Abstract
Background: To investigate whether extrinsic antioxidant seleno‐glutathione peroxidase mimic ebselen (PZ51) can protect endothelium and vascular structure of stroke‐prone spontaneously hypertensive rats (SHRsp) during the chronic process of hypertension. Methods: Twenty‐two 8‐week‐old SHRsp were randomized into a PZ51 group and a control group, and administered by gavage for 6 weeks. We examined the level of nitric oxide (NO) and malonaldehyde (MDA) in plasma. The intima‐media thickness (IMT) of the common carotid artery (CCA) was measured by an image‐analysis system. The endothelium of the CCA was observed by scanning electron microscopy. The eNOS protein of the major artery was assayed by immunohistochemistry and western blotting. Results: Compared with the control group, PZ51 decreased plasma MDA (7.88±1.06 vs 10.88±1.73 nmol/l, p<0.001) and increased plasma NO (40.02±9.74 vs 22.22±10.05 µmol/l, p<0.001), increased eNOS protein expression (8.25±2.36 vs 4.46±3.14, p = 0.026), decreased IMT (69.85±5.47 vs 76.60±6.53 µm, p<0.05) significantly and alleviated the damage to the endothelium of the CCA. Conclusion: Administration of PZ51 for 6 weeks can protect the endothelium and inhibit vascular remodeling, maybe due to its suppression of lipid peroxide formation and increase in eNOS protein expression.
Introduction
Vascular endothelium plays a key role in the maintenance of vascular tone and blood pressure by regulating the release of several vasoactive substances, including nitric oxide (NO). Endothelial nitric oxide synthase (eNOS)‐derived NO, generated within the vascular endothelium, is considered the most important vasodilator in arteries.
During the past decade, it has become apparent that both endothelial cells and vascular smooth muscle cells are capable of producing excessive reactive oxygen species (ROS) from a variety of enzymatic and non‐enzymatic sources in the hypertensive state Citation[1], Citation[2]. Patients with essential hypertension have associated impairment of anti‐oxidant status or an increased response to oxidative stress or both Citation[3]; they have lower levels of endogenous antioxidant glutathione peroxidase (GPX) in their plasma compared with the normotensive patients Citation[4].
New antioxidant ebselen (PZ51), 2‐phenyl‐1,2‐benzisoselenazol‐3[2H]‐one, is a lipid‐soluble seleno‐organic compound that potently inhibits lipid peroxidation through a GPX‐like action Citation[5], Citation[6], but only in acute circumstances (such as ischemia reperfusion after acute ischemic stroke or acute myocardial infarction), does its short‐term role appear certain Citation[7–10]; there is no study of the long‐term effect of PZ51 on the chronic process of hypertension.
Since stroke‐prone spontaneously hypertensive rats (SHRsp) develop hypertension and cardiovascular disease (CVD) spontaneously, and increased ROS generation is involved in the pathogenesis and maintenance of hypertension and vascular injury, it is an appropriate experimental model for studying not only the pathogenesis but also the prevention of CVD in hypertension. The purpose of the present study is to investigate whether long‐term administration of PZ51 can protect endothelium and inhibit vascular remodeling during the chronic development of hypertension. This study also explores possible mechanisms of this effect.
Materials and method
Animals and experimental protocol
Treatment of the animals was based on the “Guide for the care and use of laboratory animals” published by the US National Institutes of Health (NIH publication NO.85‐23, revised 1985). Eight‐week‐old SHRsp weighing 160±20 g were housed in a humidity and temperature‐controlled semi‐barrier space with 12‐h light and dark cycles in the experimental animal department of the Cardiovascular Institute and Fuwai Hospital. The animals were given 1.0% saline water to accelerate the progression of hypertension and organ damage. Twenty‐two SHRsp were randomized to a PZ51‐treatment group or a control group. Body weight was measured every 2 days. Based on previous findings, PZ51 at a dose of 60 mg/kg/day was considered sufficient to exert an antioxidant activity in rats Citation[8], Citation[11], Citation[12]. Rats in the PZ51 group received PZ51 dissolved in polyethylene glycol at 60 mg/kg/day by gastric gavage for 6 weeks, whereas those in the control group received vehicle solution instead. Resting blood pressure (tail‐cuff method) and heart rate were measured in the morning once weekly under quiet conditions.
Preparation of tissue sample
At the end of 6 weeks, the rats were anesthetized with intraperitoneal injection of pentobarbital sodium at a dose of 50 mg/kg. Blood was obtained by abdominal artery puncture. Thoracic artery and common carotid arteries (CCA) were harvested immediately, cleaned thoroughly with 4°C normal saline to remove the contaminating blood. The thoracic aorta was promptly frozen in liquid nitrogen for western blot determination; right and left CCA were put into paraformaldehyde and glutaraldehyde, respectively.
Measurement of the plasma NO and malonaldehyde (MDA)
The NO kit and MDA kit were purchased from Nanjing Jiancheng Biotechnology Co. (Nanjing, China). We measured the plasma NO by the method of Tracey et al. Citation[13] and Ling et al. Citation[14]. NO was determined by its oxidation products nitrite and nitrate. Nitrate present in plasma is reduced to nitrite by reduced nicotinamide adenine dinucleotide phosphate in the presence of the enzyme nitrate reductase. The nitrite formed reacts with sulfanilamide and N‐(1‐naphtyl)‐ethyl‐enediamine dihydrochloride to give a red–violet diazo dye. Finally, the diazo dye is measured on the basis of its absorbance at 550 nm by a spectrophotometer.
The concentration of MDA was measured by thiobarbituric acid reactive substance assay (TBARS) according to the method of Kato et al. Citation[15]; 200 µl of plasma was piped into a 10‐ml centrifuge tube and 10 µl of butylate‐hydroxytoluene was added to the sample to prevent oxidation during the subsequent boiling step. Then, 200 µl of 8.1% sodium lauryl sulfate, 1500 µl of 20% acetic acid (pH = 3.5) and 1500 µl of 0.8% thiobarbituric acid were added to the sample. The sample was then boiled for 1 h at 95°C and centrifuged at 3000g for 10 min. The absorbance of the supernatant was measured by spectrophotometer at 532 nm comparing to that of a standard consisting of 200 µl of 1,3,3‐tetramethoxy propane in place of the plasma.
Tissue histology, immunohistochemistry and scanning electron microscopy
The right CCA was fixed in paraformaldehyde, then embedded in paraffin. Sections 5 µm thick were cut, dyed with hematein and eosin, and viewed under a microscope to evaluate the intima‐media thickness (IMT) and ratio of media cross‐sectional area to lumen area (M/L). M was calculated as π⋅(De2−Di2)/4, where De and Di were external and lumen diameters respectively. Immunohistochemistry was performed with standard techniques. Briefly, sections were blocked in 3% H2O2, boiled in citrate buffer to restore the antigenicity, then blocked with 20% goat serum and incubated overnight at 4°C in a humidified box with polyclonal antibody against eNOS (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:120. For negative control, the primary antibody was replaced with a phosphate‐buffered saline (PBS). The sections were then incubated in Biotinylated anti‐rabit‐IgG for 30 min followed by streptavidin conjugated to horseradish peroxide for another 30 min. Color was developed by the addition of DAB. Sections were viewed with a microscope and scored in the Universal Image Analysis Software system by an independent observer who was unaware of the experiment.
For scanning electron microscopy examination (SEM), after fixation in glutaraldehyde, the left CCA was then refixed in osmium tetroxide and embedded in isoamyl acetate. The sections were observed with a scanning electron microscope (Hitachi S‐320).
Western blotting
Thoracic aorta was homogenized with polytron in a lysis buffer (pH = 7.6) consisting of 1 mol/l Tris–HCl, 0.01 mmol/l EDTA and 0.01 µg/ml PMSF. After the homogenate was centrifuged at 20,000g for 30 min, protein concentration was determined using the Bradford method; 50 µg/lane of protein was size‐fractioned on an 8% polyacrylamide gel by electrophoresis using a Mini‐Protean II Dual stab cell, then electrotransferred onto Hybond‐ECL membranes (Amersham Life Science Inc., Arlington Heights, IL, USA) in the glycine/methanol buffer. The membrane was prehybridized in 20 ml of buffer A (10 mmol/l Tris–HCl, 100 mmol/l NaCl, 0.05%Tween‐20 and non‐fat milk powder) for 1 h and then hybridized in the same buffer containing anti‐eNOS polyclonal antibody (1:400) (Santa Cruz Biotechnology) in 4°C overnight. The membrane was washed with wash buffer A without non‐fat milk for 1 h in a shaking bath, changed every 10 min. The membrane was then incubated in buffer A plus horseradish peroxidase‐conjugated anti‐rabit‐IgG at 1:4000 for 2 h. Finally, after washing for another hour, the membrane was developed with an ECL‐plus chemiluminescence detection system by exposing to Roentgen‐film in a dark room to visualize the bands. β‐Actin was used as protein quality control. The concentration of protein was obtained from the densitometry value of the corresponding band measured by Totallab software (Nonlinear Dynamics, Durham, NC, USA).
Data analysis
The results are presented as the mean±SEM for each group of rats. Statistical analysis was performed with SPSS software, version 10.0. The normality of the distribution of the data was checked. Statistical analysis was carried out using one‐way ANOVA. Values of p<0.05 were considered statistically significant.
Results
Body weight, resting blood pressure and heart rate
Body weight, resting blood pressure and heart rate were similar between the two groups at baseline. Six weeks administration of PZ51 had no effect on them compared with the control group (Table ).
Table I. Body weight, blood pressure and heart rate in the PZ51 group and the control group.
Plasma MDA and NO
The production of MDA is an indicator of the development of oxidative stress. We assayed the level of plasma MDA formation to monitor the oxidative stress and extent of lipid peroxidation. MDA content was 10.88±1.73 nmol/l in control group compared to only 7.88±1.06 nmol/l in the PZ51 group (p<0.001), demonstrating that PZ51 lowered the oxidative stress. The plasma NO of the PZ51 group (44.02±9.74 µmol/l) increased significantly compared with the control group (22.22±10.05 µmol/l, p<0.001).
Vascular structure
As shown in Table , IMT of CCA was thinner in the PZ51 group than that of the control group (p<0.05) and the M/L ratio showed the same trend but did not attain significance.
Table II. Intima‐media thickness (IMT) and media/lumen ratio (M/L) of common carotid artery.
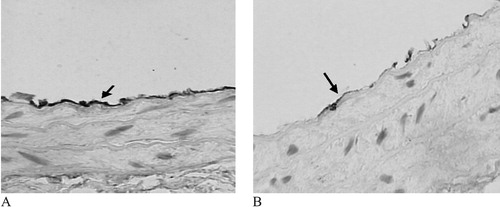
Immunohistochemistry
As shown in Figure , in the PZ51‐treated group, the signal corresponding to eNOS protein increased (8.25±2.36 vs 4.46±3.14, p = 0.026) significantly compared with that in control group.
Western blotting
Further support of the increased expression of eNOS in artery in the PZ51 group compared with that in control group came from the result of western blot shown as Figure .
Scanning electron microscopy examination
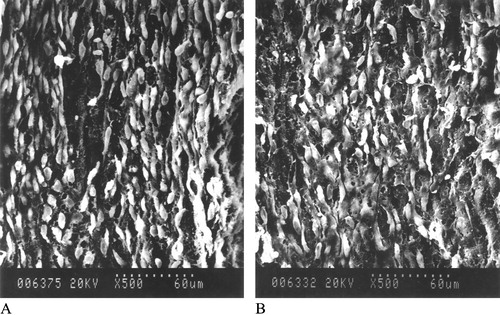
Figure shows the results of the SEM. In the control group, the lesions of endothelium were severe, with endothelial cells swollen and crater‐like ulcer and pinpole, even with endothelium desquamating. PZ51 alleviated these injuries. In the PZ51 group, the spindle‐shaped endothelial cells aligned regularly with few lesions.
Figure 3 Effect of PZ51 on endothelium of CCA shown by SEM. The PZ51 group(A), the endothelium is spindle‐shaped, aligned along the direction of blood flow regularly. The control group (B), the lesions of endothelium are severe with endothelial cells swelling and crater‐like ulcers and pinpoles, even with endothelium desquamating (×500).
Discussion
The major findings of the present study are as follow: (i) administration of PZ51 on SHRsp for 6 weeks resulted in a significant increase of plasma NO and a decrease of MDA; (ii) PZ51 decreased the IMT of the CCA; (iii) PZ51 increased eNOS protein expression from endothelium of artery; and (iv) PZ51 protected the endothelium from being impaired.
Many experiments have demonstrated that antioxidant vitamins and enzymes can lower the level of ROS in hypertensive animal models and have a protective effect against cardiovascular diseases. But results from long‐term large‐scale clinical trials of antioxidants on hypertensive patients are conflicting Citation[16], Citation[17]. In these clinical trials, hard endpoints such as stroke, myocardial infarction and cardiovascular death are used to evaluate the long‐ term clinical efficacy of these drugs. Meta‐analyses of clinical trials of antioxidant vitamins on hypertensive patients produced negative results. New antioxidants, especially mimics of endogenous antioxidant enzymes, such as superoxide dismutase (SOD) and GPX, have been developed and studied. GPX inhibits oxidative reactions by scavenging oxygen‐derived free radicals and/or interfering with the chain reaction of peroxidation Citation[18]. PZ51 is a seleno‐GPX mimic that has potent antioxidant activity. PZ51 is reported to reach inside cells as a result of its reactive binding to the intracellular thiol groups such as glutathione. It inhibits the peroxidation of membrane phospholipids, inhibits lipoxygenase and blocks the production of superoxide anions by activated leukocytes Citation[19].
ROS from diverse origins is thought to contribute to the pathogenesis and maintenance of hypertension by several mechanisms, including lipid peroxidation, inactivation of NO and formation of peroxynitrite (OONO‐). An increase in the production of ROS breaks the tenuous balance between ROS and antioxidant defense system that exists in the vessel wall of normotensive individuals. ROS avidly reacts with and inactivates NO at a speed six times faster than its reaction rate with an intrinsic antioxidant enzyme. NO inactivation contributes to hypertension and endothelial dysfunction. The balance between NO and ROS during the mutual scavenging is more important than each of them in the pathophysiological process of hypertension Citation[20].
The extent of lipid peroxidation caused by ROS attacking cell membrane can be assessed by MDA, a product reduced from lipid peroxidation, and a reliable and reproducible biomarker of lipid peroxide in various organs Citation[21]. In our study, we evaluated the antioxidant efficacy of PZ51 by measuring the plasma concentration of MDA. The result showed that PZ51 administration markedly reduced the plasma level of MDA.
Stroke and coronary artery disease are vascular diseases in which endothelial damage is an incipient sign that can be detected by modern techniques. Intact vascular endothelium contributes to local blood flow regulation and is important for protection against arterial thrombosis. Endothelial cells of SHRsp are constantly exposed to oxidative damage from ROS. Oxidative stress also plays a role in the pathogenesis of pressure‐induced atherosclerosis Citation[22]. Damaged endothelium and thickened intima are observed in hypertensive mammals. Even worse, endothelial cells denude and the underlying collagen is exposed to the blood flow, eliciting a series of adverse reaction. In the present study, SEM showed that PZ51 protected the endothelium from being impaired. This result is the same as Tomas et al.'s experiment, in which increased activity of GPX enhances resistance to oxidative damage in bovine aortic endothelial cells Citation[23].
Diminished production of NO caused by impaired expression of eNOS has been implicated in the pathogenesis of hypertensive endothelial dysfunction Citation[24]. In our study, PZ51 increased eNOS protein expression significantly. Production of NO via eNOS is essential for healthy endothelium. Increased NO from eNOS may upregulate manganese–SOD expression in adjacent vascular smooth muscle cells (VSMC) via cyclic guanosine monophospate (cGMP)‐mediated pathways, thus preventing superoxide‐induced degradation of NO Citation[25], Citation[26]. However, we are not sure which is the cause and which is the result: the change of endothelial cells or the increased eNOS expression.
IMT of the CCA is an independent and strong predictor of stroke occurrence Citation[27], Citation[28]. In our study, light microscopic examination showed that PZ51 administration for 6 weeks reduced the IMT of the CCA significantly, with a trend (but non‐significant) reduction of M/L ratio. Much of this may be due PZ51's inhibition of protooncogene expression by reducing ROS. Studies in chronic models of hypertension have shown that ROS increased mRNA of protooncogene such as c‐myc and c‐fos Citation[29], Citation[30] and thickened the media of arteries mainly through VSMC hypertrophy and proliferation. VSMC from SHRsp showed greater growth rate than those from Wistar–Kyoto Citation[31], which contribute to the arterial remodeling in hypertension Citation[32]. Increased eNOS‐derived NO also plays a role in inhibiting VSMC proliferation stimulated by protooncogene Citation[33].
Previous studies of antioxidants on the antihypertensive effect have been conflicting. Most antioxidants prevented blood pressure elevation significantly in humans Citation[34], Citation[35], Dahl salt‐sensitive hypertensive rats Citation[36] and SHRsp Citation[37–40]. Some results are contrary Citation[41], Citation[42]. However, in the present study, we only found a non‐significant reduction in blood pressure. This was likely due to extreme hypertension in SHRsp ingesting a high salt load. The protective effect on endothelium and vascular structure of PZ51 is independent of blood pressure in these animals.
In conclusion, the present study showed that PZ51 administration suppressed lipid peroxidation, elevated plasma level of NO and enhanced expression of eNOS. Maybe by means of these mechanisms, PZ51 improved vascular remodeling and protected the endothelium. Data from this experiment provided important evidence for the use of antioxidant PZ51 in the protection of artery and we inferred that PZ51 may have a preventive effect against stroke in hypertensive subjects.
Acknowledgements
We thank Professor Xi Chen and Xiang‐feng Cong of the Biochemical Division, and Professor Ying‐mao Ruan of the Pathological Division of Fu Wai Hospital for their excellent technical assistance. We also thank Dr. Robert Detrano of the Los Angeles Biomedical Research Institute for reading this manuscript.
References
- Suzuki H., DeLano F. A., Parks D. A., Jamshidi N., Granger D. N., Ishii H., et al. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci USA 1998; 95: 4754–4759
- Suzuki H., Swei A., Zweifach B. W., Schmid‐Schonbein G. W. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats: Hydroethidine microfluorography. Hypertension 1995; 25: 1083–1089
- Russo C., Olivieri O., Girelli D., Faccini G., Zenari M., Lombardi S., et al. Anti‐oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertension 1998; 16: 1267–1271
- Mihailovic M. B., Avramovic D. M., Jovanovic I. B., Pesut O. J., Matic D. P., Stojanov V. J. Blood and plasma selenium levels and GSH‐PX activities in patients with arterial hypertension and chronic heart disease. J Environ Pathol Toxicol Oncol 1998; 17: 285–289
- Hattori R., Inoue R., Sase K., Eizawa H., Kosuga K., Aoyama T., et al. Preferential inhibition of inducible nitric oxide synthase by ebselen. Eur J Pharmacol 1994; 267: R1–2
- Masumoto H., Sies H. The reaction of ebselen with peroxynitrite. Chem Res Toxicol 1996; 9: 262–267
- Hideaki I., David I. G., Hiroyuki M., Iseavall M. M. Antioxidant ebselen reduces oxidative damage in focal cerebral ischemia. Free Rad Biol Med 2003; 34: 56–63
- Dawson D. A., Masayasu H., Graham D. I., Macrae I. M. The neuroprotective efficacy of ebselen (a glutathione mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci Lett 1995; 185: 65–69
- Ogawa A., Yoshimoto T., Kikuchi H., Sano K., Saito I., Yamaguchi T., et al. Ebselen in acute middle cerebral artery occlusion: A placebo‐controlled, double‐blind clinical trial. Cerebrovasc Dis 1999; 9: 112–118
- Maulik N., Yoshida T., Das D. K. Oxidative stress developed during the reperfusion of ischemic myocardium induces apoptosis. Free Rad Biol Med 1998; 24: 869–875
- Takasago T., Peters E. E., Graham D. I., Masayasu H., Macrae I. M. Neuroprotective efficacy of ebselen, an antioxidant with anti‐inflammatory actions, in a rodent model of permanent middle cerebral artery occlusion. Br J Pharmacol 1997; 122: 1251–1256
- Namura S., Nagata I., Takami S., Masayasu H., Kikuchi H. Ebselen reduces cytochrome c release from mitochondria and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke 2001; 32: 1906–1911
- Tracey W. R., Tse J., Carter G. Lipopolysaccharide‐induce changes in plasma nitrite and nitrate concentrations in rat and mice: Pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther 1995; 272: 1011–1015
- Li L., Shen Y‐M., Yang X‐S., Wu W‐L., Wang B‐G., Chen Z‐H., et al. Effect of spiramine T on antioxidant enzymatic activities and nitric oxide production in cerebral ischemia‐reperfusion gerbils. Brain Res 2002; 944: 205–209
- Kato N., Yanaka K., Nagase S., Hirayama A., Nose T. The antioxidant EPC‐K1 ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischaemia. Acta Neurochir 2003; 145: 489–493
- The Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E supplementation and cardiovascular events in high‐risk patients. N Eng J Med 2000; 342: 154–160
- Stephens N. G., Parsons A., Schofield P. M., Kelly F., Cheeseman K., Mitchinson M. J. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996; 347: 781–786
- Kok F. J., van Poppel G., Melse J., Verheul E., Schouten E. G., Kruyssen D. H., et al. Do antioxidants and polyunsaturated fatty acids have a combined association with coronary atherosclerosis. Atherosclerosis 1991; 86: 85–90
- Ichikawa S., Omura K., Katayama T., Okamura N., Ohtsuka T., Ishibashi S., et al. Inhibition of superoxide anion production in guinea pig polymorphonuclear leukocytes by a seleno‐organic compound, ebselen. J Pharmacobio‐dyn 1987; 10: 595–597
- Guzik T. J., West N. E. J., Pillai R., Taggart D. P., Channon K. M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 2002; 39: 1088–1094
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–358
- Yasunari K., Maeda K., Nakamura M., Yoshikawa J. Carvedilol inhibits pressure‐induced increase in oxidative stress in coronary smooth muscle cells. Hypertens Res 2002; 25: 419–425
- Tomas J. P., Geiger P. G., Girotti A. W. Lethal damage to endothelial cells by oxidized low density lipoprotein: Role of selenoperoxidases in cytoprotection against lipid hydroperoxide‐ and iron‐mediated reactions. J Lipid Res 1993; 34: 479–489
- Liao J. K., Shin W. S., Lee W. Y., Clark S. L. Oxidized low‐density lipoprotein decreases the expression of endothelial nitric oxide synthase. J Biol Chem 1995; 270: 319–324
- Fukuo K., Yang J., Suzuki T., Kaimoto T., Takemura Y., Yasuda O., et al. Nifedipine upregulates manganese superoxide dismutase expression in vascular smooth muscle cells via endothelial cell‐dependent pathways. Hypertens Res 2003; 26: 503–508
- Fukai T., Siegfried M. R., Ushio‐Fukai M., Cheng Y., Kojda G., Harrison D. G. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 2000; 105: 1631–1639
- Bots M. L., Hoes A. W., Koudstaal P. J., Hofman A., Grobbee D. E. Common carotid intima‐media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997; 96: 1432–1437
- Chambless L. E., Folsom A. R., Clegg L. X., Sharrett A. R., Shahar E., Nieto F. J., et al. Carotid wall thickness is predictive of incident clinical stroke: The Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000; 151: 478–487
- Rao G. N., Lassegue B., Griendling K. K., Alexander R. W. Hydrogen peroxide stimulates transcription of c‐jun in vascular smooth muscle cells: Role of aracidonic acid. Oncogene 1993; 8: 2759–2764
- Eddie L. G., Victoria V., Ayad A. J. Role of reactive oxygen species in bradykinin‐induced mitogen‐activated protein kinase and c‐fos induction in vascular cells. Hypertension 2000; 35: 942–947
- Yamori Y., Igawa T., Kanbe T., Nara Y., Tagami M. Enhanced growth rate of cultured smooth muscle cells from spontaneously hypertensive rats. Heart Vessels 1988; 4: 94–99
- Intengan H. D., Schiffrin E. L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fivrosis. Hypertension 2001; 38((3 pt 2))581–587
- Yang D., Tan Z., Pan J. Y., Wang T. H. 17beta‐Estradiol inhibits vascular smooth muscle cell proliferation and c‐fos expression: Role of nitric oxide. Sheng Li Xue Bao 2002; 54: 17–22
- Ceriello A., Giugliano D., Quatraro A., Lefebvre P. J. Anti‐oxidants show anti‐hypertensive effect in diabetic and hypertensive subjects. Clin Sci 1991; 81: 739–742
- Galley H. F., Thornton J., Howdle P. D., Walker B. E., Webster N. R. Combination oral antioxidant supplementation reduces blood pressure. Clin Sci 1997; 92: 361–365
- Meng S., Cason G. W., Gannon A. W., Racusen L. C., Manning R. D Jr. Oxidative stress in Dahl salt‐sensitive hypertension. Hypertension 2003; 41: 1346–1352
- Park J. B., Touyz R. M., Chen X., Schiffrin E. L. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt‐loaded stroke‐prone spontaneously hypertensive rats. Am J Hypertens 2002; 15((1 Pt 1))78–84
- Noguchi T., Ikeda K., Sasaki Y., Yamamoto J., Seki J., Yamagata K., et al. Effect of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke‐prone spontaneously hypertensive rats. Hypertens Res 2001; 24((6))735–742
- Negishi H., Xu J. W., Ikeda K., Njelekela M., Nara Y., Yamori Y. Black and green tea polyphenols attenuate blood pressure increases in stroke‐prone spontaneously hypertensive rats. J Nutr 2004; 134: 38–42
- Chen X., Touyz R. M., Park J. B., Schiffrin E. L. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke‐prone SHR. Hypertension 2001; 38((3 Pt 2))606–611
- Gil‐Longo J., Fernandez‐Grandal D., Alvarez M., Sieira M., Orallo F. Study of in vivo and in vitro resting vasodilator NO tone in normotensive and genetically hypertensive rats. Eur J Pharmacol 1996; 310: 175–183
- Miyashita F., Kawaguchi A., Sugimoto K., Kitoh Y., Tsutsumi H., Fujimura A. Failure of probucol to prolong survival in salt‐loaded stroke‐prone spontaneously hypertensive rats. Hypertens Res 2000; 23: 497–501