Abstract
This study aimed to assess blood pressure (BP) profile, BP control, left ventricular hypertrophy (LVH) and albumin/creatinine ratio (ACR) in urine after 5 years of antihypertensive treatment in subjects with newly diagnosed essential hypertension. Fifty‐four subjects were included and prescribed calcium‐channel blocker in monotherapy during an 8‐week period, and later 46 subjects (34 men, 12 women, 53.1±8.6 years) attended a 5‐year follow‐up visit at the hypertension clinic. They underwent 24‐h ambulatory BP monitoring (ABPM), ECG and ACR at baseline and after 5 years. Echocardiography performed after 5 years revealed LVH in 54% of the subjects, while there was no change in Cornell product, an ECG criterion for LVH. BP control assessed by office BP was 33%, and only 20% using 24‐h ABP. Night‐time fall in BP was significantly attenuated from 13.2±5.9% to 10.7±6.0%, p = 0.01 for systolic BP and from 13.3±6.9% to 9.8±6.8%, p = 0.004 for diastolic BP. The number of dippers decreased after 5 years, but this did not reach statistical significance. In contrast to the lack of change in Cornell product, there was a significant decrease in ACR, and 93% of the subjects had ACR<1.5 mg/mmol after 5 years compared with 57% at baseline (p<0.001). Thus, ABPM should be encouraged in the follow‐up of all hypertensive subjects as it reveals better inadequate BP control than office BP and gives information about night‐time fall, as this may explain the high prevalence of LVH. The diversity in development of LVH and ACR during antihypertensive treatment needs to be verified.
Introduction
Hypertension (HT) is associated with development of organ damage and cardiovascular diseases (CVD) Citation[1], Citation[2]. Ambulatory blood pressure (ABP) has been found to be a better predictor of cardiovascular morbidity and mortality than office blood pressure (BP) in subjects with treated HT in the general population and in older patients Citation[3], Citation[4]. We have previously reported associations between ABP and left ventricular mass (LVM) and albumin excretion in urine in the early phases of uncomplicated essential HT and before antihypertensive treatment was initiated Citation[5]. Reduced night‐time fall in BP has been associated with CVD Citation[6], Citation[7]. Furthermore, smoothness index (SI), a measure of duration and homogeneity of BP reduction, has been associated with organ damage Citation[8], Citation[9]. However, there is a scarcity of data regarding progress and development of ABP profile, SI and possible relations to organ damage in newly diagnosed hypertensive subjects. An increase in albumin excretion rate in the urine, but still within the reference values, might be an early sign of hypertensive organ damage Citation[10], Citation[11]. It has recently been shown that reduction in albumin/creatinine ratio (ACR) in urine translates to reduction in CVD during antihypertensive treatment in patients with left ventricular hypertrophy (LVH) Citation[12].
Thus, the aim of the present study was to assess ABP profile and BP control, LVH and ACR after 5 years of antihypertensive treatment in subjects with essential HT.
Material and methods
Study population
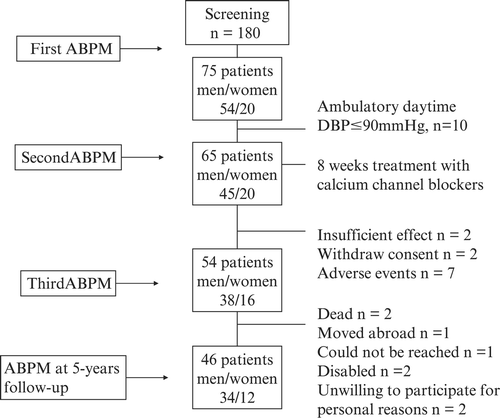
A detailed description of the study population has been given previously Citation[13]. The subjects (all >18 years) who entered the study were considered to be in need of antihypertensive therapy. The inclusion criteria were newly diagnosed, untreated HT with mean daytime diastolic (D) BP>90 mmHg on 24‐h ABP monitoring (M) after 4 weeks of observation without medication. To enter the study, the office DBP should be >95 mmHg as described previously Citation[13]; this was done to ensure that only patients necessitating pharmacological treatment should be included. The same argument was used when only patients with ambulatory daytime DBP>90 mmHg were included Citation[13].
Patients with diabetes mellitus, valvular heart disease, self‐reported myocardial infarction, serum creatinine ⩾130 μmol/l, positive urine dipstick test for albumin or glucose were excluded. Of 180 screened, 65 subjects were included and antihypertensive treatment with calcium‐channel blockers was started. They were followed for 8 weeks at the HT clinic; 24‐h ABPM and office BP was performed after 4 and 8 weeks of treatment Citation[14]. Thereafter they were followed by general practitioners. Five years later, the subjects were invited for a follow‐up visit at the HT clinic and underwent clinical examination, ECG, urinalysis, blood sampling, echocardiography, office BP and ABPM. Forty‐six subjects attended the follow‐up, i.e. 62% of those initially included in the study, see flowchart, Figure . Characteristics of the study population are given in Table . None of the participants had been admitted to hospital during the 5‐year period and they were all apparently healthy. At the time of the follow‐up, 14 (30%) of the subjects used statins and 40 of 46 subjects used antihypertensive drugs in either monotherapy (n = 20) or as part of a combination therapy with two or more drugs (n = 20), 25 subjects used calcium‐channel blockers, 19 used angiotensin‐converting enzyme inhibitors or angiotensin II receptor antagonists, and 11 diuretics, both loop and thiazide, while beta‐adrenergic or alpha‐adrenergic receptor blockers were prescribed in 11 and two of the subjects, respectively. All subjects received oral and written information and signed an informed consent. The National Committee for Medical Research Ethics in Norway approved the protocol.
Table I. Demographic characteristics, blood pressures (BP), night‐time fall in BP, white coat effect (WCE) and heart rate at baseline and at 5‐year follow‐up in hypertensive subjects (n = 46).
BP measurements
Office BP was measured after a 5‐min rest in a sitting position with arm supported at heart level and using an appropriate‐sized cuff. All measurements were performed between 07.00 and 09.00 h. The mean value of three consecutive BP measurements was used for all statistical analyses. The measurements were performed with a semi‐automatic oscillometric device Omron M4 (Omron Healthcare Europe, Hoofdorp, The Netherlands) validated using the protocols of the British Society of Hypertension and the Association for the Advancement of Medical Instrumentation Citation[15]. The 24‐h ABPMs were performed on a working day using the non‐dominant arm for measurements with the auscultatory device Tycos Quiet Trak [Welch Allyn/Tycos Instuments Inc., Skaneateles Falls, NY, USA Citation[16]]. BP was recorded at 20‐min intervals during the daytime period (07.00–22.00 h) and at 30‐min intervals during the night (22.00–07.00 h). During all measurements, the patients were instructed to keep their arm still to ensure good quality recordings. The same protocol for ABPM was used both at the initial study and after 5 years. Pulse pressure (PP) was calculated as the difference between systolic BP (SBP) and DBP.
The night‐time fall in BP was calculated as the percentage decline in mean SBP and DBP at night compared with daytime mean values. A cut‐off point of 10% was chosen for both SBP and DBP and used for defining dipping and non‐dipping pattern. Fixed time limits were used for night‐time BP, since the results should be compared with the measurements at baseline when sleeping hours were not registered. During the ABPM at 5‐year follow‐up, awake and sleep periods were noted and there was a strong agreement between fixed night‐time and individual sleep time BP measurements with r = 0.99, p<0.001 for both SBP and DBP.
White‐coat effect (WCE), indicating an additional elevation of BP in the HT clinic leading to a possible overestimation of the real BP, was calculated in two different ways: first by subtracting daytime SBP and DBP from the initial hour of measurements, when the subject was still at the HT clinic, thus comparing values using the same BP device (WCEamb). The most widely used calculation of WCE, namely the difference between office BP and daytime ABP, was also done (WCEconv). According to the European Society of Hypertension guidelines, values were considered hypertensive if mean 24‐h ABP was ⩾125/80 mmHg, daytime ABP⩾130/85 mmHg, night‐time ABP⩾120/70 mmHg or office BP⩾140/90 mmHg Citation[17].
The SI, a measure of the homogeneity of the BP reduction, was derived from the 24‐h BP measurements done after 8 weeks Citation[14] and 5 years of treatment. The SI was obtained by first calculating the average BP values for each hour of the monitoring period; these BP values were subtracted and the average of all hourly BP values (ΔH) were computed together with its SD, using the formula ΔH/SDΔH, as described by Parati et al. Citation[8]. SI should be higher with increasing efficacy of drug‐induced BP lowering; thus, optimum BP control is provided by drugs with a SI close to 1 Citation[8]. Separate calculations were made for SBP and DBP.
Urinary albumin excretion
Urinary albumin excretion was determined using albumin/creatinine ratio (ACR) in the first voided morning urine sample before and after the 24‐h ABPM, and the mean value of the two measurements was used for statistical analyses. Patients were categorized as having low normoalbuminuria (ACR<1.5 mg/mmol), high normoalbuminuria (1.5⩽ACR<3.0 mg/mmol) or microalbuminuria (ACR⩾3.0 mg/mmol). Albumin in the urine was measured turbidimetrically on a Hitacchi 912 autoanalyser, using Tinaquant reagents from Roche, Basel, Switzerland, and creatinine in urine was measured with an enzymatic method on a routine clinical chemistry analyser (Cobas Integra, Roche Basel, Switzerland).
Echocardiography
Echocardiography was done by one experienced investigator blinded for patient information using a GE‐Vivid 7 echocardiograph (GE VingMed, Horten, Norway) with 1.7‐MHz probe in second harmonic mode. End‐diastolic LV dimensions were used to calculate LVM by an anatomically validated formula (r = 0.9 vs necropsy LVM) Citation[18]. LVH was defined as LVM>215 g Citation[18] and LVM index (LVMI) >134 g/m2 in men or >110 g/m2 in women Citation[19]. Relative wall thickness (RWT), a measure of concentric remodelling, was calculated as 2×posterior wall thickness in diastole (PWTd)/LV internal diameter in diastole (LVIDd). Increased RWT was present when this ratio was >0.43 Citation[20]. Standard 12‐lead ECG was recorded at 50 mm/s. For each individual, the sex‐specified Cornell voltage – QRS duration product Citation[21] was calculated and LVH was defined as Cornell product >2440 mm⋅ms.
Statistical analysis
All values are expressed as mean±SD or percentages, if data were skewed as medians and 25% and 75% percentiles (Q1:Q3), and differences as 95% confidence intervals (CI). Within‐group and between‐group comparisons were done using Student's t‐test or Wilcoxon's test if data was not normally distributed. For comparison of proportions of subjects within different categories, Mc Nemar's test was applied. Simple relationships between variables were examined using Pearson (for normally distributed data) or Spearman's correlation coefficient. Data analyses were performed with SPSS statistical package version 12.0 (SPSS Inc., Illinois, USA). Two‐tailed p<0.05 was considered statistically significant.
Results
BPs
Data on BP are given in Table .
BP control assessed by office BP was 33% and 20% using 24‐h ABP. Compared with ABPM, office BP measurement overestimated the proportion of subjects with SBP control (56 vs 26%, p = 0.002), while no difference was observed for DBP (33 vs 20%, ns). Normal night‐time ABP was found in 9% of the subjects.
Overall, mean night‐time fall in BP decreased with time (Table ). Although the proportion of dippers was numerically reduced during the 5‐year period, this did not reach statistical significance (SBP 69% vs 56%, ns; DBP 67% vs 47%, ns). SI for SBP was significantly lower after 5 years of antihypertensive treatment compared with the initial 8 weeks of treatment (0.9±0.8 vs 0.5±0.9, p = 0.02), no change was observed for DBP (0.9±0.7 vs 0.8±0.9, ns).
WCEconv (using office BP compared with daytime ABP) decreased significantly for SBP during the 5‐year follow‐up (Table ). No change occurred regarding WCEconv for DBP (35% vs 35%, ns), and no WCEamb was observed either at baseline or at the 5‐year follow‐up (Table ).
Albumin/creatinine ratio
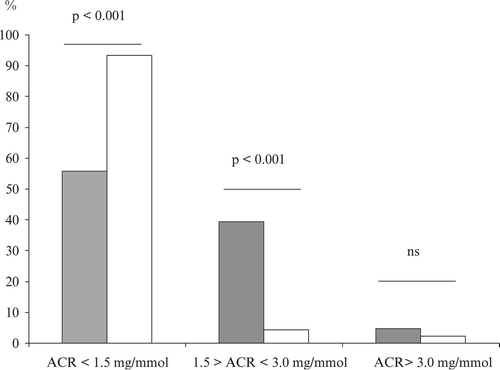
ACR decreased significantly with Δ1.1 mg/mmol (95% CI 0.8–1.3, p<0.001) during the 5‐year period, and a reduction was found after 8 weeks of treatment Δ0.3 mg/mmol (95% CI 0.1–0.5, p = 0.004). The distribution of patients between the three defined ACR categories changed during the period as can be seen from Figure with a majority entering the low normoalbuminuric category (<1.5 mg/mmol) after 5 years.
LVM and BP
Echocardiography was only performed at the 5‐year follow‐up. LVH was observed in 25 (54%) and RWT>0.43 in 21 (46%) of the subjects. Associations were observed between RWT and the change in 24‐h SBP (r = 0.37, p = 0.015) and 24‐h DBP (r = 0.40, p = 0.008). Echocardiographic‐assessed LVM was significantly related to change in ABP during the 5‐year period (Δ24‐h DBP, r = −0.34, p = 0.020, Δnight‐time DBP, r = −0.33, p = 0.030) and also to night‐time fall (%) in SBP after 5 years (r = 0.32, p = 0.035).
LVH based on Cornell voltage QRS duration product, was diagnosed in three patients at baseline and seven after 5 years. Overall, no significant change in Cornell voltage QRS duration product was observed during the follow‐up period (1513.4±581.5 vs 1559.8±682.0 mm⋅ms, ns). However, SI assessed at the 5‐year follow‐up and changes in ABP during the 5‐year period were related to change in Cornell voltage QRS duration product during the 5 years (Table ).
Table II. Associations between (a) left ventricular mass (LVM) assessed by Cornell voltage QRS duration product and blood pressure (BP), and (b) the change in LVM and the change in BP during 5‐year follow‐up, (n = 46).
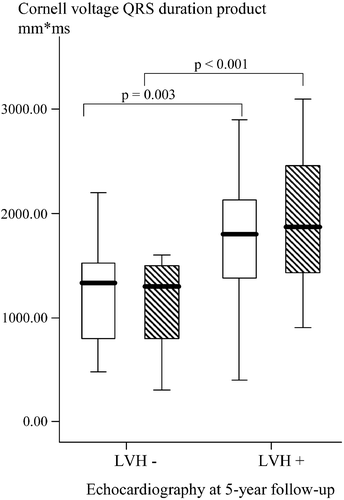
Echocardiographic assessed LVM and Cornell voltage QRS duration product was significantly correlated (r = 0.70, p<0.001), and this relationship persisted when correcting for BSA (r = 0.52, p<0.001). Furthermore, those with echocardiographic verified LVH after 5 years had significantly higher left ventricular size assessed by Cornell voltage QRS duration product at baseline (1748.8±584.7 vs 1244.4±456.5 mm⋅ms, p = 0.003) and after 5 years (1901.6±650.6 vs 1130.9±432.3 mm⋅ms, p<0.001) compared with those without LVH (Figure ).
Figure 3 Boxplots of left ventricular mass(LVM) assessed by Cornell voltage QRS duration product in hypertensive subjects with and without left ventricular hypertrophy (LVH) assessed by echocardiography at 5‐year follow‐up. Data are given both at baseline (open boxes) and at 5‐year follow‐up (hatched boxes).
Discussion
The most unexpected result of the study was the high prevalence of LVH based on echocardiography as it was observed in 54% of the subjects. This was much higher than the 20–30% reported by others Citation[22], Citation[23]. In a 20‐year follow‐up study of hypertensive men, LVH was found in 45% of the subjects Citation[24]. Our patients had mild to moderate HT of 5–6 years' duration, and were without signs of hypertensive complications at the time of diagnosis. They had been considered to be in need of antihypertensive treatment based on clearly elevated diastolic ABP>90 mmHg, and this could explain part of the discrepancy in LVH prevalence between our study and others'.
Furthermore, the cut‐off value for detecting echocardiographic LVH varies in the literature. This has been discussed by Abergel et al. Citation[25], who found that the prevalence of LVH varied from 17% to 35% depending on the partition value applied. The high prevalence of LVH among our patients occurred despite use of a high partition value of 110 g/m2 and 134 g/m2 for women and men, respectively Citation[19], Citation[22]; lower cut‐off values have been applied by others Citation[23], Citation[24]. Although ECG has poor sensitivity Citation[26] and underestimates the occurrence of LVH compared with echocardiography Citation[27], the easy accessibility has led to widespread use in the work‐up of patients with essential HT in general practice. Even in the early phases of HT, an association between BP and Cornell voltage QRS duration product has been shown, indicating early hypertensive organ damage Citation[5]. A clear relationship between ECG‐based LVM and BP was also observed in the large‐scale LIFE (Losartan Intervention For Endpoint reduction in hypertension) study, which included established hypertensive subjects with LVH Citation[21].
Poor BP control using office BP was present in 67% of the subjects. This disappointing result is in accordance with other studies from Norway including a high number of hypertensive subjects in general practice Citation[28], Citation[29]. When using ABP as a measure of BP control, only one‐fifth of the patients had satisfactory BP control in our study. There is scarcity of data using ABP to assess BP control over a prolonged time period. In the PAMELA (Pressioni Arteriose Monitorate E Loro Associazioni) study, 79% of the subjects were either poorly controlled on treatment or were untreated with elevated 24‐h ABP ⩾125/80 mmHg Citation[23]. We have previously observed that office BP overestimates BP control compared with ABP in hypertensive renal transplant patients Citation[30], and this finding has now been extended to subjects with essential HT of limited duration. The importance of accurate BP measurements in the diagnosis and follow‐up of hypertensive subjects is crucial Citation[15], Citation[17].
There was an attenuation of the night‐time fall in both SBP and DBP during the 5‐year period, and only 9% had ABP<120/70 mmHg during night‐time. A significant relationship was observed between echocardiographic LVM and the night‐time fall in SBP. Thus, together with the elevated BP, these observations could in part explain the high proportion of subjects with LVH. Also the SI developed in a less favourable way during the 5‐year period indicating a non‐homogeneous BP reduction.
The reduction in urinary albumin excretion rate in our study occurred within the normoalbuminuric range. Even small changes in ACR in the microalbuminuric range translated to changes in risk of cardiovascular morbidity and mortality in the LIFE study Citation[12]. The reason for the divergent results regarding cardiac and renal influence of HT 5 years after the diagnosis is not apparent to us. One could speculate that a more pronounced BP reduction is necessary to influence LVM than ACR. Furthermore, LVH is also determined by non‐haemodynamic factors such as genetic and neurohumoral influence. Although we did not perform echocardiography at baseline, one cannot exclude that left ventricular geometry might already have been altered. This is supported by the difference in left ventricular size assessed by ECG between those who had echocardiographic LVH or not 5 years later. However, the results should be interpreted with caution, as ECG has low sensitivity in detecting LVH, and thus may underestimate the true prevalence of LVH. Furthermore, ACR exhibits day‐to‐day variations although great care was taken in determination of the ACR regarding standardization and multiple sampling.
There are several limitations in the study that should be recognized. There is a problem with the small sample size, albeit from a large group of screenees. On the other hand, the study population is well characterized, especially regarding duration of HT as they prior to the study had normal BP during yearly follow‐up. The hypertensive subjects were selected based on short duration of HT (<1 year), and they were considered to be in need of antihypertensive treatment based on ABP. They should not have signs of hypertensive organ damage or secondary HT. Furthermore, the absence of LVH was based on ECG which has low sensitivity compared with echocardiography.
In the current study, LVH verified by echocardiography was present in a substantial portion of the subjects, most likely due to poor BP control. Office BP overestimated BP control compared with 24‐h ABP and failed to give information about night‐time BP and the homogeneity of diurnal BP reduction. The divergent results on urinary albumin excretion and LVM after 5 years of BP treatment are not clear to us, but could suggest that more pronounced BP reduction is needed for regression of cardiac damage.
Acknowledgement
This study has been supported by a grant from the Norwegian Society of Nephrology, and by grants from the Research Forum at Ullevål University Hospital.
References
- Koren M. J., Devereux R. B., Casale P. N., Savage D. D., Laragh J. H. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114: 345–352
- Schillaci G., Verdecchia P., Porcellati C., Cuccurullo O., Cosco C., Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35: 580–586
- Clement D. L., De Buyzere M. L., De Bacquer D. A., de Leeuw P. W., Duprez D. A., Fagard R. H., et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med 2003; 348: 2407–2415
- Staessen J. A., Thijs L., Fagard R., O'Brien E. T., Clement D., de Leeuw P. W., et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999; 282: 539–546
- Bulatov V. A., Stenehjem A., Os I. Left ventricular mass assessed by electrocardiography and albumin excretion rate as a continuum in untreated essential hypertension. J Hypertens 2001; 19: 1473–1478
- Kario K., Matsuo T., Kobayashi H., Imiya M., Matsuo M., Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension 1996; 27: 130–135
- Verdecchia P., Porcellati C., Schillaci G., Borgioni C., Ciucci A., Battistelli M., et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994; 24: 793–801
- Parati G., Omboni S., Rizzoni D., Gabiti‐Rosei E., Mancia G. The smoothness index: A new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens 1998; 16: 1685–1691
- Rizzoni D., Muiesan M. L., Salvetti M., Castellano M., Bettoni G., Monteduro C., et al. The smoothness index, but not the trough‐to‐peak ratio predicts changes in carotid artery wall thickness during antihypertensive treatment. J Hypertens 2001; 19: 703–711
- Boulatov V. A., Stenehjem A., Os I. Association between albumin:creatinine ratio and 24‐hour ambulatory blood pressure in essential hypertension. Am J Hypertens 2001; 14: 338–344
- Romundstad S., Holmen J., Hallan H., Kvenild K., Kruger O., Midthjell K. Microalbuminuria, cardiovascular disease and risk factors in a nondiabetic/nonhypertensive population. The Nord‐Trondelag Health Study (HUNT, 1995–97), Norway. J Intern Med 2002; 252: 164–172
- Ibsen H., Olsen M. H., Wachtell K., Borch‐Johnsen K., Lindholm L. H., Mogensen C. E., et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45: 198–202
- Stenehjem A. E., Os I. Reproducibility of blood pressure variability, white‐coat effect and dipping pattern in untreated, uncomplicated and newly diagnosed essential hypertension. Blood Press 2004; 13: 214–224
- Stenehjem A. E., Os I. Clinical utility and applicability of smoothness index, normalized smoothness index and individualized RDH index during treatment of essential hypertension. Blood Press 2006; 15: 281–290
- O'Brien E., Waeber B., Parati G., Staessen J., Myers M. G. Blood pressure measuring devices: Recommendations of the European Society of Hypertension. BMJ 2001; 322: 531–536
- Modesti P. A., Costoli A., Cecioni I, I., Toccafondi S., Carnemolla A., Serneri G. G. Clinical evaluation of the QuietTrak blood pressure recorder according to the protocol of the British Hypertension Society. Blood Press Monit 1996; 1: 63–68
- European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053
- Devereux R. B., Alonso D. R., Lutas E. M., Gottlieb G. J., Campo E., Sachs I., et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458
- Devereux R. B., Lutas E. M., Casale P. N., Kligfield P., Eisenberg R. R., Hammond I. W., et al. Standardization of M‐mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol 1984; 4: 1222–1230
- Ganau A., Devereux R. B., Roman M. J., de S. G., Pickering T. G., Saba P. S., et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 1992; 19: 1550–1558
- Wachtell K., Olsen M. H., Dahlof B., Devereux R. B., Kjeldsen S. E., Nieminen M. S., et al. Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE study. J Hypertens 2002; 20: 405–412
- Koren M. J., Ulin R. J., Koren A. T., Laragh J. H., Devereux R. B. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens 2002; 15: 1021–1028
- Mancia G., Carugo S., Grassi G., Lanzarotti A., Schiavina R., Cesana G., et al. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control: Data from the PAMELA population. Pressioni Arteriose Monitorate E Loro Associazioni. Hypertension 2002; 39: 744–749
- Strand A. H., Gudmundsdottir H., Os I., Smith G., Westheim A. S., Bjornerheim R., et al. Arterial plasma noradrenaline predicts left ventricular mass independently of blood pressure and body build in men who develop hypertension over 20 years. J Hypertens 2006; 24: 905–913
- Abergel E., Tase M., Bohlender J., Menard J., Chatellier G. Which definition for echocardiographic left ventricular hypertrophy?. Am J Cardiol 1995; 75: 498–502
- Molloy T. J., Okin P. M., Devereux R. B., Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage‐duration product. J Am Coll Cardiol 1992; 20: 1180–1186
- Levy D., Labib S. B., Anderson K. M., Christiansen J. C., Kannel W. B., Castelli W. P. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81: 815–820
- Tonstad S., Furu K., Rosvold E. O., Skurtveit S. Determinants of control of high blood pressure. The Oslo Health Study 2000–2001. Blood Press 2004; 13: 343–349
- Westheim A., Klemetsrud T., Tretli S., Stokke H. P., Olsen H. Blood pressure levels in treated hypertensive patients in general practice in Norway. Blood Press 2001; 10: 37–42
- Stenehjem A. E., Gudmundsdottir H., Os I. Office blood pressure measurements overestimate blood pressure control in renal transplant patients. Blood Press Moni 2006; 11: 125–133