Abstract
The male reproductive system of the bobtail squid Neorossia caroli (Cephalopoda: Sepiolidae) is described in detail, based on observations of 90 mature males caught from 500 to 1600 m depth in Sardinian waters (western Mediterranean Sea). Reproductive organs in mature specimens accounted for up to 6% of total body weight. Of this, 70% was represented by the spermatophoric complex. Up to 83 spermatophores were found inside the Needham's sac. Mean spermatophore length was 16.7 mm. Sperm mass, cement body, and ejaculatory apparatus represented 63.2%, 13.1%, and 23.7% of the total spermatophore length, respectively. Inverted spermatophores, empty spermatophore sheaths, and spermatangia were also found in the sac, and their presence is discussed. Spermatangia implanted in several parts of the bodies of males (e.g., head, funnel, and eyes) were recorded and their occurrence is discussed. The spermatophoric reaction was induced in the laboratory by submerging spermatophores in seawater, and it is described briefly.
Introduction
Reproduction is a key phase in the life history of all living organisms and understanding the different reproductive strategies of each species is a key to shedding light on their entire life cycle. This is certainly true for many cephalopods, given their fast growth and relatively short life span compared to those of the vertebrates that share the same habitat and compete with them.
The Carol bobtail squid Neorossia caroli (Joubin 1902) is distributed in the Mediterranean Sea and in the eastern Atlantic Ocean from south-western Iceland and Ireland, southwards to the Gulf of Guinea, and the Namibian coast of southern Africa; doubtful and sporadic records exist for the western Atlantic, from the southern slope of the Great Newfoundland Bank, the slope of Nova Scotia, and the Gulf of Mexico (Reid and Jereb Citation2005). It is the deepest living sepiolid, and it is collected at the depths of 1744 m in the western Mediterranean basin (Villanueva Citation1992), and to 1535 m in the eastern Atlantic (Collins et al. Citation2001).
Though of minor commercial importance, usually it is taken as a trawl fishery by-catch and it is sold fresh and frozen in fish markets, mixed with Rossia macrosoma and other bobtail squids. Separate statistics are not reported, but captures of this species, along with those of other sepiolids, can be quite consistent in some Mediterranean areas (e.g., Jereb et al. Citation1998), where they represent a non-negligible contribution to local fisheries.
In spite of its wide distribution and rather common presence in Mediterranean bottom trawl catches, the main information on the species’ life cycle and reproductive biology is still from the studies carried out in the early 1960s in the Catalan Sea (Mangold-Wirz Citation1963a, Citation1963b), with some additional information available from a few other studies (e.g., Reid Citation1991; Jereb et al. Citation1998; Cuccu et al. Citation2007; Krstulovic-Sifner et al. Citation2007). The need to re-describe the species (Reid Citation1991) and the more recent tentative attribution of Neorossia specimens recorded from Falkland Islands’ waters to another subspecies, N. caroli jeannae (Nesis et al. Citation2001), emphasize the importance of improving our knowledge on the genus Neorossia and the species that comprise it.
Observations on the reproductive biology of females of the Sardinian population (western Mediterranean) were recently carried out, and a continuous spawning strategy was suggested (Cuccu et al. Citation2007). Several sperm reservoirs (spermatangia) were found embedded in the mantle of mated females (Cuccu et al. Citation2007). While the implantation of spermatangia is a common sperm transfer strategy (Nesis Citation1995), the mechanism responsible for spermatangia implantation, recently investigated in Rossia moelleri (Hoving et al. Citation2009), is not yet fully understood. The aim of the research reported here is to improve our knowledge of the reproductive biology of N. caroli of the southern Sardinian waters, focusing on the male reproductive system.
Materials and methods
Samples of N. caroli were collected during daytime by bottom trawling on sandy and muddy bottoms in Sardinian waters, throughout the year in 2008, at depths between 500 and 1600 m. Upon collection, specimens were deep frozen. Dorsal mantle length (ML; to the nearest 0.1 mm) and total weight (TW; to the nearest 0.01 g) were recorded on thawed animals. Males were considered mature when spermatophores were present in the Needham's sac. Total gonad weight (GW), testis weight (TeW), and spermatophoric complex weight (SpCW) to the nearest 0.01 g were recorded on a sample of 90 mature gonads after preservation in 4% formalin. The gonadosomatic [GSI = GW × 100/TW] and Hayashi [HI = SpCW/(SpCW + TeW)] indices were also computed.
Spermatophores and spermatangia length (i.e., SpL; SgL; to the nearest 0.01 mm) were measured on a sample of 200 spermatophores from 40 specimens (ML: 24.0–51.0 mm; TW: 11.3–56.6 g) and the number of both structures was recorded. The spermatophore length index was also computed (SpLI = SpL × 100/ML).
The male reproductive system of five freshly caught specimens was photographed for details. After dissection, a few spermatophores were submerged in artificial seawater in order to observe in vitro the spermatophoric reaction. Following Mann (Citation1984), this is defined as “the extrusion of the ejaculatory apparatus, the advance (and evagination) of the sperm rope and the formation of the spermatic bladder (or spermatangium), the bulbous structure formed by the evaginated tunic, into which the sperm mass is propelled”.
Results
Ninety mature males were examined. They were caught year-round, their size varying between 22 and 52 mm ML (i.e., 36.6 ± 8.5 mm) and their weight between 11.1 and 56.6 g TW (i.e., 29.2 ± 10.6 g; ). The reproductive system represents approximately 6% of the TW in mature specimens, and the HI values indicate that the spermatophoric complex gives the largest contribution in weight to the gonad (). In this study, 1 to 83 spermatophores (28 ± 26) were found inside 90 Needham's sacs analyzed. Spermatophores length ranged between 12.03 and 21.53 mm (16.69 ± 2.38 mm) and SpLI varied between 30.2% and 66.9% (42.9 ± 9.4%). Sperm mass, cement body, and ejaculatory apparatus constituted 63.2%, 13.1%, and 23.7% of the total spermatophore length, respectively (). In most of the examined Needham's sacs (i.e., 84%), intact spermatophores were found along with inverted spermatophores (1–69), empty spermatophore sheaths (1–30), and spermatangia (1–8; ; ). As shown in , spermatangia, the final product of the spermatophoric reaction, lack the empty spermatophore sheets and the external tunic that covers the trailing end in the inverted spermatophores. Spermatangia measured between 8.56 and 8.60 mm (mean 8.58 ± 0.01 mm; 20 spermatangia from 10 specimens) in length and consisted of an oral sperm mass (21%) and a trailing end (79%; ). Fresh spermatophores submerged in seawater inverted after 30–90 s. No spermatangia resulted from the spermatophoric reaction induced in this study. Spermatangia were found implanted in several parts of the bodies of 13 males. The number of spermatangia per specimen varied between 1 and 12 (). They were found around the eyes, on the head, arms, funnel, and the neck ().
Figure 1. (A) Internal anatomy of a mature N. caroli male: testis (ts) and spermatophoric complex (sc); (B) Spermatophore: ejaculatory apparatus (ea), cement body (cb), and sperm mass (sm); (C) Inverted spermatophore: sperm mass (sm), trailing end (te), and empty spermatophore sheath (es); (D) Spermatangium: sperm mass (sm) and trailing end (te); and (E) Empty spermatophore sheath.
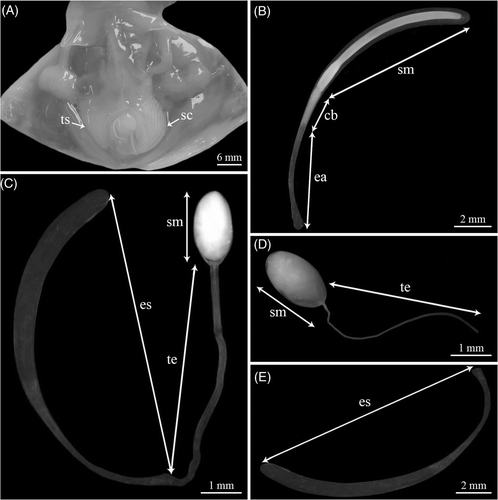
Figure 2. Spermatangia implanted on the head of male N. caroli: behind the eyes (A) and in different locations of the head (B).
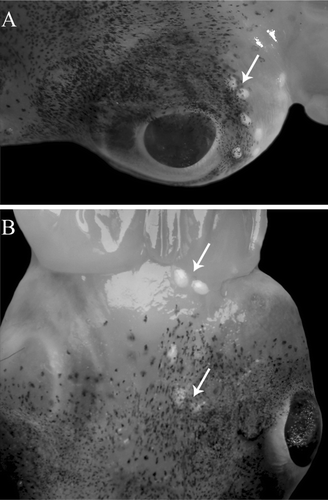
Table 1. Measurements and computed indices for the analyzed specimens.
Table 2. Percentage occurrence and numbers of the different structures found inside the Needham's sacs.
Discussion
Our observations indicate that males of N. caroli of the Sardinian waters mature at smaller sizes than those previously reported from other areas of the Mediterranean Sea (Mangold-Wirz Citation1963a, Citation1963b; Reid Citation1991; Jereb et al. Citation1998), confirming the previous record of a mature male of 19 mm ML caught at 1600 m (Cuccu et al. Citation2007). These findings may indicate that smaller mature individuals prefer the deeper waters of bathyal bottoms beyond 1000 m, where sampling and/or fishery activities are far less common, which may also explain why such small mature specimens have not been previously recorded. The general morphology of the male reproductive system conforms to the description given for the species by Reid (Citation1991; specimen caught off Banyuls sur Mer, western Mediterranean Sea). The number and size of the spermatophores and the SpLi index values are also similar to data and observations by Mangold-Wirz (Citation1963a, 1963b). The mean value of gonadosomatic index registered in mature males is higher (5.8%) than those reported in literature for other sepiolid species (Sepiola intermedia: 1.78%; Sepiola robusta: 1.67%, and Sepiola Steenstrupiana: 1.32%) (Salman and Önsoy Citation2004).
Our record of the coexistence of a number of different sexual products inside Needham's sacs appears to be the first for this species. As shown in , these structures are all well defined: clearly identifiable inverted spermatophores, empty spermatophore sheaths, and spermatangia. Therefore, they are not false spermatophores as reported for other cephalopods (e.g., Laptikhovsky and Nigmatullin Citation1987; Nigmatullin and Sabirov Citation2002; Hoving et al. Citation2004). The spermatophoric reaction is a phenomenon driven by the contraction of the spermatophore elastic tunics and by the hydrostatic pressure caused by the uptake of water through osmotic processes (Drew Citation1919; Austin et al. Citation1964; Mann Citation1984). In decapod cephalopod species in general, this reaction is known to be fast (Drew Citation1919; Austin et al. Citation1964; Mann Citation1984; Takahama et al. Citation1991; Hoving et al. Citation2009), which is confirmed in the Carol bobtail squid (30–90 s) from our results.
The contemporary presence of spermatophores, inverted spermatophores, empty spermatophore sheaths, and spermatangia in the Needham's sac may indicate that in N. caroli, the spermatophoric reaction could take place inside the reproductive organ, probably due to uptaking of water in the Needham's sacs during mating (natural process) and/or as a result of the fishery capture trauma (an artificial process, e.g., variations of pressure, specimens are squeezed, and water may run in the Needham's sac). In a natural situation, the hectocotylus of N. caroli probably removes spermatophores from the Needham's sac to transfer them to the female. It is possible that the spermatophoric reaction initiates when the spermatophores are removed from the Needham's sac by the hectocotylus which transfers inverting spermatophores on the female's body, as suggested for R. moelleri by Hoving et al. (Citation2009). At present, there is no evidence that N. caroli transfer inverting spermatophores by the hectocotyli; however, this has been observed, recently, in Sepietta oweniana (Cuccu et al. Citation2010). The fact that no spermatangia and only inverted spermatophores were obtained in this study by submerging the spermatophores in seawater may indicate that organic fluids or tissue contact is needed to complete the process; according to Hoving et al. (Citation2009); sufficiently long and proper contact with the female tissue seems an important factor to ensure implantation. Implanting spermatangia in the female body is known to be an efficient way to allow sperm storage, due to the lack of a functional bursa copulatrix or seminal receptacle (Nesis Citation1995). Females of N. caroli have been observed to carry from 5 to 24 spermatangia deeply implanted in their mantle tissues, in the anterior ventral area overlying the oviduct (Cuccu et al. Citation2007), and also in other body parts (head, arms, funnel, and neck) (Cuccu, personal observation Citation2010). The large number of spermatophores observed in mature males may indicate that males have the potential to mate with several females as also observed for Sepietta oweniana (Cuccu et al. Citation2010).
In this article we report for the first time the presence of implanted spermatangia on the head, arms, funnel, and neck of N. caroli males; however, no spermatangia were found on the anterior ventral mantel area, which is the main area where spermatangia are implanted in females. The occurrence of spermatangia implants on the bodies of males, as noted in this case, is apparently not an uncommon phenomenon in deepwater cephalopods (e.g., Hoving et al. Citation2004, Citation2008). Some authors suggest that it is caused either by trauma during capture or by accident, as an auto-implantation during mating (Hoving et al. Citation2004, Citation2008). Contrary to what has been reported for other decapods (e.g., Sepia species), in fact, no courtship has been observed in the field or in the aquarium for sepiolid species; sepiolid males simply “grasp the female on the head or the neck and turn her more or less upside down” (Mangold Citation1987, p. 165) in a fast and apparently traumatic event during which auto-implantation can occur. However, we cannot exclude the possibility that a male-to-male mating occurs in N. caroli as also suggested for Octopoteuthis sicula (Hoving et al. Citation2008). To the best of the authors’ knowledge, in fact, copulation between males has been observed for other cephalopods, both in natural conditions (e.g., Illex coindetii; Lordan and Casey Citation1997) and in captivity (e.g., Idiosepius paradoxus, Idiosepius thailandicus, Sepia esculenta; Kasugai and Segawa Citation2005).
Acknowledgements
The authors are grateful to the two referees whose comments and suggestions helped in improving this contribution.
References
- Austin , CR , Lutwak-Mann , C and Mann , T . 1964 . Spermatophores and spermatozoa of the squid Loligo pealii . Proceeding of the Royal Society of London B: Biological Science , 161 : 143 – 152 .
- Collins , MA , Yau , C , Allcock , L and Thurson , MH . 2001 . Distribution of deep-water benthic and bentho-pelagic cephalopods from the north-east Atlantic . Journal of the Marine Biological Association of the United Kingdom , 81 : 105 – 117 .
- Cuccu , D , Mereu , M , Cannas , R , Follesa , MC , Cau , A and Jereb , P . 2007 . Egg clutch, sperm reservoirs and fecundity of Neorossia caroli (Cephalopoda: Sepiolidae) from the southern Sardinian Sea (western Mediterranean) . Journal of the Marine Biological Association of the United Kingdom , 87 : 971 – 976 .
- Cuccu , D , Mereu , M , Cannas , R , Marcias , S , Cau , A and Jereb , P . 2010 . An unusual finding of Sepietta oweniana (Cephalopoda: Sepiolidae) egg clutch . Scientia Marina , 74 ( 3 ) : 555 – 560 .
- Drew , GA . 1919 . Sexual activities of the squid Loligo pealii (Les.). II. The spermatophore; its structure, ejaculation and formation . Journal of Morphology , 32 : 379 – 435 .
- Hoving , HJT , Lipiński , MR and Videler , JJ . 2008 . Reproductive system and the spermatophoric reaction of the mesopelagic squid Octopoteuthis sicula (Rüppell 1844) (Cephalopoda: Octopoteuthidae) from southern African waters . African Journal of Marine Science , 30 : 603 – 612 .
- Hoving , HJT , Nauwelaerts , S , Van Genne , B , Stamhuis , EJ and Zumholz , K . 2009 . Spermatophore implantation in Rossia moelleri Steenstrup, 1856 (Sepiolidae; Cephalopoda) . Journal of Experimental Marine Biology and Ecology , 372 : 75 – 81 .
- Hoving , HJT , Roeleveld , MAC , Lipinski , MR and Melo , Y . 2004 . Reproductive system of the giant squid Architeuthis in South African waters . Journal of Zoology, London , 264 : 153 – 169 .
- Jereb , P , Mazzola , A and Di Stefano , M . 1998 . Rossiinae (Mollusca, Cephalopoda) from the Strait of Sicily . Bollettino Malacologico , 33 : 157 – 160 .
- Kasugai , T and Segawa , S . 2005 . Life cycle of the Japanese pygmy squid Idiosepius paradoxus (Cephalopoda: Idiosepiidae) in the Zostera Beds of the temperate coast of central Honshu, Japan . Phuket Marine Biological Center Research Bulletin , 66 : 249 – 258 .
- Krstulovic-Sifner , S , Isajlovic , I and Vrgoc , N . 2007 . On the occurrence of Neorossia caroli (Jouben, 1902) in the central Adriatic Sea (Croatian waters) . Acta Adriatica , 48 : 95 – 100 .
- Laptikhovsky , VV and Nigmatullin , ChM . 1987 . “ Phenomenon of tentative spermatophores in cephalopods ” . In Molluscs, results and perspectives of investigation , Edited by: Starobogatov , YaI , Golikov , AN and Likharev , IM . 240 – 242 . Leningrad : Nakua. .
- Lordan , C and Casey , J . 1997 . The first record of “accidental” copulation between male squid of the genus Illex . Journal of Molluscan Studies , 63 : 556 – 558 .
- Mangold-Wirz , K . 1963a . Contribution a l’étude de Rossia caroli Joubin . Vie et Milieu , 14 : 205 – 224 .
- Mangold-Wirz , K . 1963b . Biologie des Céphalopodes benthiques et nectoniques de la Mer Catalane . Vie et Milieu , 13 ( Suppl ) : 118 – 125 .
- Mangold , K . 1987 . “ Reproduction ” . In Cephalopod life cycles. Comparative reviews , Edited by: Boyle , PR . Vol. 2 , 157 – 200 . London : Academic Press .
- Mann , T . 1984 . Spermatophore development, structure, biochemical attributes and role in the transfer of spermatozoa . Zoophysiology , 15 : 1 – 217 .
- Nesis , KN . 1995 . Mating, spawning and death in oceanic cephalopods: a review . Ruthenica , 6 : 23 – 64 .
- Nesis , NK , Arkhipkin , AI , Nikitina , IV , Middleton , DAJ and Brickle , P . 2001 . A new subspecies of the bathyal sepiolid cephalopod Neorossia caroli (Joubin 1902) from the southwestern Atlantic off the Falkland Islands . Ruthenica , 11 : 51 – 56 .
- Nigmatullin , ChM and Sabirov , RM . 2002 . “ Cephalopod spermatophores, their function, ontogenetic and evolutionary aspects (example: ommastrephid squids) ” . In International Symposium ‘Coleoid cephalopods through time’ , Edited by: Warnke , K . Vol. 1 , 85 – 87 . Berlin : Palaobiologische Abhandlungen .
- Reid , A . 1991 . Taxonomic review of the Australian Rossiinae (Cephalopoda: Sepiolidae), with a description of a new species, Neorossia leptodons, and redescription of N. caroli (Joubin, 1902 . Bulletin of Marine Science , 49 : 748 – 831 .
- Reid , A and Jereb , P . 2005 . “ Family Sepiolidae Leach, 1817 ” . In Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Chambered Nautiluses and Sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae) , Edited by: Jereb , P and Roper , CFE . Vol. 1 , 153 – 203 . Rome : FAO .
- Salman , A and Önsoy , B . 2004 . Analysis of fecundity of some bobtail squid of the genus Sepiola (Cephalopoda: Sepiolida) in the Aegean Sea (eastern Mediterranean) . Journal of the Marine Biological Association of the United Kingdom , 84 : 781 – 782 .
- Takahama , H , Kinoshita , T , Sato , M and Sasaki , F . 1991 . Fine structure of the spermatophores and their ejaculated forms, sperm reservoirs, of the Japanese common squid, Todarodes pacificus . Journal of Morphology , 207 : 241 – 252 .
- Villanueva , R . 1992 . Deep-sea cephalopods of the north-western Mediterranean: inclination of up-slope ontogenetic migration in two bathybenthic species . Journal of Zoology, London , 227 : 267 – 276 .