Abstract
The wider application of the plasma aldosterone to renin activity ratio (ARR) test has led independent groups to report a 10‐fold or higher prevalence in the detection and prevalence of primary aldosteronism than previously suggested, although such figures have been contested. We determined the prevalence of a raised ARR in an unselected group of patients who were referred to the hypertension clinic at Aberdeen Royal Infirmary. Over a 4‐month period, all newly referred patients had an ARR, urea and electrolytes, and 24‐h ambulatory blood pressure monitoring (ABPM) performed in addition to a detailed clinical examination. One hundred and twenty‐two patients (mean age 51±16 years) were examined over the study period; 57 (47%) were receiving no anti‐hypertensive medication, 32(26% of total) had a normal 24‐h ABPM of which 15 patients were receiving antihypertensive medication (“controlled” hypertensives) and 17(14%) were receiving no anti‐hypertensive medication (“white‐coat hypertensives). Twenty patients (mean age 58±11 years) were found to have a raised ARR (>750), of which 10 patients were receiving beta‐blocker therapy as part of their anti‐hypertensive regimen. Patients with a raised ARR were more likely (odds ratio 3.6, 95% confidence interval 1.2–13.2, p<0.05) to be classified as a “non‐dipper” compared with those whose blood pressure fell at night. The proportion of newly referred hypertensive patients with a raised ARR is still significant and confirms that of previous studies The ratio appears to be significantly driven by a suppressed renin value and further investigation is required to clarify the status of those patients receiving anti‐hypertensive medications, particularly beta‐blockers.
Introduction
It has previously been thought that primary aldosteronism was a rare cause of secondary hypertension, accounting for less than 1% of the hypertensive population. The wider application of the plasma aldosterone to renin activity ratio (ARR) test has led independent groups to report 10‐fold or higher increases in the detection and prevalence of primary aldosteronism Citation[1], although such figures have been contested Citation[2]. The majority of patients in these series were normokalaemic and thus masqueraded as if they had so‐called “essential hypertension” and would have escaped detection if the diagnosis had not been specifically sought. A raised ARR has been reported to be 93% sensitive for detecting primary aldosteronism when judged against the traditional methods of identifying this condition (such as salt loading and the fludrocortisone suppression test) and predicts the blood pressure (BP) responsiveness to the aldosterone antagonist, spironolactone. A high ARR is also associated with an increased prevalence of target organ damage and therefore early detection may prevent such damage occurring Citation[3]. Given the multisystem target organ damage and increased cardiovascular risk associated with uncontrolled hypertension, a readily treatable cause such as hyperaldosteronism is an important diagnosis to make. A previous study of treated hypertensives in primary care suggested a prevalence of a raised ARR of 14.4%, with patients with a raised ARR being treated with significantly more anti‐hypertensive drugs but having poorer BP control Citation[4]. We wished to determine the prevalence of a raised ARR ratio among an unselected group of patients, newly referred from primary care to the Hypertension clinic at Aberdeen Royal Infirmary.
Methods
One hundred and twenty‐two newly referred patients to the hypertension clinic at Aberdeen Royal infirmary for assessment of hypertension had ambulant aldosterone (pmol/l) to renin (ng/ml/h) ratio (ARR) estimation and 24‐h ambulatory BP monitoring (ABPM) recording performed using the same type of ABPM monitor (Spacelabs Medical®, Redmond, Washington, USA). “Dipping” status was confirmed by a greater than 10% difference in systolic or diastolic BP between daytime (09.00–21.00 h) and night‐time (21.00–09.00 h) average BP measurements. This definition of “dipping” status was chosen as a previous study has indicated that individuals with a diminished nocturnal decline in BP have a greater risk of cardiovascular mortality compared with those with a nocturnal BP decline of at least 10% Citation[5]. The ambulant ARR was measured with the patient seated for 5 min after at least 2 h of upright posture. A raised ambulant ARR was defined as a ratio of aldosterone to renin of 750 or more Citation[4]. Antihypertensive medication use was recorded for all patients.
Statistical analysis
Descriptive statistics are presented. Student's t‐test was performed to identify differences between patients with a raised (>750) and normal (<750) ARR. Logistic regression was performed to estimate the odds for patients with a raised ARR to have a “non‐dipping” profile to their ABPM. Statistical significance was defined by a p‐value <0.05.
Results
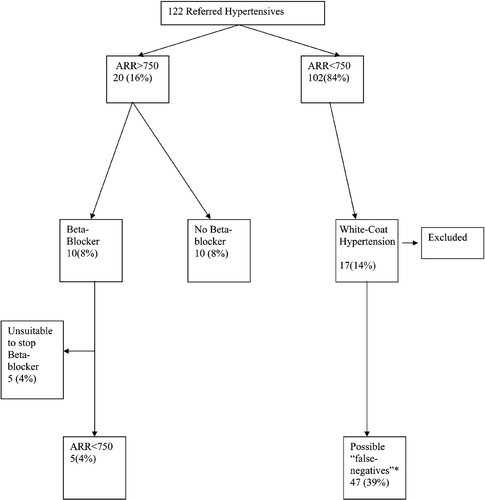
A total of 122 patients (mean age 51±16 years) were examined over a 4‐month period (June–September 2003). Fifty‐seven (47%) were receiving no anti‐hypertensive medication, 32 (26% of total) had a normal 24‐h ABPM of which 15 patients were receiving antihypertensive medication (“controlled” hypertensives) and 17(14%) were receiving no anti‐hypertensive medication (“white‐coat hypertensives”). Patients with a diagnosis of white‐coat hypertension were excluded from further analysis. Twenty patients (mean age 58±11 years) were found to have a raised ARR (>750) of whom 10 patients were receiving beta‐blocker therapy as part of their anti‐hypertensive regimen. Of the 10 patients receiving beta‐blocker therapy, it was deemed suitable to withdraw beta‐blocker therapy over at least a 2‐week period in five patients whose ARR was found to be normal on retesting (). Patients with a raised ARR were older, had higher 24‐h systolic and diastolic BPs, lower serum potassium and lower renin levels compared with those with a reduced ARR (). Such patients also received more antihypertensive medications compared with patients with an ARR <750. Patients with a raised ARR were more likely (odds ratio 3.6, 95% confidence interval 1.2–13.2, p<0.05) to be classified as a “non‐dipper” compared with those whose BP fell at night. Forty‐seven (55%) of the patients with a normal ARR were also receiving medications that could lead to a false‐negative ARR result. The prevalence of a raised ARR ranged from 12% in hypertensive patients who were receiving no medications to 16% for all referrals to the hypertension clinic to 19% if patients with white‐coat hypertension were excluded.
Table I. Characteristics of newly referred hypertensive patients.a
Discussion
Our results suggest a prevalence of a raised ARR of between 12% and 19% in patients referred to a specialist hypertension clinic, which are similar to those reported previously Citation[1],Citation[4],Citation[6]. It has previously been suggested that hypokalaemia was necessary to stimulate the investigation for hyperaldosteronism Citation[7,8]. However, whilst potassium levels tended to be lower in patients with a raised ARR, the majority of our patients (75%) had a normal serum potassium. Indeed, Conn, within a few years of his first description of primary hypertension, remarked that hypertension due to primary hyperaldosteronism may exist for several years before hypokalaemia becomes evident Citation[9]. Increased aldosterone levels within the physiological range have been shown to predispose patients to hypertension Citation[10]. A potential medical “cure” therefore exists for this group of patients who tend to have poorer BP control than other hypertensives, despite multiple drug therapy Citation[11]. Furthermore, aldosterone excess may induce cardiovascular injury through mechanisms that may be independent of its effects on BP Citation[12]. Successful medical treatment with spironolactone or amiloride can be achieved without side‐effects by using low doses over a number of weeks to demonstrate effect. The BP‐lowering efficacy of spironolactone in primary aldosteronism has been impressive in small uncontrolled studies, with BP falls of 40–60 mmHg systolic and 10–20 mmHg diastolic not uncommonly reported, and nearly 50% of patients with primary aldosteronism can be maintained on monotherapy Citation[13], allowing one to reduce the number of anti‐hypertensive medications that a patient is receiving with improvement in hypertensive control Citation[13].
Our results suggest that up to 26% of patients referred to the hypertension clinic presented with either white‐coat hypertension or controlled hypertension and therefore would require no further therapy. ABPM has developed over the past 40 years and is now widely used in the assessment of hypertensive patients Citation[14]. ABPM improves the precision and reproducibility of BP measurement, eliminates observer errors and bias, and allows assessment of white‐coat hypertension and white‐coat responses. Ambulatory BP levels are also a more sensitive predictor of target organ damage with numerous studies linking ambulatory BP to left ventricular hypertrophy, microalbuminuria, retinal hypertensive changes and cerebrovascular disease Citation[15]. The majority of people show a substantial fall in BP during the night (nocturnal dip). A mean difference between average day and night pressures of 10% is generally regarded as normal (the patient is a “dipper”). Hypertensive patients who do not dip at night are more likely to have target organ damage than dippers. In a subgroup of patients in the SYST‐EUR study who had ABPM, the strongest association between cardiovascular risk and BP was with the night‐time readings Citation[16]. Furthermore, patients with secondary hypertension have been shown to dip less at night Citation[17], a finding confirmed by our results, which suggest that patients with a raised ARR are less likely to have a fall in BP at night.
Our results also demonstrate the importance of performing ARR in patients naïve to drug therapy to avoid false‐positive and false‐negative results, although it is often not possible to withdraw all medication in a patient with severe hypertension who is taking several classes of anti‐hypertensive medications. Furthermore, as primary aldosteronism often presents as moderate to severe hypertension, patients tend to be screened when they are already receiving anti‐hypertensive therapy. In particular, beta‐blockers can decrease plasma renin levels to a greater extent compared with plasma aldosterone levels Citation[18,19], whereas diuretics, angiotensin‐converting enzyme (ACE) inhibitors, dihydropyridine calcium‐channel blockers and angiotensin II receptor blockers (ARBs) tend to reduce the ARR Citation[20–24]. We found that beta‐blockers in particular led to an increased ARR, primarily by reducing the renin level. However, we also accept that a number of normal ARRs could have been influenced by drug therapy leading to potential false‐negative results, although it has been argued that ACE inhibitors, diuretics, calcium‐channel blockers and ARBs may improve the discriminatory power of the ARR due to the autonomous nature of aldosterone excess Citation[25].
In conclusion, the prevalence of a raised ARR in patients referred to a specialist hypertension clinic is significant. We suggest that the ARR is best measured in patients naïve to antihypertensive therapy and suggest that this be part of the routine investigation of newly diagnosed hypertensive patients. Furthermore, ABPM should be performed in all newly referred hypertensive patients to prevent inappropriate antihypertensive therapy.
Acknowledgement
The authors are grateful to TENOVUS Scotland for the support they provided for this study.
References
- Lim P., Dow E., Brennan G., Jung R., MacDonald T. High prevalence of primary aldosteronism in the Tayside hypertension clinic population. J Human Hypertens 2000; 14: 311–315
- Kaplan N. Cautions over the current epidemic of primary aldosteronism. Lancet 2001; 357: 953–954
- Rayner B., Opie L., Davidson J. The aldosterone/renin ratio as a screening test for primary aldosteronism. South African Med J 2000; 90: 394–400
- Lim P., Rodgers P., Cardale K., Watson A., MacDonald T. Potentially high prevalence of primary adosteronism in a primary‐care population. Lancet 1999; 353: 40
- Ohkubo T., Hozawa A., Yamaguchi J., Kikuyi M., Ohmori K., Michimata M., et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: The Ohasama study. J Hypertens 2002; 20: 2183–2189
- Gordon R., Ziesk M., Tunny T., Stowasser M., Klemm S. Evidence that primary aldosteronism may not be uncommon: 12% incidence among antihypertensive drug trial volunteers. Clin Exp Pharmacol Physiol 1993; 20: 296–298
- Ganguly A. Primary aldosteronism. N Engl J Med 1998; 339: 1828–1834
- Kaplan N. Clinical hypertension. Williams and Wilkins, Baltimore 1994
- Conn J. Plasma renin activity in primary aldosteronism. Importance in differential diagnosis and in research of essential hypertension. JAMA 1964; 190: 222–225
- Vasan R. S., Evans J. C., Larson M. G., Wilson P. W. F., Meigs J. B., Rifai N., et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med 2004; 351: 33–41
- Lim P., Young W., MacDonald T. A review of the medical treatment of primary aldosteronism. J Hypertens 2000; 19: 353–361
- Weber K., Sun Y., Campbell S., Slight S., Ganham V., Griffing G., et al. Chronic mineralocorticoid excess and cardiovascular remodelling. Steroids 1995; 60: 125–132
- Lim P., Jung R., MacDonald T. Raised aldosterone to renin ratio predicts ant‐hypertensive efficacy of spironolactone. A prospective cohort follow‐up study. Br J Clin Pharmacol 1999; 48: 756–760
- Prasad N., Isles C. Fortnightly review: Ambulatory blood pressure monitoring: A guide for general practitioners. BMJ 1996; 313: 1535–1541
- Gibbs C., Murray S., Beevers D. The clinical value of ambulatory blood pressure monitoring. Heart 1998; 1998: 225–228
- Pickering T. Ambulatory blood pressure monitoring. Curr Hypertens Rep 2000; 2: 558–564
- Middeke M., Schrader J. Nocturnal blood pressure in normotensive subjects and those with white coat, primary, and secondary hypertension. BMJ 1994; 308: 630–632
- Buhler F., Laragh J., Baer L., Vaughan E., Brunner H. Propranolol inhibition of renin secretion: A specific approach to diagnosis and treatment of renin‐dependent hypertensive diseases. N Engl J Med 1972; 287: 1209–1214
- Gordon M., Williams G., Hollenberg N. Renal and adrenal responsiveness to angiotensin II: Influence of beta‐blockage. Endocr Res 1992; 18: 115–131
- Stowasser M., Gordon R., Rutherford J., Nikwan N., Daunt N., Slater G. Diagnosis and management of primary aldosteronism. J renin angiotensin aldosterone Syst 2001; 2: 156–169
- Young W. Primary aldosteronism: Update in diagnosis and treatment. The Endocrinologist 1997; 7: 213–221
- Gordon R. Primary aldosteronism. J Endocrinol Invest 1995; 18: 495–511
- Fiad T., Cunningham S., Hayes F., McKenna T. Effects of nifedipine treatment on the renin–angiotensin–aldosterone axis. J Clin Endocrinol Metab 1997; 82: 457–460
- Brown M., Hopper R. Calcium‐channel blockade can mask the diagnosis of Conn's syndrome. Postgrad Med J 1999; 82: 457–460
- Foo R., O'Shaughnessy K. M., Brown M. J. Hyperaldosteronism: Recent concepts, diagnosis, and management. Postgrad Med J 2001; 77: 639–644