Abstract
What mechanisms underlie aging? One theory, the wear-and-tear model, attributes aging to progressive deterioration in the molecular and cellular machinery which eventually lead to death through the disruption of physiological homeostasis. The second suggests that life span is genetically programmed, and aging may be derived from intrinsic processes which enforce a non-random, terminal time interval for the survivability of the organism. We are studying an organism that demonstrates both properties: the colonial ascidian, Botryllus schlosseri. Botryllus is a member of the Tunicata, the sister group to the vertebrates, and has a number of life history traits which make it an excellent model for studies on aging. First, Botryllus has a colonial life history, and grows by a process of asexual reproduction during which entire bodies, including all somatic and germline lineages, regenerate every week, resulting in a colony of genetically identical individuals. Second, previous studies of lifespan in genetically distinct Botryllus lineages suggest that a direct, heritable basis underlying mortality exists that is unlinked to reproductive effort and other life history traits. Here we will review recent efforts to take advantage of the unique life history traits of B. schlosseri and develop it into a robust model for aging research.
Keywords:
Introduction
Botryllus schlosseri is an ascidian, a member of the Tunicata, invertebrate chordates that are thought to be the sister group to the vertebrates, and grow in marinas throughout the world (Delsuc et al. Citation2006). Embryogenesis results in a tadpole larva with a number of chordate characteristics, including a notochord, dorsal hollow nerve tube, post-anal tail, striated musculature and pharynx with gill slits. After a free-swimming phase, larvae settle and undergo a dramatic metamorphosis during which most of these characteristic chordate structures are resorbed, resulting in a sessile invertebrate adult. In addition, B. schlosseri belongs to a subset of ascidian species that are colonial and grow, not by increasing in size, but by a lifelong asexual budding process that eventually gives rise to a colony of genetically identical individuals, called zooids. Zooids arrange themselves into star-shaped structures called systems, and a colony can consist of one to hundreds of systems (Figure ).
Figure 1. Life history of B. schlosseri: Top panels show the chordate tadpole larva immediately following hatching, which after a short free-swimming phase settles onto a suitable substrate, then undergoes metamorphosis into an invertebrate body plan, called an oozooid. This is followed by a weekly, coordinated asexual budding cycle which gives rise to a colony of genetically identical individuals (called zooids, bottom middle), and linked by a common vasculature. Zooids are filter-feeders which organize themselves into star-shaped structure called systems (bottom middle), and a genotype can consist of multiple systems (bottom right). Bottom left shows the vasculature visualized following the injection of fluorescent dye. The vasculature runs throughout the colony, and at the periphery terminates in finger-shaped protrusions called ampullae.
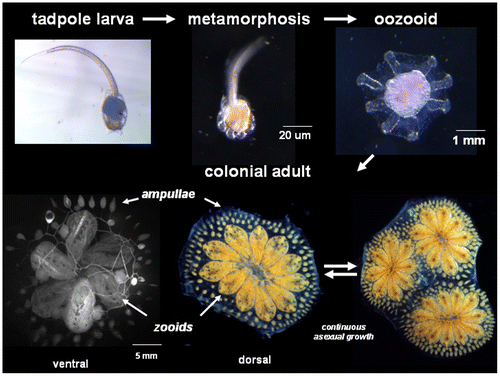
Each zooid within a Botryllus colony is an independent, filter-feeding individual with a complex body plan, including incurrent and excurrent siphons, pharynx, gastrointestinal tract, nervous (both peripheral and central) and endocrine systems, and a germline. For the latter, when sexually mature, Botryllus is an ovoviparous hermaphrodite, and each zooid reproduces sexually each week, giving rise to 1–3 tadpole larvae (reviewed in Manni et al. Citation2007).
As shown in Figure , all the zooids are connected by an extracorporeal vasculature that runs throughout the colony, terminating in the periphery of the colony in structures called ampullae. However, while linked to each other, the zooids are not dependent on each other and pieces of a colony can be surgically separated, placed on an independent substrate, and will continue to grow. This sets up a unique experimental situation, as a colony can be repeatedly divided (subcloned), and an individual can be used in multiple experiments concurrently. More specifically for aging research, pieces of an individual genotype can be isolated and analyzed throughout its lifespan. The ability to study multiple isogenic subclones in a variety of conditions over time is a unique and powerful characteristic of Botryllus for studies on aging. Colonial ascidians are the only chordates with this ability.
Asexual reproduction
While a genotype can survive from three months to several years, the zooids are transient structures. Under laboratory conditions (18 °C), zooids have a three week lifespan. Development takes two weeks (described below), followed by one week as feeding, sexually reproducing adults. During that week, each zooid is asexually reproducing in a process called blastogenesis. This process is coordinated throughout the colony and arranged spatially: the center of each system is occupied by the zooids, which are actively feeding and capable of sexually reproducing. They are joined peripherally via the vasculature to primary buds, which are completing their development of both somatic and germline tissues. In turn, these are connected to secondary buds, which are in the initial stages of development. After feeding and reproducing sexually for one week, zooids die in a massive wave of apoptosis called takeover (reviewed in Manni et al. Citation2007). Development and takeover are coordinated throughout the colony: all stages of budding occur simultaneously, and during takeover all the zooid bodies simultaneously undergo apoptosis and are removed via phagocytic cells in the blood (Burighel & Schiavinato Citation1984; Lauzon et al. Citation1992, Citation1993). During takeover, the primary buds migrate into the newly vacated center region of the colony, opening their siphons and becoming an adult zooid, the secondary bud becomes the primary bud, and a new secondary bud begins to develop.
Thus, the life history of Botryllus consists of a constant succession of individual zooids, each with a three-week lifespan- 2 weeks of development, and one week as an adult. Each week, each zooid can generate between 1 and 4 buds, so the colony asexually expands over the substrate, and can form large colonies consisting of thousands of zooids. Thus in contrast to how we normally think of development and aging, whereby organisms are more or less static and new tissues are replaced by a long-lived pool of stem cells with limited potential, for example hematopoietic stem cells in mammals, Botryllus can be thought of as a pool of pluripotent stem cells in which the ‘body’ is transient and remade anew each week. This massive regeneration and turnover continues for the life of the genotype.
Stem cells and regeneration
B. schlosseri undergoes a natural transplantation reaction which occurs when two colonies grow into each other (reviewed in De Tomaso Citation2006). Juxtaposed ampullae (Figure ) will either fuse, forming a hematopoietic chimeric colony which shares a common circulation (parabiosis); or the ampullae will reject- an active, blood-based, inflammatory reaction during which the interacting ampullae are destroyed, thus preventing blood transfer, an immune-type response. This reaction is controlled by a single, highly polymorphic locus called the fuhc (for fusion/histocompatibility; Sabbadin Citation1962; Scofield et al. Citation1982). Two colonies will fuse together if they share one or both fuhc alleles, and will reject each other if there are no alleles in common. Natural populations contain tens to hundreds of fuhc alleles, limiting the chance of fusion to kin. After vascular fusion of compatible parabiosed partners become chimeric and will remain so for the lifespan of the individual (> 6mos; Sabbadin & Zaniolo Citation1979; Pancer et al. Citation1995; Stoner et al. Citation1999). Thus, mobile, long-lived progenitors from two colonies contribute to the development of germline and/or somatic tissues in the newly developing buds.
In addition, following fusion one genotype may contribute disproportionately to new germline development, enabling one genotype to dominate the gametic output of the fused bud (Sabbadin & Zaniolo Citation1979; Pancer et al. Citation1995, Stoner & Weissman Citation1996). This process, called germ cell parasitism (GCP), is a repeatable and heritable trait (Stoner et al. Citation1999), with winner and loser genotypes in both lab-reared and field colonies. These parasitic abilities are autonomous to the cells themselves, retaining their parasitic phenotype upon transplantation (Laird et al. Citation2005; Brown et al. Citation2009), and allowing an experimental analysis of the cell properties responsible for regeneration (Brown et al. Citation2009).
The relationship of natural parabiosis and stem cell parasitism suggests that a highly polymorphic allorecognition system evolved in Botryllus to control vascular fusion, blocking GCP winner genotypes from sweeping through a population. As described below, the situation may be more complicated, involving a co-operative association between juveniles and adults, and a potential rejuvenation via natural transplantation of young stem cells (De Tomaso Citation2006).
Aging and death
Given the constant regeneration and turnover of zooids, how and why does a colony die? One explanation is that the stem cells responsible eventually burn out metabolically, or accumulate mutations such that downstream developmental processes become imbalanced. An alternative may be systemic factors secreted by the permanent colonial vasculature that control regenerative processes that themselves become imbalanced over time, affecting growth. Clues to these aging processes come from observations on both the lifespan of genotypes, as well as the events that precede organismal death (Chadwick-Furman & Weissman Citation1995; Grosberg Citation1981, Citation1988; Lauzon et al. Citation2000). For the former, there appears to be three broad groups of lifespan in the lab, either short (3–7 months), intermediate (7–14 months), and long (≥1.5 years). This correlates with ecological observations from natural populations: summer is a high growth, high density season, after which the majority of individuals die, with a remnant group surviving throughout the winter to seed the population for the next year (Grosberg Citation1988). In one study two distinct life-history morphs in a natural population were identified in an east coast population. One morph (semelparous) grew rapidly, reproduced at a young age with high reproductive effort, and then died. The other morph (iteroparous) grew slower, put less effort into reproduction (reproducing later and with lower clutch sizes), and survived longer. Both lab and field experiments suggested that these were genetically determined phenotypes and could be observed over multiple years (Grosberg Citation1988). Distinct phenotypes with intermediate lifespans have been observed. In addition, a minority can survive for over 4 years, with a record of nine years, but this long-lifespan has never been observed in natural populations (Grosberg Citation1988; Chadwick-Furman & Weissman Citation1995). However, on average, the lifespan is short enough to allow multiple strategies to be taken to dissect the basis of aging in the lab, using both genetic and in vivo approaches.
The process of organismal death is easy to observe, and the first indication of senescence is observed 4–6 weeks prior to death, when budding slows to only a single bud per zooid, often accompanied by a lengthening of the blastogenic cycle to 9–10 days. Approximately 1–4 weeks prior to death, the colony shrinks, with zooids visibly decreasing in size about 10–20%. This stage is variable and not 100% reliable for predicting death, as colonies often recover and begin to grow robustly within a few weeks (Lauzon et al. Citation2000). However, 7–10 days prior to death, a colony begins to go through a very well defined set of morphological changes. The initial features of senescence are a colony-wide constriction of the colonial vasculature, with an accumulation of pigmented cells throughout the colony and a major reduction in blood flow. Normally, pigmented cells are found at the tips of the ampullae and within the zooid, with few actually circulating. This changes dramatically within a 12 h period, and the colony suddenly becomes heavily pigmented. Interestingly, the onset of senescence is not linked to blastogenesis, and senescence can start at any stage of asexual development (Brunetti Citation1974; Lauzon et al. Citation2000). Within 48 h of this change in pigmentation, zooids lose about 30% of their size, and buds appear to separate from the zooids as the vascular connection lengthens. At this point the heartbeat becomes slower and weaker and within the next 24 h begins to beat infrequently. At about 96 h, the siphons, which usually have a robust response to touch (usually withdrawing immediately and re-extending after ca. 30 s), become unresponsive. During the next 24 h, the colony becomes disorganized, and the zooids no longer come together into systems, but instead are spread out throughout the tunic. Within the next 24 h all zooids in the colony are dead: all hearts have stopped beating, no blood flow is apparent in the extracorporeal vasculature, and both oral and atrial siphons are closed. During senescent death there is no apoptosis or other characteristics that occur during zooid death in the weekly takeover cycle (Lauzon et al. Citation2000). In addition, comparison of senescence in both lab-reared and natural populations reveal that senescent death is equivalent, regardless of environment (Grosberg Citation1981, Citation1988; Chadwick-Furman & Weissman Citation1995; Lauzon et al. Citation2000).
Seasonal life span and non-random senescence
While organismal death is easily visualized, another aspect of the lifespan makes B. schlosseri a potentially powerful model to study aging: non-random senescence during genotypic death. During initial genealogical analysis of 41 lab-reared strains, senescence of genotypes manifested itself in either a random (24/41) or non-random (17/41) fashion (Rinkevich et al. Citation1992). For random senescent genotypes, individual subclones of a single genotype died haphazardly, with a standard deviation of about six months. In other words, after separation, some subclones would die after 2–3 months; some would die after a year, and others in between. In contrast, nearly half the genotypes showed non-random senescence, where independent subclones of a single genotype would die simultaneously, initiating senescence and dying within a 48 h period of each other. This simultaneous senescence could occur even months after separation. The same study compared the standard deviation of the lifespan of independent subclones of a single genotype to the lifespan of the genotype, and another interesting observation was made: the standard deviation of short-lived genotypes (ca. 4 months) was very small, and a significant percentage of those showed non-random senescence. The same was true of many of the longer lived colonies, although fewer subclones died non-randomly. Only a single example of a genotype with intermediate lifespans (7–14 mos.) showed non-random senescence. The presence of short- and long-lived phenotypes correlates with previous studies in natural populations which described the semelparous and iteroparous life histories (described above; Grosberg Citation1988). In summary, about 45% of both short and long-lived phenotypes show non-random senescence, while the vast majority of intermediate-lived phenotypes senesce randomly.
Given the fact that we observe both short and long life spans, coupled to random and non-random senescence, two questions are: (1) what initiates the senescence program; and (2) is lifespan genetically determined?
In a recent two-year longitudinal study on two B. schlosseri populations reared under laboratory and field conditions, the founding populations were assembled from lab-reared colonies crossed with wild type colonies collected from the field. Life history traits and subsamples were collected for 1600 individuals over their entire lifespan. A weekly census was taken including the following data for every colony: (1) asexual investment (the number of buds/asexual generation; (2) sexual investment (the presence of testes and eggs) and the (3) collection and labeling of hatches (offspring). Throughout the study, temperature, salinity, and dissolved oxygen were also tracked, enabling the analysis of field and lab differences and also seasonal variation in the field.
Comparing field versus laboratory populations revealed that the majority of individuals have short lifespans (45–65 weeks), but a number of individuals can live significantly longer (>2 years). Using Interquartile Range (IQR) as an appropriate measure of variability, the two populations nevertheless appear to be very similar. The main disparity is found in longevity, with 63 weeks as the maximum age of a field animal versus 45 weeks for the lab. Serial subclones of multiple individuals were collected from both populations, including extremely long-lived individuals. In other studies in this and other labs, life spans of years as opposed to weeks may be the unintended consequence of selecting particular strains for experimentation and then housing those strains in proximity to one another, a potentially selective breeding process that was not completely controlled. Botryllus tightly regulates timing of sperm and egg release to prevent selfing but broadcast spawning does allow for a large amount of inbreeding due to proximity, especially within mariculture conditions. In contrast, those conditions were removed during this study as animals were allowed to outcross with wild type under field conditions. These changes in longevity under similar environmental conditions could help to reveal the interactions of genes and environment that play a role in determining longevity.
Many other aspects of the data collected in this experiment remain to be analyzed, including seasonal patterns of longevity, effects of seasonal temperatures, relationships between age of both first testes and egg production and longevity, etc. These analyses will explore the theory that timing of reproduction is not as critical in influencing longevity as balancing the relationship between investments in growth versus investments in reproduction.
On-going current studies and future goals
Current studies are investigating the basic processes described above, but with increasingly sophisticated tools and approaches. The following is a brief summary of the goals and expected implications of these on-going studies.
Re-examining non-random senescence
Previous studies of non-random senescence (see above) typically preserved very few clones. Current studies of 60 individuals subcloned on multiple dates both recapitulated the previous study and now enables transcriptome analysis of changes occurring throughout the lifespan of animals with non-random senescent phenotypes.
Transcriptome profiling of aging
mRNA-seq transcriptome analysis of individuals during the entire regenerative cycle in both infertile and fertile conditions has established determination of baseline gene expression during regeneration. These results can now be used to reveal changes in gene expression during the asexual cycle in single genotypes at different ages. Preliminary intriguing data have thus far revealed no phenotypic signs of aging. In other words, a two-year old colony has buds that seem equivalent to those of a 2 month old colony. No analog of gray hair or wrinkled skin in the new buds has yet to be identified.
Enrichment and transcriptome profiling of stem cell populations
Transcriptome profiling of enriched germline stem cell populations is creating a baseline for characterizing differences between different competitive GCP phenotypes. An exciting preliminary result is the discovery that GCP winner phenotypes are significantly enriched in processes such as epithelial to mesenchyme transition, and a number of cancer metastasis phenotypes have been observed. This analysis will be coupled with functional data from reconstitution following reciprocal transplantation experiments (Laird et al. Citation2005; Brown et al. Citation2009).
Identification of a germline niche
Analysis of differential transcriptome expression in a population of follicle cells that surround germline progenitors have identified a number of genes that are male or female specific, and others that differentiate in step with the gametes. These and other experiments have identified the germline niche in which aging and niche/germline interactions can be analyzed.
Vascular regeneration and aging
Using a novel labeling methodology, experiments are investigating a new hypothesis that aging is due to changes in the vasculature (Braden et al. Citation2014). The hypothesis is supported by preliminary data showing that the only long-lived organ in colonies is the vasculature. Other new methods to isolate vascular cells by FACS will enable the study of vasculature genetic changes over time.
Juvenile-adult parabiosis
Parabiosis (fusion) experiments are being used to examine roles of stem cells in rejuvenating buds. These experiments reveal a significant advantage to a juvenile in a juvenile/adult parabiosis, an intriguing result given the limited space in which in most natural Botryllus habitats. The advantage to a juvenile that can land on adjacent (or even on top of) an adult which has established itself and undergo parabiosis is an interesting ecological question. Current experiments are examining the mutual (or otherwise) benefits of parabiosis of juveniles into adults of different ages in natural and laboratory environments.
In summary, the life history of B. schlosseri offers a number of unique traits to characterize the processes underlying aging. The colonial nature enables the analysis of genetic changes over time in a single individual by serial collection of subclones. A genomics infrastructure to do this analysis has been established. In addition, enrichment and transplantation of stem cells between individuals, and natural parabiosis processes provide means to dissect intrinsic versus extrinsic changes in regeneration. With the germline niche now identified, critical genes in its function can now be characterized in single individuals over time. Each of these traits is experimentally accessible in a species which can be reared in both the lab and natural populations. B. schlosseri is thus expected to have the potential to provide unique insights into aging processes.
Funding
This work was supported by the NIH [grant number AG037966] and [grant number AI041588].
References
- Braden BP, Taketa DA, Pierce JD, Kassmer S, Lewis DD, De Tomaso AW. 2014. Vascular regeneration in a basal chordate is due to the presence of immobile, bi-functional cells. PLoS One. 9:e95460.
- Brown FD, Tiozzo S, Roux MM, Ishizuka K, Swalla BJ, De Tomaso AW. 2009. Early lineage specification of long-lived germline precursors in the colonial ascidian Botryllus schlosseri. Development. 136:3485–3494.10.1242/dev.037754
- Brunetti R. 1974. Observations of the life cycle of Botryllus schlosseri (Pallas) (Ascidiaceae) in the Venetian Lagoon. Bollettino di Zoologia. 41:225–251.
- Burighel P, Schiavinato A. 1984. Degenerative regression of the digestive tract in the colonial ascidian Botryllus schlosseri (Pallas). Cell and Tissue Research. 235:309–318.
- Chadwick-Furman NE, Weissman IL. 1995. Life histories and senescence of Botryllus schlosseri (Chordata, Ascidiacea) in Monterey Bay. Biological Bulletin. 189:36–41.10.2307/1542199
- De Tomaso AW. 2006. Allorecognition polymorphism versus parasitic stem cells. Trends in Genetics. 22:485–490.10.1016/j.tig.2006.07.001
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. 2006. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 439:965–968.10.1038/nature04336
- Grosberg RK. 1981. Life Histories of the Colonial Ascidian Botryllus-Schlosseri. American Zoologist. 21:1019–1025.
- Grosberg RK. 1988. Life-history variation within a population of the colonial ascidian Botryllus-Schlosseri. 1. The genetic and environmental-control of seasonal-variation. Evolution. 42:900–920.10.2307/2408907
- Laird DJ, De Tomaso AW, Weissman IL. 2005. Stem cells are units of natural selection in a colonial ascidian. Cell. 123:1351–1360.10.1016/j.cell.2005.10.026
- Lauzon RJ, Ishizuka KJ, Weissman IL. 1992. A cyclical, developmentally-regulated death phenomenon in a colonial urochordate. Developmental Dynamics. 194:71–83.10.1002/aja.v194:1
- Lauzon RJ, Patton CW, Weissman IL. 1993. A morphological and immunohistochemical study of programmed cell death in Botryllus schlosseri (Tunicata, Ascidiacea). Cell and Tissue Research. 272:115–127.10.1007/BF00323577
- Lauzon RJ, Rinkevich B, Patton CW, Weissman IL. 2000. A morphological study of nonrandom senescence in a colonial urochordate. Biological Bulletin. 198:367–378.10.2307/1542692
- Manni L, Zaniolo G, Cima F, Burighel P, Ballarin L. 2007. Botryllus schlosseri: a model ascidian for the study of asexual reproduction. Developmental Dynamics. 236:335–352.10.1002/(ISSN)1097-0177
- Pancer Z, Gershon H, Rinkevich B. 1995. Coexistence and possible parasitism of somatic and germ cell lines in chimeras of the colonial urochordate Botryllus schlosseri. Biological Bulletin. 189:106–112.10.2307/1542460
- Rinkevich B, Lauzon RJ, Brown BW, Weissman IL. 1992. Evidence for a programmed life span in a colonial protochordate. Proceedings of the National academy of Sciences of the United States of America. 89:3546–3550.10.1073/pnas.89.8.3546
- Sabbadin A. 1962. Le basi genetiche dell capacita di fusione fra colonie in Botryllus schlosseri (Ascidiacea) Atti Accad. Naz Lincei Rend. 32:1031–1035.
- Sabbadin A, Zaniolo G. 1979. Sexual differentiation and germ cell transfer in the colonial ascidian Botryllus schlosseri. Journal of Experimental Zoology. 207:279–301.
- Scofield VL, Schlumpberger JM, West LA, Weissman IL. 1982. Protochordate allorecognition is controlled by a MHC-like gene system. Nature. 295:499–502.10.1038/295499a0
- Stoner DS, Weissman IL. 1996. Somatic and germ cell parasitism in a colonial ascidian: possible role for a highly polymorphic allorecognition system. Proceedings of the National academy of Sciences of the United States of America. 93:15254–15259.10.1073/pnas.93.26.15254
- Stoner DS, Rinkevich B, Weissman IL. 1999. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proceedings of the National academy of Sciences of the United States of America. 96:9148–9153.10.1073/pnas.96.16.9148
