Abstract
Objective. An index of large artery stiffness (SIDVP) simply derived from the digital volume pulse (DVP) was developed recently. However, the role of the SIDVP in untreated hypertensive patients was not well elucidated. Methods. We enrolled 124 untreated hypertensive patients (mean age 55.4±13.1 years, 57 men). The DVP was measured in right index finger by a photoplethysmography. The SIDVP was formulated as body height divided by transition time from early systolic peak to the inflection point of reflection wave. Two functional indices of aortic compliance, stiffness index (SI) and distensibility (DI), were also used for measurement of aortic stiffness. Results. The SIDVP was significantly correlated with blood urea nitrogen (BUN), and left ventricular mass index (LVMI). Patients with vascular diseases had higher level of SIDVP (10.12±2.97 vs 8.45±1.78, p<0.001), SI (13.76±7.63 vs 10.87±8.88, p = 0.116), BUN (28.4±24.7 vs 14.5±4.6, p<0.001) and lower level of DI (1.34±0.88 vs 1.93±1.12, p = 0.010) than those without vascular diseases. By multivariate analysis, only the SIDVP was significantly associated with vascular diseases (OR 1.39, 95% CI 1.06–1.82, p = 0.016). Conclusions. SIDVP, SI and DI were significantly correlated with target organ damage in untreated hypertension. However, only the SIDVP was independently associated with presence of vascular diseases. SIDVP simply derived from the DVP can be used as a marker for risk stratification in untreated hypertensive patients.
Introduction
Increased stiffness of large arteries is associated with an increased cardiovascular risk in hypertension Citation[1]. Pulse wave velocity (PWV) is now recognized as a standard method for measurement of aortic stiffness Citation[2], Citation[3]. An elevated aortic PWV was not only associated with increased cardiovascular risks in hypertension Citation[3], but also could be a prognostic factor in end‐stage renal disease patients Citation[4]. Aortic stiffness increases with age Citation[5–8] and cardiovascular risk factors Citation[9]. An index of large artery stiffness (SIDVP) derived from the digital volume pulse (DVP) measured by photoplethysmography was well correlated with PWV and age Citation[10–13]. Photoplethysmography can measure DVP easily by using transmission of infrared light through the finger pad Citation[11], Citation[14]. However, the role of the SIDVP in hypertensive patients was not well elucidated. This study used SIDVP derived from DVP and two aortic function indices derived from M‐mode echocardiography to investigate the clinical significances of these indexes in untreated hypertension and to compare their differences in the associated role for vascular complications.
Methods
Subjects
One hundred and twenty‐four consecutive patients (mean age 55.4±13.1 years, 57 men) with untreated essential hypertension from a hypertension clinic were included in this study. Hypertension was diagnosed if blood pressure more than 140/90 mmHg on two separate occasions. All patients received the appropriate evaluation to exclude secondary hypertension and did not take any anti‐hypertension agents before the study. All patients received detail examination for the presence of end organ damage and vascular diseases. Vascular diseases were diagnosed if patients had coronary artery disease (defined as patients with any history of angina pectoris, myocardial infarction, coronary revascularization or congestive heart failure), cerebral vascular diseases (defined as patients with any history of transient ischemic attack, ischemic stroke or cerebral hemorrhage) or renal disease (defined as plasma creatinine concentration >2.0 mg/dl).
Acquisition of the SIDVP
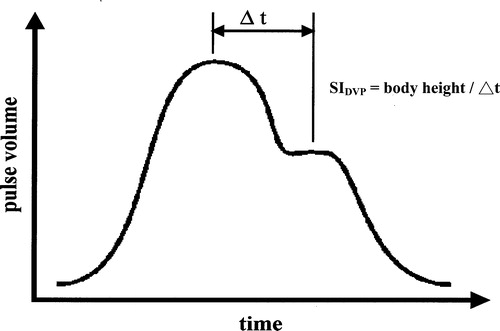
The DVP was measured in right index finger by a photoplethysmography (Micro Medical, Gillingham, UK) transmitting infrared light at 940 nm. Frequency response of the photoplethysmography was flat to 10 Hz. Digital output from the photoplethysmography was recorded through an analogue‐to‐digital converter (12 bits, sampling frequency 100 Hz). The DVP waveforms were recorded for 10 s and SIDVP was formulated automatically by computer as body height (m) divided by transition time (s) from the first systolic peak to the inflection point of reflection waveform (). All measurements were made with the subject supine in a temperature‐controlled laboratory at 25±1°C. All subjects were allowed to acclimatize to this temperature for at least 10 min before recordings commenced Citation[13].
Determination of stiffness index (SI) and distensibility (DI)
All patients received trans‐thoracic echocardiography. The studies were performed by means of a commercially available ultrasound system (Hewlett‐Packard Sonos 1000, Andover, MA, USA) with a 2.5‐MHz phased array transducer. Two functional indices of aortic compliance, SI and DI, were derived from measurements of aortic diameters at 3 cm above aortic valve by M‐mode echocardiography and sphygmomanometric brachial artery blood pressure. The SI and DI were calculated from following formulas: SI = ln(systolic blood pressure/diastolic blood pressure)/(changes in aortic diameter/diastolic diameter); DI = 2(changes in aortic diameter)/(diastolic diameter)(pulse pressure) Citation[15–17].
Determination of left ventricular mass index (LVMI)
We assessed left ventricular mass by using a standard method, which was previously reported Citation[18]. Two skilled cardiologists performed two‐dimensional and M‐mode echocardiography, using a Hewlett Packard Sonos 1000 with a 2.5‐MHz transducer. A single observer who was unaware of the results of blood pressure measurements read the echocardiograms and videotape recordings. Measurements of end‐systolic and end‐diastolic left ventricular internal dimensions, end‐diastolic interventricular septal thickness, end‐diastolic posterior wall thickness, aortic root dimension, and left atrial dimension were made according to the American Society of Echocardiography guidelines Citation[19]. Left ventricular mass was determined by the method of Devereux et al. Citation[20], with LVMI calculated by dividing left ventricular mass by body surface area.
Assessment of transmitral Doppler flow velocity
The heart was scanned in the standard apical four‐chamber view so that the diameter of the mitral annulus was maximized. This imaging study was followed by Doppler interrogation of the mitral inflow velocities with the sample volume at the tip of mitral leaflets Citation[21]. The following parameters were measured from the mitral flow velocity spectrum: peak early filling velocity, peak atrial velocity and early filling to atrial velocity ratio (E/A). E/A was used as an index of left ventricular diastolic function.
Statistical analysis
The relation between measurements of aortic stiffness and target organ damage was assessed using Pearson's correlation. Differences between patients with or without vascular diseases were compared with Student's t‐test, for continuous variables, or the chi‐square test, for categorical variables. Multiple logistic regression analysis was used for assessment of the most independent marker for vascular diseases. All data are presented as the mean±standard deviation (SD). A p‐value of less than 0.05 was considered statistically significant. All analysis was performed with the SPSS 10.0 for Windows (SPSS Institute, Chicago, IL, USA).
Results
The SIDVP was significantly correlated with SI (r = 0.314, p<0.001) and DI (r = −0.340, p<0.001). Among risk factors, SIDVP was significantly correlated with systolic blood pressure (r = 0.162, p = 0.063), diastolic blood pressure (r = 0.202, p = 0.024) and serum triglyceride level (r = 0.275, p = 0.008). SI was significantly correlated with systolic blood pressure (r = 0.171, p = 0.050), diastolic blood pressure (r = −0.219, p = 0.015) and duration of hypertension (r = 0.357, p<0.001). DI was significantly correlated with duration of hypertension (r = −0.291, p = 0.002), systolic blood pressure (r = −0.334, p = <0.001) and serum cholesterol level (r = −0.249, p = 0.015). Both SI and DI were significantly correlated with age (r = 0.450, p<0.001; r = −0.514, p<0.001, respectively) (). Sex, smoking, diabetes mellitus or history of hyperlipidemia did not affect SIDVP, SI and DI (). Among measurements of target organ damage, SIDVP was significantly correlated with BUN (r = 0.230, p = 0.023) and LVMI (r = 0.177, p = 0.050). SI was significantly correlated with serum creatinine level (r = 0.279, p = 0.005) and E/A (r = 0.279, p = 0.002). DI was significantly correlated with BUN (r = –0.231, p = 0.023) and LVMI (r = –0.239, p = 0.008) (). There were 29 (23%) patients with vascular diseases; these had a higher level of SIDVP (10.12±2.97 vs 8.45±1.78 m/s, p<0.001), LVMI (114.4±40.6 vs 96.8±35.1, p = 0.026), BUN (28.4±24.7 vs 14.5±4.6 mg/dl, p<0.001) and lower level of DI (1.34±0.88 vs 1.93±1.12×10−6 cm2/dyne, p = 0.010) than those without vascular diseases (). By multivariate logistic regression analysis, only SIDVP was significantly associated with vascular diseases (OR 1.39, 95% CI 1.06–1.82, p = 0.016) ().
Table I. Correlations between clinical characteristics and aortic stiffness.
Table II. Risk factors and aortic stiffness.
Table III. Correlation between aortic stiffness and measurements of end organ damage.
Table IV. Comparison between patients with or without vascular diseases.
Table V. Multiple logistic analysis for association of vascular diseases.
Discussion
The present study showed that all three indices of aortic stiffness were significantly correlated with target organ damage and age. An increased aortic stiffness has been noted to be associated with target organ damage in hypertension Citation[1], Citation[3]. PWV is frequently used for measuring aortic stiffness; however, measuring PWV is time‐consuming and skill‐dependent. Measuring PWV also needs specialized devices Citation[3], Citation[4], making this method difficult to be used extensively in clinical practice. The methods that we used in this study, including SI and DI derived from M‐mode echocardiography and SIDVP from plethysmography, were more simple, convenient, skill‐independent and time‐saving in comparison with PWV. SIDVP was noted to be correlated with aging Citation[11]. SI and DI were noted to be associated with microalbuminemia Citation[22] and LVH Citation[23] in essential hypertension. However, this is the first time that the correlation between these indices of aortic stiffness and target organ damage in hypertension has been extensively demonstrated.
This analysis also showed that SIDVP was significantly associated with vascular diseases but not SI and DI after multivariate analysis controlling blood pressure. One of the possible reasons was that SI and DI were pressure‐dependent measurements Citation[24]. SI, DI and other factors lost their association with vascular diseases when we analyzed them controlling blood pressure in multivariate analysis. The other possible reason was that good image acquisition was needed to measure diameter changes in the ascending aorta from M‐mode echocardiography. SI and DI were more subjective to variation when the image was not perfect.
This study was the first to demonstrate the clinical significance of SIDVP in hypertension. SIDVP could be obtained easily and objectively from commercially available photoplethysmography with automatically formulated software Citation[11], Citation[13]. However, good and steady DVP was necessary for this measurement. DVP patients with deformed fingers, arrhythmias or peripheral occlusive artery diseases were difficult to detect by photoplethysmography. SIDVP could not be applied in these patients.
Our study was limited by a cross‐section design rather than a longitudinal study. However, SIDVP was still an independent factor associated with vascular diseases after carefully evaluation by multivariate analysis considering all possible factors. Another limitation was that we only included untreated hypertension patients. The influences of anti‐hypertensive drugs on these aortic stiffness indices were not studied. Further large‐scale studies are warranted to study the drug effects on these stiffness indices and their impact on prognosis.
In conclusion, we found that the SIDVP was independently associated with the presence of vascular diseases among untreated hypertensive patients. The SIDVP simply derived from the DVP can be used as a marker for risk stratification in untreated hypertension and it is suitable for use in large‐scale trials because the DVP contour analysis is simple, time‐saving, operator‐independent and relatively inexpensive.
Acknowledgements
This study was supported by Grant NSC 90‐2324‐B‐006‐085 from the National Science Council, and Grand A‐19‐B‐FA09‐2‐4 from the Ministry of Education, Executive Yuan, Taipei, Taiwan.
References
- Laurent S., Boutouyrie P., Asmar R. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241
- Bramwell J. C., Hill A. V. Velocity of transmission of the pulse‐wave and elasticity of the arteries. Lancet 1992; i: 891–892
- Blacher J., Asmar R., Djane S., London G. M., Safar M. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33: 1111–1117
- Blacher J., Guerin A. P., Pannier B., Marchais S. J., Safar M., London G. Impact of aortic stiffness on survival in end‐stage renal disease. Circulation 1999; 99: 2434–2439
- Avolio A. P., Chen S. G., Wang R. P., Zhang C. L., Li M. F., O'Rourke M. F. Effects of ageing on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 1983; 68: 50–58
- Avolio A. P., Deng F. Q., Li W. Q. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: Comparison between urban and rural communities in China. Circulation 1985; 71: 202–210
- Kelly R. P., Hayward C., Avolio A. P., O'Rourke M. F. Non‐invasive determination of age‐related changes on the human arterial pulse. Circulation 1989; 80: 1652–1659
- van der Heijden‐Spek J. J., Staessen J. A., Fagard R. H., Hoeks A. P., Boudier H. A., van Bortel L. M. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: A population study. Hypertension 2000; 35: 637–642
- Lehmann E. D., Hopkins K. D., Rawesh A. Relation between number of cardiovascular risk factors/events and noninvasive Doppler ultrasound assessments of aortic compliance. Hypertension 1998; 32: 565–569
- Millasseau S. C., Guigui F. G., Kelly R. P. Non‐invasive assessment of the digital volume pulse: Comparison with the peripheral pressure pulse. Hypertension 2000; 36: 952–956
- Millasseau S. C., Kelly R. P., Ritter J. M., Chowienczyk P. J. Determination of age‐related increases in large artery stiffness by digital pulse contour analysis. Clin Sci 2002; 103: 371–377
- Dillon J. B., Hertzman A. B. The form of the volume pulse in the finger pad in health, atherosclerosis, and hypertension. Am Heart J 1941; 21: 172–190
- Chowienczyk P. J., Kelly R. P., MacCallum H. Photoplethysmographic assessment of pulse wave reflection. J Am Coll Cardiol 1999; 34: 2007–2114
- Greenwald S. E. Pulse pressure and arterial elasticity. Q J Med 2002; 95: 107–112
- Haouzi A., Berglund H., Pelikan P. C. D. Heterogeneous aortic response to acute β‐adrenergic blockade in Marfan syndrome. Am Heart J 1997; 133: 60–63
- Stefanadis C., Wooley C. F., Bush C. A. Aortic distensibility abnormalities in coronary artery disease. Am J Cardiol 1987; 59: 1300–1304
- Hirai T., Sasayama S., Kawasaki T., Yagi S. Stiffness of systemic arteries in patients with myocardial infarction: A noninvasive method to predict severity of coronary atherosclerosis. Circulation 1989; 80: 78–86
- Wang M. C., Tseng C. C., Tsai W. C. Blood pressure and left ventricular hypertrophy in patients on different peritoneal dialysis regimens. Peritoneal Dialysis Int 2001; 21: 36–42
- Sahn D., DeMaria A., Kisslo J., Weyman A. The committee on M‐mode standardization of the American Society of Echocardiography, “Recommendations regarding quantization in M‐mode echocardiography:results of a survey of echocardiographic measurements”. Circulation 1978; 58: 1072–1083
- Devereux R. B., Alonso D. R., Lutas E. M. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458
- Tsai W. C., Tsai L. M., Teng J. K. Prognostic value of Doppler‐derived mitral deceleration time in postinfarction patients with left ventricular ejection fractions of 35% or more. J Formos Med Assoc 1999; 98: 70–72
- Tsioufis C. P., Lambrou S. G., Stefanadis C. I. Microalbuminemia is associated with abnormal thoracic aortic mechanics in essential hypertension. Am J Cardiol 2000; 86: 797–801
- Bouthier J. D., DeLuca N., Safar M. E., Simon A. Cardiac hypertrophy and arterial distensibility in essential hypertension. Am Heart J 1985; 109: 1345–1352
- Yin F. C. P., Ting C. T. Compliance changes in physiological and pathological states. J Hypertension 1992; 10(Suppl 6)S31–S33