Abstract
Acromegaly is characterized by major cardiovascular alterations. Although the underlying mechanisms of these vascular modifications have not been elucidated, recent studies have focused on endothelial dysfunction. Nitric oxide (NO) may contribute to increased vascular resistance, reduced platelet aggregation, inhibition of smooth muscle cell proliferation, and reduction of lipoxygenase activity. At present, no data on NO production in acromegalics are available. The aim of this study was to evaluate the effect of high levels of growth hormone (GH) and insulin‐like growth factor‐1 (IGF‐1) present in acromegaly on NO pathway to investigate the role played by this molecule in the cardiovascular changes experienced by these patients. We studied 13 acromegalics and 12 sex‐ and age‐matched normotensive controls. Platelet NO levels were measured in the supernatant of lysed platelets. Endothelial NO synthase (eNOS) was determined by Western blot analysis of platelets. NO concentrations were significantly reduced in patients (p<0.0001). There were no differences between male and female patients, nor were platelet NO levels and the presence/absence of hypertension related in acromegalics; by contrast, NO concentrations inversely correlated with GH (p = 0.03) and IGF‐1 (p = 0.04) levels, and with disease duration (p = 0.04). eNOS protein concentrations were significantly reduced in the platelets of patients compared with controls (p<0.0001). This study demonstrates for the first time a strong reduction in platelet NO concentrations in acromegalic patients due to reduced eNOS expression. Moreover the inverse correlation of NO levels with GH, IGF‐1 and disease duration suggests that reduced levels of platelet NO linked to GH excess may contribute to the vascular alterations affecting patients with acromegaly.
Introduction
Acromegaly is a rare condition, almost uniquely due to a primary pituitary adenoma growth hormone (GH) secreting, characterized by increased morbidity and mortality from cardiovascular disease (CVD), which may be early observed Citation[1], Citation[2].
The high prevalence of CVD in acromegalic patients has been related to concomitant disorders such as hypertension, high glucose and insulin levels and dyslipoproteinaemia Citation[2].
In addition to these recognized cardiovascular risk factors, in vitro and in vivo studies have widely demonstrated the deleterious effects of GH/insulin‐like growth factor‐1 (IGF‐1) excess directly on the heart, in terms of structural changes and functional impairment Citation[3]. Furthermore, the vascular consequences of acromegaly have been explored, demonstrating an increased intima‐media thickness of common carotid arteries Citation[4] and hypertrophic remodelling of subcutaneous small resistance arteries in acromegalic patients Citation[5].
Along with these changes, endothelial dysfunction, in particular flow‐mediated dilatation impairment (which is an early marker of atherosclerosis) has been described in active acromegaly Citation[1].
In the context of vascular alterations, a pivotal role is played by nitric oxide (NO), produced by endothelial NO synthase (eNOS), which is fundamental in vascular tone regulation and acts as a potent vasodilator. It also reduces platelet aggregation and activation, it inhibits proliferation and migration of vascular smooth muscle cells, and reduces the adhesion of leukocytes to endothelium Citation[6].
Decreased NO levels in platelets of both diabetic and hypertensive patients have been previously described by our group Citation[7], Citation[8].
Not much data is available on GH and NO reciprocal interactions. In vitro and in vivo studies indicate that isosorbide dinitrate and sodium nitroprusside (i.e. NO donor and releaser, respectively) seem to stimulate GH secretion in acromegalic patients Citation[9]. Conversely, NO‐mediated dilation is impaired in patients with acquired GH deficiency, and restored by GH replacement therapy Citation[10]. GH deficiency of hypopituitaric patients is in fact characterized not only by increased intima‐media thickness in conduit arteries Citation[11], but also by a trend toward impaired flow‐mediated dilation Citation[12], and decreased NO generation Citation[13].
Extensive literature data show that cardiac and vascular alterations contribute to excess mortality in patients with both acromegaly and adult GH deficiency Citation[14], Citation[15].
As data on NO production in acromegalics are not available, the present study was undertaken to evaluate the effect of high levels of GH and IGF‐1 on NO pathway, in order to investigate the role of this molecule in the cardiovascular alterations experienced by these patients.
Materials and methods
Patients
We investigated 25 subjects: 13 patients (six men and seven women) with active acromegaly and 12 sex‐ and age‐matched control subjects (six men and six women). All acromegalic women and all female controls were in post‐menopause. All subjects gave their written informed consent to take part in the study according to local ethical committee guidelines.
The diagnosis of acromegaly was based on typical clinical findings: high levels of IGF‐1 in relation to age and/or elevated GH levels not suppressible below 1 μg/l after a 75‐g oral glucose tolerance test (OGTT).
The presence of insulin resistance was established through the determination of the homeostasis model assessment of insulin resistance index (HOMA‐IR), as follows: fasting insulin (mcU/ml)×fasting glucose (mmol/l)/22.5 Citation[16].
Normal mean HOMA‐IR values in the Italian population are 1.13, range 0.72–1.72 Citation[17].
Eight patients were hypertensive, five had impaired glucose tolerance and three had diabetes mellitus, according to WHO criteria. Duration of acromegaly was established by interviewing patients and by comparing photographs taken in the different decades.
The 12 healthy subjects were normotensive (blood pressure below 140/90 mmHg); they had no history of CVD disease and had not taken medications for at least 6 weeks.
Patients and controls were also matched for smoking habits.
Determination of eNOS activity
Peripheral venous blood was collected after overnight fasting and immediately mixed with anticoagulant citrate dextrose. After platelets isolation, NO levels were determined in the supernatants of lysed platelets, as described by Chen Citation[18], as previously reported Citation[7].
eNOS activity, used as an index of NO production, was expressed in nmol NO produced/min/mg protein. Protein concentrations were determined as described by Lowry Citation[19] using albumin as standard.
Western blot analysis was performed as previously described Citation[20]. Blots were incubated for 2 h at room temperature with eNOS rabbit polyclonal antibody diluted 1:200, and washed using TTBS. The antigen‐antibody immunocomplex was detected with protein A125 I (Amersham Pharmacia Biotech) labelled by incubation for 45 min at room temperature.
Membranes were thoroughly washed and subjected to autoradiography for 72 h at −70°C.
Statistical analysis
Data are reported as mean±SE. Statistical analysis was performed using analysis of variance, Student's unpaired t‐test, and linear correlation analysis (r). Significance was set at p<0.05.
Results
The clinical and biochemical characteristics of patients and control subjects are shown in .
Table I. Clinical and biochemical characteristics of acromegalic patients and control subjects.
GH levels were calculated as the mean of four different GH values obtained from blood samples withdrawn in the morning (08.00–14.00 h).
Mean disease duration was 12.2±1.7 years (range 2–26 years).
There were no significant differences between the two groups in age, body mass index, plasma glucose and total cholesterol, whereas blood pressure, triglycerides and lipoprotein (Lp)(a) levels were higher in patients. Mean high‐density lipoprotein (HDL) cholesterol was significantly lower in patients than in controls (p<0.05).
HOMA‐IR was significantly higher in patients (p<0.05), and was inversely correlated with NO levels (p<0.05).
Platelet NO levels were significantly decreased in patients compared with controls (0.51±0.02 vs 5.1±0.2 nmol NO/m/mg protein, p<0.0001) ().
Figure 1. (A) Nitric oxide synthase (NOS) activity evaluated as nmol nitric oxide (NO) produced/min/mg protein, in platelets of acromegalic patients and healthy subjects. Data are expressed as mean±SE. (B) Endothelial NOS (e‐NOS) expression determined through Western Blot analysis. Values are given as mean±SE.
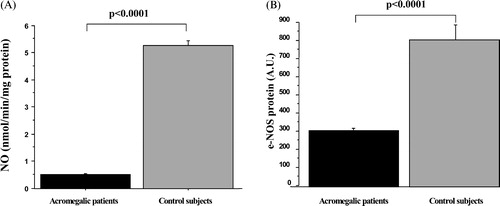
Western blot analysis showed that differences in NO levels were due to reduced expression of eNOS protein (acromegalics vs controls: 285±13 vs 785±84 AU, p<0.0001) ().
There were no differences in NO levels between male and female patients (0.48±0.02 vs 0.55±0.4 nmol NO/m/mg protein, respectively, p = 0.875). Male controls had slightly lower NO levels than females (4.6±0.2 vs 5.5±0.4 nmol NO/m/mg protein, p = 0.072). No differences were noted among patients between hypertensives and normotensives, between diabetics and non‐diabetics, and between smokers and non‐smokers.
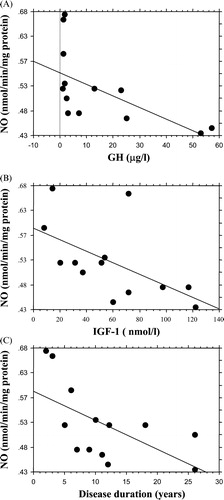
In patients, NO concentrations inversely correlated with GH levels (r = 0.60, p = 0.03), IGF‐1 levels (r = 0.56, p = 0.04) and with disease duration (r = 0.6, p = 0.04) ().
Figure 2. Regression analysis showing the negative correlation observed in acromegalic patients between nitric oxide (NO) concentrations and (A) growth hormone (GH) levels (r = 0.60, p = 0.03), (B) insulin‐like growth factor‐1 (IGF‐1) levels (r = 0.56, p = 0.04) and (C) disease duration (r = 0.6, p = 0.04).
Discussion
This is, to our knowledge, the first study analysing the effects of GH and IGF‐1 excess on NO production in patients with acromegaly.
The study showed a 10‐fold reduction of platelet NO levels in acromegalics compared with control subjects, which was due to reduced expression of eNOS protein. These data provide new insights into the pathophysiology of cardiovascular complications developed by acromegalic patients.
The increased CVD risk of acromegalics is generally attributed to the concomitant high prevalence of risk factors for atherosclerosis, such as hypertension, dyslipoproteinaemia, and high glucose and insulin levels Citation[2]. However, the significant inverse correlation observed between NO concentrations and GH and IGF‐1 levels as well as disease duration, also suggests that the increased atherosclerotic risk in these patients may be linked to abnormal GH secretion, and increased IGF‐1 rather than to classical risk factors.
This hypothesis is supported by a recent paper from Colao and collaborators Citation[21] showing that somatostatin analogue therapy can reverse cardiomyopathy in non‐hypertensive non‐diabetic acromegalic patients only in young subjects with short disease duration. These evidences highlight the importance of the duration of disease and of high levels of GH and IGF‐1 in determining the cardiovascular alterations seen in acromegaly.
Moreover, the capillaroscopic morphological alterations observed by Brevetti et al. in the nailfold of acromegalics, including patients without diabetes mellitus or hypertension, suggest that acromegaly per se plays a significant role in determining microcirculation abnormalities Citation[22].
The reduced expression of eNOS protein found in the platelets of acromegalic patients could underline their reduced NO production, as this molecule is mainly produced by eNOS in platelets.
Although in vitro studies have shown that physiological doses of IGF‐1 stimulate eNOS expression, it is possible that supra‐physiological levels of IGF‐1 can inhibit this enzyme through a down‐regulation phenomenon. It is well known that platelets express IGF‐1 receptors Citation[23], so that is possible to hypothesize a direct effect of IGF‐1 on platelets NO production. Molecular studies are needed to elucidate the mechanism of reduced platelets eNOS expression.
Moreover, the finding that GH replacement therapy in hypopituitaric patients restores NO‐mediated vasodilation does not contrast with our data. GH deficiency induces vascular alteration such increased intima‐media thickness of conduit arteries and decreased NO generation Citation[11], Citation[13], complications that are somehow similar to the ones observed in acromegalic patients with GH/IGF‐1 excess. Both acromegalics and GH deficiency patients suffer from high mortality mainly due to cardiovascular disease Citation[14], Citation[15].
It is indeed likely that there is a balance between adverse and beneficial effects of GH and IGF‐1 according to their circulating and tissues levels.
Interestingly, decreased platelet NO content has been observed in both diabetic and hypertensive patients. In a previous study, we observed a roughly fivefold reduction in NO levels in hypertensive compared with control subjects Citation[7]. However, in the present study, we found no differences between hypertensive and normotensive acromegalic patients, nor between diabetics and non‐diabetics, and this could be connected with their marked reduction in NO levels suggesting that the negative effect of high GH/IGF‐1 levels might overcome the influence of hypertension or hyperglicaemia.
Moreover, no differences in NO levels were observed between smokers and non‐smokers acromegalic patients, suggesting that the NO deficiency and the consequent CVD risk could be linked to GH/IGF‐1 levels and to metabolic GH‐related risk factors rather than to other well‐established risk factors. However, it is noteworthy that the small number of patients with acromegaly, a rare disease, could have influenced the statistical results.
As shown, the lipid profile and blood pressure values, were significantly different between acromegalics and controls; this is likely to be due to acromegaly itself and to GH and IGF‐1 excess that are responsible for the development of several complications, and among them hypertension and dyslipoproteinaemia.
To this regard, as expected, our patients exhibited reduced HDL cholesterol levels, which may be due to reduced HDL‐mediated NO levels, as is possible to hypothesized on the basis of recent studies Citation[24]. On the other hand, data on the possible role of increased triglycerides levels, as found in our acromegalics, in vascular alterations are conflicting Citation[25].
The correlation analysis performed in order to investigate the possible relationship between total and HDL cholesterol, triglycerides, and NO levels, showed no statistically significant results.
A further, established atherogenic risk factor – elevated levels of Lp(a), that correlates with the development of atherosclerotic lesions Citation[26] – exhibited significant difference between patients and controls.
The finding of high Lp(a) levels is in agreement with previous data showing an increase in Lp(a) concentrations in acromegalic patients Citation[27] and indicating Lp(a) as a possible independent risk factor for CVD in acromegaly. Indeed, in our patients we found no correlation between NO and Lp(a) levels, even though recent in vitro studies have demonstrated inhibition of NO synthesis by oxidized Lp(a) Citation[28]. The mechanisms proposed to explain this dysfunction are hyperinsulinism and reduced post‐heparin lipase activity Citation[29]; in addition, increases in Lp(a) are reversed by therapy with the somatostatin analogue octreotide Citation[27].
Finally, an important issue is the possible role of insulin in NO production. Our data show that acromegalic patients are insulin‐resistant as defined by the HOMA‐IR index Citation[16] compared with control subjects, and that insulin levels correlate with GH and IGF‐1 concentrations. Hyperinsulinaemia is indeed a common feature observed in acromegalic patients Citation[2], although our patients exhibited a smaller degree of hyperinsulinaemia compared with diabetic patients referring to our unit (data not shown).
Although insulin enhances endothelium‐dependent vasodilation via stimulation of NO release Citation[30], mounting evidence suggests that insulin resistance leads to impaired endothelium‐dependent vasodilation, explaining the insulin‐induced vasodilation impairment affecting patients with obesity, hypertension and diabetes mellitus type 2, all conditions commonly associated with hyperinsulinism. Moreover, such impairment appears to be directly related to metabolic insulin resistance Citation[31].
In vitro studies also show that insulin‐mediated NO release and glucose metabolism share common signalling pathways in vascular endothelial cells, suggesting that the vascular and metabolic actions of insulin may be coupled Citation[32].
To what extent the peripheral insulin resistance in acromegaly contributes to platelet NO production is unclear. Our data seem to suggest that insulin resistance linked to GH excess may contribute to NO reduction observed in our patients.
In conclusion, we demonstrate for the first time a striking reduction in NO levels associated with reduced eNOS expression in the platelets of acromegalic patients, which is inversely correlated with GH and IGF‐1 levels. These data suggest that low amounts of platelet NO may contribute to the increased atherogenic risk and to the vascular alterations affecting acromegalics. The possible increase of NO bioavailability in cured acromegalic patients remains to be established.
Acknowledgments
We are grateful to Dr Silvia Modena for her assistance in the revision of this manuscript.
References
- Brevetti G., Marzullo P., Silvestro A., Pivonello R., Oliva G., Di Somma C. Early vascular alterations in acromegaly. J Clin Endocrinol Metab 2002; 87: 3174–3179
- Melmed S. Acromegaly. N Engl J Med 1990; 322: 966–977
- Colao A., Marzullo P., Di Somma C., Lombardi G. Growth hormone and the heart. Clin Endocrinol 2001; 54: 137–154
- Colao A., Spiezia S., Cerbone G., Pivonello R., Marzullo P., Ferone D. Increased arterial intima‐media thickness by B‐M mode echo‐doppler ultrasonography in acromegaly. Clin Endocrinol 2001; 54: 515–524
- Rizzoni D., Porteri E., Giustina A., De Ciuceis C., Sleiman I., Baori G. E. M. Acromegalic patients show the presence of hypertrophic remodeling of subcutaneous small resistance arteries. Hypertension 2004; 43: 1–5
- Ignarro L. J. Physiology and pathophysiology of nitric oxide. Kidney Int 1996; 55: S2–S5
- Camilletti A., Moretti N., Giacchetti G., Faloia E., Martarelli D., Mantero F. Decreased nitric oxide levels and increased calcium content in platelets of hypertensive patients. Am J Hypertens 2001; 14: 382–386
- Rabini R. A., Staffolani R., Martarelli D., Fumelli P., Ravaglia F., Dousset N. Influence of low density lipoprotein from insulin‐dependent diabetic patients on platelet functions. J Clin Endocrinol Metab 1999; 84: 3770–3774
- Cuticca C. M., Giusti M., Bocca L., Sessarego P., De Martini D., Valenti S. Nitric oxide modulates in vivo and in vitro growth hormone release in acromegaly. Neuroendocr 1997; 66: 426–431
- Capaldo B., Guardasole V., Pardo F., Matarazzo M., Di Rella F., Numis F. Abnormal vascular reactivity in growth hormone deficiency. Circulation 2001; 103: 520–524
- Markussis V., Beshyah S. A., Fisher C., Sharp P., Nicolaides A. N., Johnston D. G. Detection of premature atherosclerosis by high‐resolution ultrasonography in symptom‐free hypopituitary adults. Lancet 1992; 340: 1188–1192
- Boger R. H., Skamira C., Bode Boger S. M., Brabant G., Von zur Muhlen A., Frolich J. C. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double‐blind, placebo controlled study. J Clin Invest 1996; 98: 2706–2713
- Pfeifer M., Verhover R., Zizek B., Prezelj J., Poredos P., Clayton R. N. Growth hormone (GH) treatment reverses early atherosclerotic changes in GH‐deficient adults. J Clin Endocrinol Metab 1999; 84: 453–457
- Rosen T., Bengtsson B. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet 1990; 336: 285–288
- Bulow B., Hagmar L., Mikoczy Z., Nordstrom C. H., Erfurth E. M. Increased cerebrovascular mortality in patients with hypopituitarism. Clin Endocrinol 1996; 46: 75–81
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. Homeostasis model assessment: Insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419
- Kiechl S., Willeit J., Poewe W., Egger G., Oberhollenzer F., Muggeo M. Insulin sensitivity and regular alcohol consumption: Large, prospective, cross sectional population study (Bruneck study). BMJ 1996; 313: 1040–1044
- Chen L. Y., Metha P., Metha J. L. Oxidized LDL decreases L‐arginine uptake and oxide synthase protein expression in human platelets. Circulation 1996; 93: 1740–1746
- Lowry O. H., Rosenbrough A., Farr A. L., Randal R. J. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–275
- Tannous M., Rabini R. A., Vignini A., Moretti N., Fumelli P., Ielinski B. Evidence for NOS‐dependent peroxynitrite production in diabetic platelets. Diabetologia 1999; 42: 539–544
- Colao A. M., Marzullo P., Cuocolo A., Spinelli L., Pivonello R., Bonaduce D. Reversal of acromegalic cardiomyopathy in young but not middle‐aged patients after 12 months of treatment with the depot long‐acting somatostatin analogue octreotide. Clin Endocrinol 2003; 58: 169–176
- Schiavon F., Maffei P., Martini C., De Carlo E., Fais C., Todesco S. Morphologic study of microcirculation in acromegaly by capillaroscopy. J Clin Endocrinol Metab 1999; 84: 3151–3155
- Isenovic E. R., Divald A., Milivojevic N., Grgurevic T., Fisher S. E., Sowers J. R. Interactive effects of insulin‐like growth factor‐1 and beta‐estradiol on endothelial nitric oxide synthase activity in rat aortic endothelial cells. Metabolism 2003; 52: 482–487
- Spieker L. E., Sudano I., Hurlimann D., Lerch P. G., Lang M. G., Binggeli C. High‐density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation 2002; 105: 1399–1402
- Lundman P., Eriksson M. J., Stuhlinger M., Cooke J. P., Hamsten A., Tornvall P. Mild‐to‐moderate hypertriglyceridaemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol 2001; 38: 111–116
- De la Pena‐Diaz A., Izaguirre‐Avila R., Angles‐Cano E. Lipoprotein Lp(a) and atherothrombotic disease. Arch Med Res 2000; 31: 353–359
- Arosio M., Sartore G., Rossi C. M., Casati G., Faglia G., Manzato E. LDL physical properties, lipoprotein and Lp(a) levels in acromegalic patients. Effects of octreotide therapy. Italian Multicentre Octreotide Study Group. Atherosclerosis 2000; 151: 551–557
- Moeslinger T., Friedl R., Volf I., Brunner M., Koller E., Spieckermann P. G. Inhibition of inducible nitric oxide synthesis by oxidized lipoprotein (a) in a murine macrophage cell line. FEBS Lett 2000; 478: 95–99
- Tan K. C. B., Shiu S. W. M., Janus E. D., Lam K. S. L. LDL subfractions in acromegaly: Relation to growth hormone and insulin‐like growth factor‐I. Atherosclerosis 1997; 129: 59–65
- Taddei S., Virdis A., Mattei P., Natali A., Ferrarini E., Salvetti A. Effect of insulin on acetylcholine‐induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation 1995; 92: 2911–2918
- Petrie J. R., Ueda S., Webb D. J., Elliott H. L., Connell J. M. C. Endothelial nitric oxide production and insulin sensitivity. A physiological link with implications for pathogenesis of cardiovascular disease. Circulation 1996; 93: 1331–1333
- Zeng G., Quon M. J. Insulin‐stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 1996; 98: 894–898