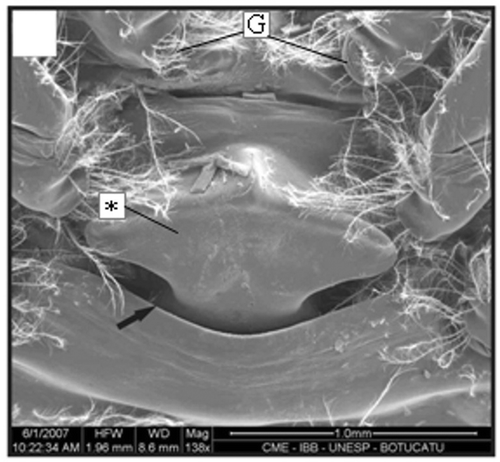
Figure 8. Thelycum of a specimen with 10.2 mm CL. Transversal ridge (arrow); gonopore (G); hood (*). Scale bar = 2 mm.

Figure 9. Gonopore of a female with 7.5 mm CL. Gonopore opening (arrow); setae (C). Scale bar = 100 µm.
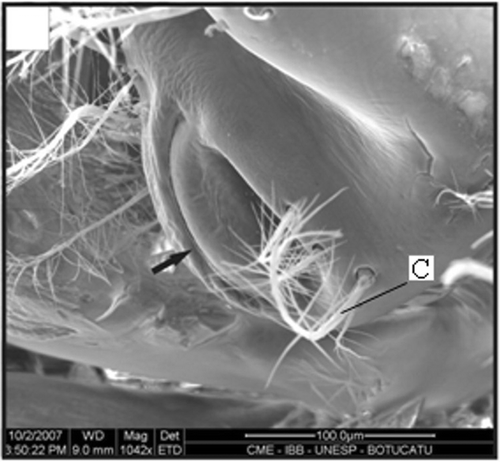
Figure 10. Gonopore of a female with 10.2 mm CL. Gonopore opening (arrow); setae (C). Scale bar = 400 µm.
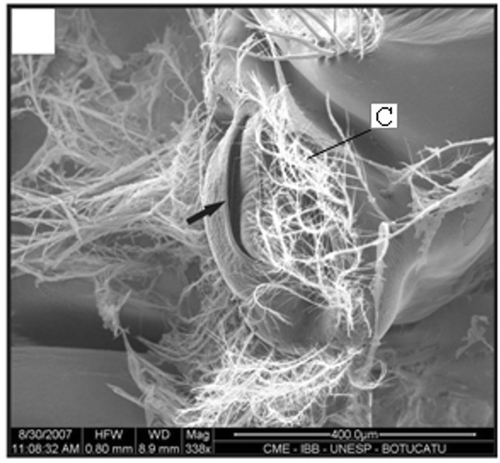
Figure 11. Gonopore of a female with 15.3 mm CL. Gonopore opening (arrow); setae (C). Scale bar = 500 µm.
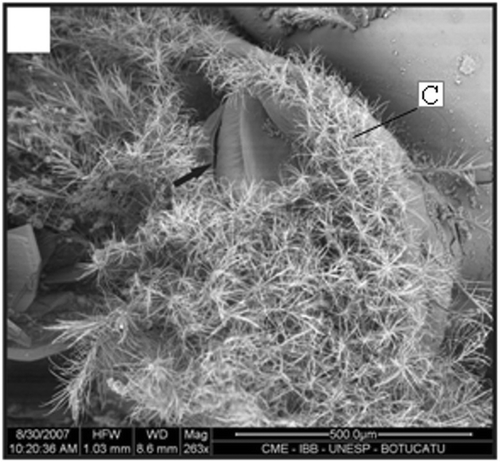
Figure 12. Dorsal view drawing of the petasma of X. kroyeri. Region with cincinnuli (arrow); terminal expansion (E). Scale bar = 1 mm. (Modified from Pérez-Farfante and Kensley Citation1997).
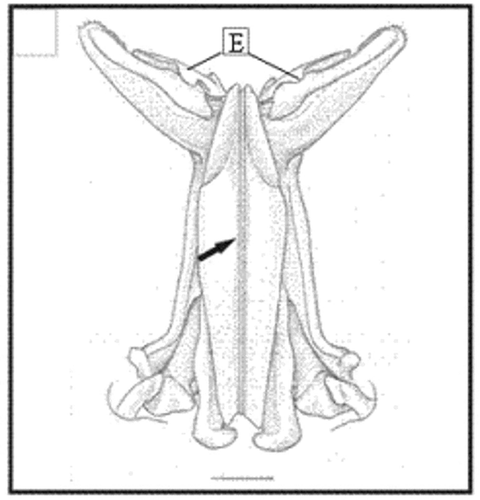
Figure 13. Macroscopy of the dorsal view of the petasma of a male (10.3 mm CL). Region with cincinnuli (arrow); terminal expansion (E). Scale bar = 1 mm.
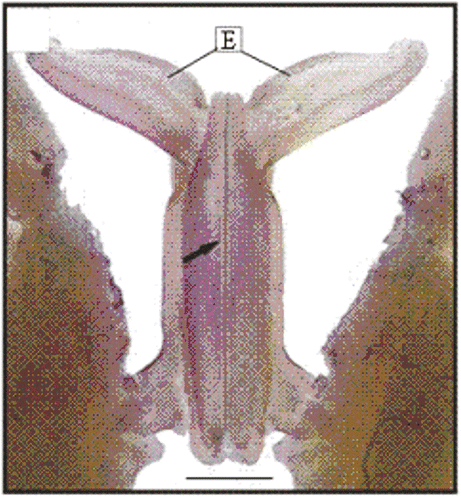
Figure 14. SEM of the dorsal view of the petasma of one specimen (22.1 mm CL). Region with cincinnuli (arrow); terminal expansion (E). Scale bar = 2 mm.
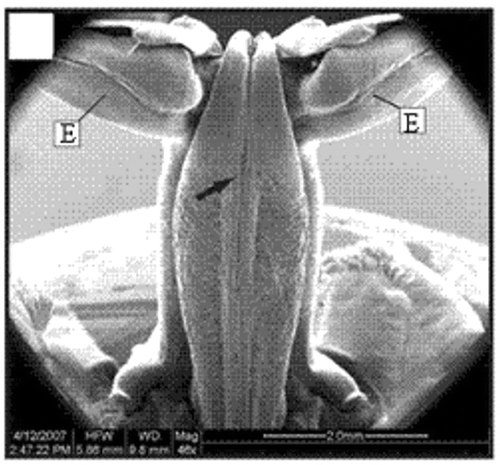
Figure 15. Ventral view of a petasma of a specimen with 22.1 mm CL. Cincinnuli (arrow). Scale bar = 2 mm.
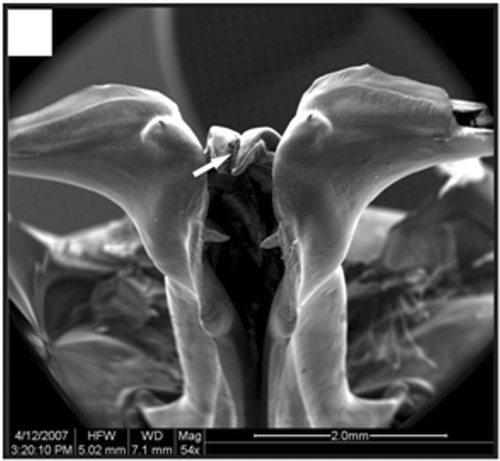
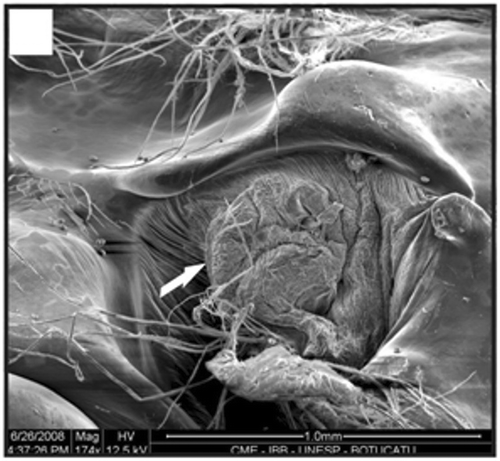
In adult males, the petasma is joined at the dorsomedial margin by a large quantity of small hooks named cincinnuli (Figures ), forming a channel. In juvenile males, the endopods of the first pleopod pairs are separated () and the cincinnuli are absent or present in a small number (). At the beginning of their development, the endopods are straight. The number of cincinnuli gradually increases until the complete union of both endopods. Additionally, the most distal portion of the endopod becomes curved and perpendicular to the proximal portion of the appendage forming the petasma, as the shrimp reaches maturity. At this stage, the petasma is T-shaped (Figures ) and its distal portion has small spines on the ventral face (). During petasma development, the gonopores grow but do not change their shape and, unlike the females, do not have setae ().
Figure 16. Detail of the dorsal view of the petasma in a specimen with 12.4 mm CL. Cincinnuli (arrow). Scale bar = 50 µm.
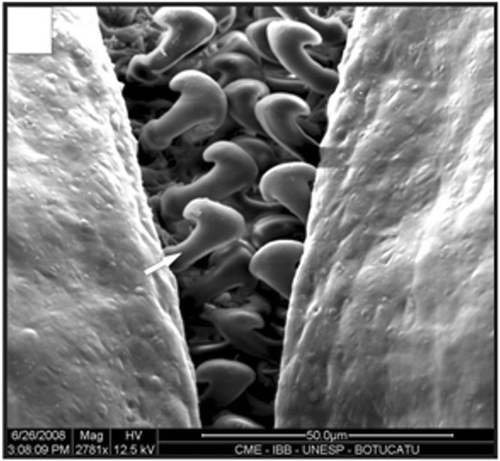
Figure 18. Detail of a petasma of a specimen with 10.1 mm CL. Cincinnuli (arrow). Scale bar = 200 µm.

Petasma and thelycum relative growth
A total of 115 males (from 5 to 26 mm CL) and 78 females (from 7.3 to 29.5 mm CL) were used for morphometric analyses.
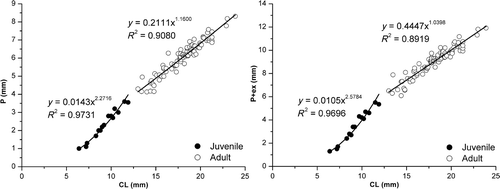
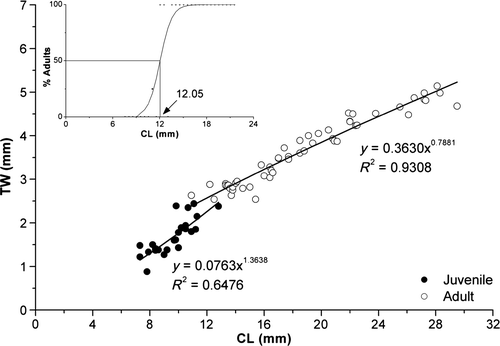
For males, the graphic representation of the relationships P versus CL and P + ex versus CL is shown in . The result of X. kroyeri petasma relative growth () shows that during the juvenile phase, the growth has a highly positive allometry (p < 0.05), and during the adult phase, it can show isometry (p > 0.05, for P + ex) or slightly positive allometry (p < 0.05, for P). The values of sexual morphological maturity for males, obtained by means of lines intersection for juveniles and adult shrimps, were 11.26 mm CL for the relationship P versus CL and 11.42 mm CL for the relationship P + ex versus CL.
Table 1. Results of the allometric analysis for X. kroyeri: Petasma length (P and P + ex) vs. CL; TL vs. CL and TW vs. CL.
For females, the graphic representation of the relationship TW versus CL is shown in . There is size overlap between juvenile and adult females. Therefore, the size of the sexual morphological maturity, in which 50% of the female specimens are mature (CL50), is 12.05 mm CL. The sizes of the smallest adult female and that of the largest juvenile female were 10.9 and 12.8 mm, respectively. The result of X. kroyeri TW relative growth () shows that during the juvenile phase, the growth is isometric (p > 0.05) and during the adult phase it shows a negative allometry (p < 0.05).
With respect to the TL versus CL relationship, there were no significant differences in the relative growth between the juveniles and adult specimens (ANCOVA, p > 0.05). Thus, the data were adjusted to just one equation (, J + A) that shows isometry (p > 0.05).
Discussion
Studies on the secondary sexual characters of penaeoidean shrimps include those of Tuma (Citation1967), Tirmizi (Citation1968), Tirmizi and Javed (Citation1976), and Hassan (Citation1981), which showed macroscopic aspects of the development of these characters in Fenneropenaeus merguiensis (=Penaeus merguiensis), Parapenaeopsis stylifera, Metapenaeus stebbingi, and Metapenaeus affinis, respectively. Our study indicates that males larger than 11 mm CL and females larger than 10 mm CL already have mature secondary sexual characters, i.e. completely formed. Thus, they are ready for reproduction and can be considered adults. According to Castro et al. (Citation2005), males and females of X. kroyeri larger than 11.6 ± 1.8 mm are adults (based on the macroscopic observation of the external morphology of their secondary sexual characters). However, in these size classes the gonads should be studied by histology to determine accurately whether such animals are capable of copulation and spawning.
Xiphopenaeus kroyeri can only be considered adult when females obtain the complete transverse ridge in the thelycum and the comma-shaped gonopore is entire, with a large quantity of setae. The increase in setation around the gonopore might be related to the protection of oocytes prior to fertilization, but there is no experimental evidence in the literature to support this assertion. Males can only be regarded as adults after the union of the endopods, which forms the petasma, considering that specimens in this size range already have mature gonads (Castro et al. Citation2005).
The type of thelycum and petasma among shrimp species is closely associated with the reproductive behaviour, the male–female disposition during mating, and the moult stage condition of females in the reproductive period (Bueno Citation1990; Dall et al. Citation1990; Misamore and Browdy Citation1996). Thus, in closed-thelycum shrimp species, the thelycum aperture direction and the petasma shape can determine the male position in relation to the female during copulation. Open-thelycum shrimp species show a complex spermatophore structure (Bauer Citation1986, Citation1991; Bauer and Cash Citation1991; Chow et al. Citation1991; Bauer and Min Citation1993). In those species, the vas deferens is divided into two parallel ducts; one contains the sperm mass and the other has accessory substances that serve to fix the spermatophoric mass to the thelycum of the respective female until fertilization of oocytes. In these cases, a semi-open petasma is generally present ().
Table 2. Comparison of petasma and thelycum types among shrimp species from the littoral of São Paulo State, Brazil (Pérez-Farfante and Kensley Citation1997).
In closed-thelycum shrimp species, spermatophores are not complex (Bauer Citation1986, Citation1991; Bauer and Min Citation1993; Subramoniam Citation1995). The vas deferens is a single tube that contains material for the formation of several small spermatophores and has a plug substance (Burkenroad Citation1934; Bauer and Min Citation1993) that is formed in a chamber of the ejaculatory duct. In this case, the petasma is semi-closed or closed ().
Seabob shrimps found in nature with incomplete petasma probably have not reproduced yet, but they were not examined with respect to their gonads in this study. Thus, further studies with respect to gonad maturation should clarify this aspect.
The application of the allometric technique to study the relative growth in shrimps has not been reported in the literature, especially concerning secondary sexual characters. This study on petasma relative growth revealed that, during the juvenile phase, the growth rate is high for both relationships used herein. In both cases, the coefficient values (b) are higher than 1, i.e. 2.3 for P and 2.6 for P + ex. This probably means that once the adult phase is reached, P grows in a similar rate to that of CL, tracking the growth of females and showing compatible sizes for mating.
The most detailed paper on the gonad development of a penaeoidean concerning secondary sexual characters is that of Tuma (Citation1967), who worked with F. merguiensis (De Man 1888). This author considered that the best criterion to identify the maturity of a shrimp is its capability to mate. Tuma (Citation1967) verified that F. merguiensis males have functional gonads right before the complete formation of the petasma, whereas females became functional only after the total formation of the thelycum.
Although shows that the adult phase for X. kroyeri males begins at approximately 11.3 mm CL, our laboratory observations have indicated that some individuals have a complete petasma from 11 mm CL. With respect to females, shows that the adult phase begins at approximately 12.05 mm CL, although some individuals have a complete thelycum morphology from 10 mm CL. These results may be due to the small number of obtained specimens, mainly in the 10–14-mm CL size range. The utilization of a larger number of individuals could either yield a graph with overlapping lines for the size classes in which the change from the juvenile to the adult phase occurs, or show a conspicuous separation between these two phases, corroborating the results of this study.
In X. kroyeri, petasma growth, relative to carapace growth, has a considerable positive allometry before puberty and isometry after it due to the attainment of the adequate proportions for the reproductive process. The petasma is integrated with the other reproductive organs, characterizing a highly efficient mechanism for spermatophore transference. Nevertheless, it is not known if the petasma actually works in such transference.
This study provides information for ecological, reproductive, systematic or phylogenetic studies. Pérez-Farfante (Citation1970) used secondary sexual characters to differentiate juveniles from three species of the genus Penaeus, proving the importance of such features for taxonomy. As mentioned by Costa et al. (Citation2003), the differentiation between two species of the genus Farfantepenaeus in the Brazilian littoral is based on the petasma and thelycum shapes. The great difficulty in the identification of juvenile shrimps is the lack of information on the early ontogeny of secondary sexual characters, which are generally very useful in the correct determination of marine shrimps.
Acknowledgements
We are grateful to Elisa Aparecida Gregório, the Centro de Microscopia Eletrônica – UNESP – Botucatu – São Paulo, Brazil, for SEM preparations; to Capes for the Master Science fellowship to the first author; to FAPESP (94/4878-4; 98/3136-4 and 04/15194-6) for sampling funding and to Gustavo Luis Hirose for his help with the statistical assistance. We are especially indebted to Dr Raymond Bauer for his valuable comments on this article. Shrimp samplings were performed according to State and Federal laws concerning wild animals.
References
- Bauer , RT . 1986 . Phylogenetic trends in sperm transfer and storage complexity in decapod crustaceans . Journal of Crustacean Biology , 6 ( 3 ) : 313 – 325 .
- Bauer , RT . 1991 . “ Sperm transfer and storage structures in Penaeoid shrimps: a functional and phylogenetic perspective ” . In Crustacean sexual biology , Edited by: Bauer , RT and Martin , JW . 183 – 207 . New York : Columbia University Press .
- Bauer , RT . 1996 . Role of petasma and appendices masculinae during copulation and insemination in the penaeoid shrimp Sicyonia dorsalis (Crustacea: Decapoda: Dendrobranchiata) . Invertebrate Reproduction and Development , 29 ( 3 ) : 173 – 184 .
- Bauer , RT and Cash , CE . 1991 . Spermatophore structure and anatomy of the ejaculatory duct in Penaeus setiferus, P. duorarum, and P. aztecus (Crustacea: Decapoda): homologies and functional significance . Transactions of the American Microscopy Society , 110 ( 2 ) : 144 – 162 .
- Bauer , RT and Min , LJ . 1993 . Spermatophores and plug substance of the marine shrimp Trachypenaeus similis (Crustacea: Decapoda: Penaeidae): formation in the male reproductive tract and disposition in the inseminated female . Biological Bulletin , 185 : 174 – 185 .
- Bueno , SLS . 1990 . Maturation and spawning of the white shrimp Penaeus schmitti Burkenroad, 1936, under large scale rearing conditions . Journal of the World Aquaculture Society , 21 ( 3 ) : 170 – 179 .
- Burkenroad , MD . 1934 . The Penaeidea of Louisiana with a discussion of the world relationships . Bulletin of the American Museum of Natural History , 68 : 61 – 143 .
- Castro , RH , Costa , RC , Fransozo , A and Mantelatto , FLM . 2005 . Population structure of the seabob shrimp Xiphopenaeus kroyeri (Heller 1962) (Crustacea: Penaeoidea) in the littoral of São Paulo, Brazil . Scientia Marina , 69 ( 1 ) : 105 – 112 .
- Chow , S , Dougherty , MM , Dougherty , WJ and Sandifer , PA . 1991 . Spermatophore formation in the white shrimps Penaeus setiferus and P. vannamei . Journal of Crustacean Biology , 11 ( 2 ) : 201 – 216 .
- Corgos , A and Freire , J . 2006 . Morphometric and gonad maturity in the spider crab Maja brachydactyla: a comparison of methods for estimating size at maturity in species with determinate growth . ICES Journal of Marine Science , 63 : 851 – 859 .
- Costa , RC and Fransozo , A . 2004 . Abundance and ecological distribution of the shrimp Rimapenaeus constrictus (Crustacea: Penaeidae) in the northern coast of São Paulo, Brazil . Journal of Natural History , 38 ( 7 ) : 901 – 912 .
- Costa , RC , Fransozo , A , Melo , GAS and Freire , FAM . 2003 . An illustrated key for Dendrobranchiata shrimps from the northern coast of São Paulo state, Brazil . Biota Neotropica , 3 ( 1 ) : 1 – 12 .
- Dall , W , Hill , BJ , Rothlisberg , PC and Staples , DJ . 1990 . The biology of the Penaeidae . Advances in Marine Biology , 27 : 1 – 489 .
- Garm , A . 2004 . Revising the definition of the crustacean seta and setal classification systems based on examinations of the mouthpart setae of seven species of decapods . Zoological Journal of the Linnean Society-London , 142 : 233 – 252 .
- Hartnoll , RG . 1974 . Variation in growth pattern between some secondary sexual characters in crabs (Decapoda, Brachyura) . Crustaceana , 27 ( 2 ) : 131 – 136 .
- Hassan , HU . 1981 . The genital organs and their development in Metapenaeus affinis (Decapoda, Penaeidae) studied through rearing them in the laboratory . Hydrobiologia , 78 : 49 – 58 .
- Huxley , JS . 1950 . Relative growth and form transformation . Proceedings of Royal Society of London , 137(B) : 465 – 469 .
- Malecha , SR and Hedgecok , D . 1989 . Prospects for the domestication and breeding of marine shrimp , Sea Grant Technical Report Honolulu, , USA : University of Hawaii Sea Grant College Program .
- Misamore , MJ and Browdy , CL . 1996 . Mating behavior in the white shrimps Penaeus setiferus and P. vannamei: a generalized model for mating in Penaeus . Journal of Crustacean Biology , 16 ( 1 ) : 61 – 70 .
- Nakagaki , JM and Negreiros-Fransozo , ML . 1998 . Population biology of Xiphopenaeus kroyeri (Heller 1862) (Decapoda: Penaeidae) from Ubatuba Bay, São Paulo, Brazil . Journal of Shellfish Research , 17 ( 4 ) : 931 – 935 .
- Pérez-Farfante , I . 1970 . Caracteristicas diagnosticas de los juveniles de Penaeus aztecus aztecus, P. duorarum y P. brasiliensis (Crustacea, Decapoda, Penaeidae) . Memórias de la Sociedad de Ciencias Naturales La Salle , 30 : 159 – 182 .
- Pérez-Farfante , I and Kensley , B . 1997 . Penaeoid and Sergestoid shrimps and Prawns of the world. Keys and diagnosis for the families and genera . Mémoires du Muséum National D’Histoire Naturelle , 175 : 1 – 233 .
- Sampedro , MP , González-Gurriarán , E , Freire , J and Muiño , R . 1999 . Morphometry and sexual maturity in the spider crab Maja squinado (Decapoda: Majidae) in Galicia, Spain . Journal of Crustacean Biology , 19 ( 3 ) : 578 – 592 .
- Subramoniam , T . 1995 . Light and electron microscopic studies on the seminal secretions and the vas deferens of the penaeiodean shrimp, Sicyonia ingentis . Journal of Biosciences , 20 ( 5 ) : 691 – 706 .
- Tirmizi , NM . 1968 . On the structure and some developmental stages of genitalia in the prawn Parapenaeopsis stylifera (H. Milne Edwards) (Decapoda, Penaeidea) . Crustaceana , 15 : 193 – 203 .
- Tirmizi , NM and Javed , W . 1976 . Study of juveniles of Metapenaeus stebbingi Nobili (Decapoda, Penaeidae) with particular reference to the structure and development of the genitalia . Crustaceana , 30 ( 1 ) : 55 – 67 .
- Tuma , DJ . 1967 . A description of the development of primary and secondary sexual characters in the banana prawn, Penaeus merguiensis de Man (Crustacea: Decapoda: Penaeidae) . Australian Journal of Marine and Freshwater Research , 18 : 73 – 88 .
- Vazzoler , AEAM . 1996 . Biologia da reprodução de peixes teleósteos: teoria e prática , 169 Maringá, , Brazil : EDUEM .
- Yano , I , Kanna , RA , Oyama , RN and Wyban , JA . 1988 . Mating behavior in the penaeid shrimp Penaeus vannamei . Marine Biology , 97 : 171 – 175 .
- Zar , JH . 1996 . Biostatistical analysis, , 3rd , 915 Upper Saddle River (NJ) : Prentice-Hall .