Abstract
Background. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (BP) provides guideline for the new category of BP levels as normal, prehypertension (PHT), and hypertension. Although PHT is associated with a markedly increased risk of developing hypertension within 4 years, its prognostic significance and predisposition to target‐organ damage is unknown. Accordingly, we evaluated the effects of normal BP, PHT and hypertension on left ventricular (LV) diastolic function and aortic elasticity, which are sensitive indicators of target‐organ damage. Methods. We evaluated LV diastolic function and aortic elastic properties of 60 subjects with PHT, 70 patients with hypertension and 50 normotensive healthy volunteers using transthoracic echocardiography. None of the subjects had any systemic disease. Results. LV diastolic function was more significantly impaired in the hypertension group than in the PHT group compared with controls, but it was not strongly different between the PHT and control group. Aortic distensibility was significantly lower, and aortic stiffness index was significantly higher in both the hypertension and the PHT group than those in the control group. However, aortic elastic properties did not significantly differ between the PHT and hypertension groups. Furthermore, we found that the presence of the PHT was significant predictor of impaired aortic elasticity in a multivariable model that adjusted for other variables. Conclusions. Aortic elastic properties are significantly and LV diastolic function is slightly impaired in subjects with PHT, and impairment of aortic elasticity is as severe as that in hypertension.
Introduction
Epidemiological studies have shown that the incidence of atherosclerotic cardiovascular disease is strongly related to the elevation of blood pressure (BP) Citation[1], Citation[2]. Prehypertension (PHT) is defined as systolic BP between 120 and 139 mmHg or diastolic BP between 80 and 89 mmHg based on two or more properly measured seated BP readings on each of two or more office visits Citation[3]. Approximately 30% of the adult population falls into this category Citation[4]. Compared with normal BP, PHT is associated with an increase in cardiovascular (CV) morbidity and mortality Citation[5], Citation[6]. The mechanism of excess risk from PHT is presumed to be the same as that from hypertension. It has also been shown that PHT is associated with subclinical atherosclerosis, including increased coronary atherosclerosis Citation[7]; however, there is no prior data regarding the impact of PHT on left ventricular (LV) diastolic function and aortic elastic properties which are surrogate markers of hypertensive target‐organ damage.
Atherosclerosis and related diseases are the most common form of target‐organ damage and the most common cause of mortality accompanied by hypertension. The aorta has an elastic structure, and it is generally affected by atherosclerosis. An increase in aortic stiffness index (AoSI) and/or decrease in aortic distensibility (AoD) may reflect the widespread nature of the atherosclerotic process Citation[8–10]. Since atherosclerosis may affect the aorta and coronary arteries simultaneously, aortic stiffness and distensibility may predict cardiovascular events Citation[8–10]. Thus, an increase in AoSI or a decrease in AoD may be an early predictor for coronary atherosclerosis, and show end organ damage in hypertensive individuals.
Although substantial evidence supports the contention that PHT associated with increased cardiovascular risk; to date, there is no study investigating LV diastolic function and aortic elastic properties in these patients. In this study, we studied these parameters in normotensive subjects, in subjects with PHT, and in newly diagnosed and never treated patients with established hypertension without excessive LVH.
Methods
Study population
The overall study population was consisted of 180 subjects: 60 subjects with PHT (group I), 70 patients with hypertension (group II) and 50 healthy volunteers with normal BP (group III). Their demographic and clinical data are shown in Table . Inclusion criterion was to be 18–55 years of age. Exclusion criteria were to have any systemic disease such as hemolytic, hepatic and renal diseases, or any disease that could cause LV diastolic function and aortic elastic properties impairment (e.g. diabetes mellitus: fasting plasma glucose level measured on three separate days in a week >126 mg/dl [7.0 mmol/l] or impaired oral glucose tolerance test: fasting plasma glucose <126 mg/dl [7.0 mmol/l] but 2‐h plasma glucose after a 75‐g oral glucose challenge >140 mg/dl [7.8 mmol/l], and excessive alcohol consumption [>50 g/day]). Normotensive healthy controls had no history of treatment with antihypertensive drugs. Subjects with PHT and patients with established hypertension have not previously taken any antihypertensive therapy. Subjects using any vasoactive drug, and those who have ST segment or T wave changes specific for myocardial ischemia, Q waves and incidental left bundle branch block on ECG were excluded from the study. In addition, the patients with LV mass index (LVMI) >126 g/m in men and >99 g/m in women Citation[11] were excluded from the study to avoid the confounding effects of LVH. Written informed consent was obtained from each subject, and the institutional ethics committee approved the study protocol.
Table I. Characteristics of subjects with PHT, hypertension and normal blood pressure.
BP measurement
According to American Heart Association guidelines, BP was measured using a mercury sphygmomanometer in an office setting; first and fifth phases of Korotkoff sounds were used for systolic and diastolic BP. Appropriate cuff sizes were chosen for each subject's arm circumference. BP was measured three times by skilled, trained physicians after 15 min of rest in the sitting position, and the average of the measurements was recorded. Physical examination included measurement of height (centimeters) and weight (kilograms), and a resting 12‐lead ECG was recorded.
Diagnosis of PHT, hypertension, and normotension
In each subject, BP was measured in at least three separate days after 15 min of comfortably sitting and averaged. Based on the JNC‐VII report Citation[3], PHT was defined as systolic BP between 120 and 139 mmHg and/or diastolic BP between 80 and 89 mmHg. Individuals who had systolic BP⩾140 mmHg and/or a diastolic BP⩾90 mmHg were diagnosed as hypertensive. Accordingly, control subjects who had systolic BP<120 mmHg and a diastolic BP<80 mmHg were diagnosed as normotensive controls.
Echocardiographic examination
Each subject was examined using an Acuson Sequoia C256® Echocardiography System equipped with a 3V2c broadband transducer with second harmonic capability (Acuson, Mountain View, CA, USA). Two‐dimensional, M‐mode, and subsequent standard and pulsed tissue Doppler echocardiographic examinations were performed on each subject in the lateral decubitus position. The echocardiographic images were recorded on VHS videotapes. Diastolic and systolic interventricular septal (IVS) thickness, posterior wall (PW) thickness, and LV end‐diastolic (LVDD) and LV end‐systolic (LVSD) diameters were measured on the parasternal long‐axis views. All measurements were performed on M‐mode images.
The pulsed Doppler sample volume was positioned at the mitral leaflet tips. Early diastolic peak flow velocity (E), late diastolic peak flow velocity (A) and E/A ratio, and E‐wave deceleration time (DT) were measured by transmitral Doppler imaging.
The Doppler tissue‐imaging (DTI) program was set to the pulsed‐wave Doppler mode. Filters were set to exclude high‐frequency signals, and the Nyquist limit was adjusted to a velocity range of –15 to 20 cm/s. Gains were minimized to allow for a clear tissue signal with minimal background noise. All DTI recordings were obtained during normal respiration. A 5‐mm sample volume was placed at the apical four‐chamber view on the lateral corner of the mitral annulus and subsequently on the medial (or septal) corner Citation[12]. The resulting velocities were recorded for 5–10 cardiac cycles at a sweep speed of 100 mm/s, and stored on VHS videotape for later playback and analysis. The following measurements were determined in each region as indexes of regional systolic function: peak velocities (cm/s), time velocity integral of myocardial systolic (Sm) wave. Myocardial early (Em) and atrial (Am) peak velocities (cm/s) and Em/Am ratio, and Sm–Em duration (isovolumic relaxation time: IVRT) were measured, as the time interval occurring between the end of Sm and the onset of Em, were determined as diastolic measurements. All diastolic parameters were measured in three consecutive cardiac cycles and averaged. The same investigator blinded for clinical data performed the echocardiography, and two cardiologists blinded for subjects' data analyzed the echocardiogram recordings.
LV mass determination
LV mass (LVM) was calculated from M‐mode records taken on parasternal long‐axis images according to the formula below (corrected American Society of Echocardiography cube method) Citation[11], Citation[13]. To take into account differences in body size that might influence cardiac size, LV mass was divided by height to create an LVMI: where IVSd is the interventricular septum thickness at diastole, PWd is the posterior wall thickness at diastole, and LVDD is the LV diastolic diameter.
AoD and stiffness calculations
Patients were examined in the left lateral position. A single operator measured internal dimensions of the ascending aorta in at least three consecutive cardiac cycles using an Acuson Sequoia C256® Echocardiography System (Acuson) equipped with a broadband transducer with second harmonic capability (3V2c). The measurements were carried out in the proximal ascending aorta, 3 cm from the origin of the aorta (Figure ). Aortic strain, AoD and AoSI were calculated using the following formulas Citation[12]: where Ds = aortic diameter at systole; Dd = aortic diameter at diastole; Ln = natural logarithm.
Figure 1 Measurements of systolic(S) and diastolic (D) aortic diameters are shown on the M‐mode tracing obtained at a level 3 cm above the aortic cusps. RV, right ventricle; LV, left ventricle; LA, left atrium; Ao, aorta; AoV, aortic valve; S, Systol; D, Diastol.
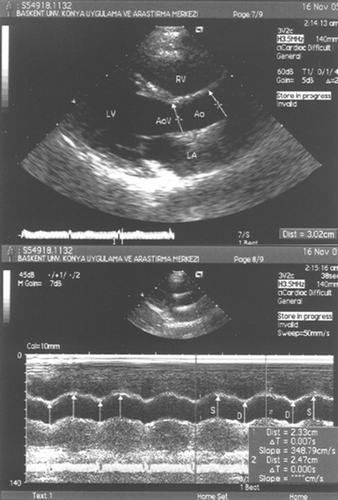
All systolic and diastolic diameter measurements were done at the same time or immediately after the BP measurement. Intraobserver intraclass correlation coefficient for aortic diameter measurement was 0.921.
Statistical analyses
The analyses were performed using SPSS 9.0 (SPSS for Windows 9.0, Chicago, IL). Data are expressed as mean±SD. The groups were compared using chi‐squared test regarding categorical variables. One‐way analysis of variance (ANOVA) followed by Tukey's test or the Kruskal–Wallis test [comparison of a characteristic across the three study groups if that characteristic did not have a normal distribution such as high‐sensitivity C‐reactive protein (hsCRP) and triglyceride] was used to compare continuous variables. Pearson's correlation test was used to test the associations between aortic elastic properties and the diastolic parameters. Multivariable analysis was used to assess associations of aortic elasticity with potential confounders via multivariate linear regression model. A p‐value less than 0.05 was considered significant.
Results
Clinical characteristics of the study population
The general characteristics and risk factors for CAD of the study population are presented in Table . Age, gender, heart rate, lipid profiles except high‐density lipoprotein (HDL)‐cholesterol and fasting glucose levels were similar within the three groups. Body mass index (BMI) was significantly greater in PHT group than that in control group; in addition, the percentage of obesity (BMI⩾30 kg/m2) was significantly higher in the PHT group compared with the other two study groups. Systolic and diastolic BP were significantly higher in hypertension group than those were in both PHT and control group; in addition, BP measurements were significantly higher in subjects with PHT compared with the controls. In the PHT group, HDL‐cholesterol was slightly lower than that in the control group and significantly lower than that in the hypertension group. Serum hsCRP level did not significantly differ between PHT and control groups, and between PHT and hypertension groups; however, it was significantly higher in hypertension group than in control group.
Analyses of the echocardiographic measurements
IVS and PW thickness, LVDD, LVSD, LV ejection fraction (EF), left atrium diameter (LAD) and LVMI were similar between the PHT and the hypertension, and the PHT and the control groups. However, IVS and PW thickness, LAD and LVMI were significantly greater in the hypertension group compared with control group (Table ).
Table II. Echocardiographic findings, and standard and tissue Doppler parameters of the left ventricle.
Standard and tissue Doppler echocardiographic analyses
Mitral E/A ratio was significantly lower in the hypertension group compared with control group. Mitral E‐wave DT was significantly greater than both PHT and control group. Standard Doppler parameters did not significantly differ between the PHT and control groups. The hypertensives had a significantly lower septal Em/Am ratio, lateral Em and lateral Em/Am ratio, and significantly higher septal IVRT and lateral Am than the controls. The PHT group had significantly higher IVRT than the control group. The other diastolic function parameters were slightly different between the PHT and control groups, but these differences did not reach statistically significance. In addition, the PHT and the hypertension groups were comparable with regard to most LV diastolic parameters obtained by standard Doppler and DTI. Only mitral E‐wave DT lateral Am and lateral Em/Am ratio significantly differed between the two hypertension groups (Table ).
Analysis of aortic parameters
Systolic aortic diameter was similar in the three groups, though diastolic aortic diameter was significantly lower in the control group than in the two hypertension groups. Aortic strain was significantly lower in the two hypertension groups than that in the control group. AoD was significantly lower and AoSI significantly higher in the both PHT and hypertension group than those in the control group; however, these parameters did not significantly differ between the PHT and hypertension groups (Table ). Furthermore, in multivariable analysis, AoD and stiffness index were separately taken as dependent, the classification status of the subjects (PHT, hypertension and control), age, BMI, lipids and other confounders including LVMI, diastolic function parameters as well as hsCRP were taken as independent, and we found that the presence of the PHT was significant predictor of impaired aortic elasticity (standardized β = −0.28, p<0.01 and β = 0.22, p<0.01; AoD and stiffnes index, respectively).
Table III Aortic elastic properties in the groups.
Reproducibility of the echocardiographic measurements
The intraobserver regression coefficient was >0.87 and interobserver regression coefficient was >0.88 for all measurements.
Relationship between aortic parameters and LV diastolic function parameters
Parameters of aortic elasticity were well correlated with LAD, mitral E/A ratio, mitral E‐wave DT, septal Em and Em/Am ratio, and lateral Em and Em/Am ratio (Table ).
Table IV. Correlations between aortic elastic properties and left ventricular diastolic function parameters.
Discussion
Although substantial evidence supports that hypertension impairs LV diastolic function and aortic elastic properties, to date, there has been no study investigating the effects of PHT on LV diastolic function and aortic elasticity in subjects without any clinical evidence of coronary artery disease. Accordingly, in the present study, we aimed to evaluate LV diastolic function and aortic elasticity in subjects with PHT, in patients with established hypertension, and in normotensive healthy controls using TTDE. We have found that aortic elasticity was significantly impaired in patients with PHT compared with normotensive subjects; furthermore, in subjects with PHT, impairment of aortic elasticity was as severely as that was in hypertension. However, LV diastolic function was not impaired as severely as that was in hypertension.
Impaired LV diastolic function is now recognized as an exceedingly common finding in hypertensive patients. It is one of the major causes of cardiac failure, ranging upwards of 74% Citation[14]. Although invasive methods are still the gold standard, the introduction of echocardiography and Doppler flow technology for the evaluation of hypertensive patients has significantly modified the means for non‐invasively assessing diastolic function Citation[15]. Transmitral flow was first used to evaluate LV diastolic function Citation[16]. Unfortunately, however, mitral flow is influenced not only by LV diastolic properties but also preload, afterload, heart rate, atrioventricular delay, pericardial restraint and ventricular interaction Citation[15]. Having evolved more recently, Doppler tissue imaging permits assessment of velocity of wall movement using a pulsed Doppler technique. This technique appears to be more sensitive than the mitral flow in detecting LV diastolic dysfunction in hypertensive patients with or without LV hypertrophy Citation[17], Citation[18].
Previous studies have shown that aortic compliance decreases in hypertensive individuals Citation[19]. By virtue of its elastic properties, the aorta influences LV diastolic function and thereby coronary blood flow Citation[20]. In addition, an increase in AoSI or a decrease in AoD may be an early predictor for coronary atherosclerosis and show target‐organ damage in hypertensive individuals. Confirming this idea, in recent years, the stiffness of large elastic arteries has been recognized as a major determinant of vascular function and cardiovascular risk Citation[9].
The new category of BP between normal BP and established hypertension “prehypertension” was recently introduced by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC‐7) Citation[1], replacing former categories of high‐normal and above‐optimal BP Citation[21]. The rationale for redefining this new category was to emphasize the excess cardiovascular risk associated with BP in this range and to focus high risk of developing hypertension in which lifestyle modifications are recommended Citation[1]. However, the absolute reduction in the incidence of new‐onset hypertension with the most successful lifestyle modification was only 8% in the Trials of Hypertension Prevention Citation[22]. The most important epidemiological data on PHT come from analyses of the Framingham Heart Study data. Compared with the optimal BP group (<120/80 mmHg), the subjects with above‐optimal (systolic BP between 120 and 129 mmHg or diastolic BP between 80 and 84 mmHg) or high‐normal BP (systolic BP between 130 and 139 mmHg or diastolic BP between 85 and 89 mmHg), which were combined into PHT under the JNC‐VII guidelines, had a markedly increased risk of developing hypertension within 4 years (aged 35–64 years, relative risk, RR = 11.6; ⩾65 years, RR = 5.5) Citation[23]. Another analysis from the Framingham Heart Study showed that compared with optimal BP, high‐normal BP is associated with a risk‐factor‐adjusted hazard ratio for cardiovascular disease of 2.5 (95% confidence interval, CI 1.6–4.1) in women and 1.6 (95% CI 1.1–2.2) in men Citation[24]. Furthermore, it has been shown that PHT is associated with a 27% increase in all cause and 66% increase in cardiovascular mortality Citation[25].
Although PHT has been widely studied in the past years, it remains a matter of controversy, especially regarding its treatment. Accordingly, its prognostic importance and risk profile for target‐organ damage have not yet clearly established, and the term PHT has yet to be widely adopted. The mechanism of excess risk from PHT is presumed to be the same as that from established hypertension. Several previous studies have demonstrated that subjects with PHT is associated with subclinical atherosclerosis and evidence of target‐organ damage, including impairment of LV relaxation Citation[24], microalbuminuria Citation[26], increased coronary atherosclerosis Citation[27] and increased carotid and brachial intima‐media thicknes Citation[28]. In line with the suggestions mentioned above, we have found that the patients with PHT had impaired aortic elastic properties than normotensive controls does, which is a surrogate marker of both subclinical atherosclerosis and hypertensive target‐organ damage.
In conclusion, these data provide evidence of strongly impaired aortic elasticity and slightly impaired LV function in subjects with PHT. Our results are consistent with the idea that PHT is associated with subclinical atherosclerosis and target‐organ damage.
References
- Stokes J 3rd., Kannel W. B., Wolf P. A., D'Agostino R. B., Cupples L. A. Blood pressure as a risk factor for cardiovascular disease. The Framingham Study – 30 years of follow‐up. Hypertension 1989; 13: I13–I18
- Tverdal A. Systolic and diastolic blood pressures as predictors of coronary heart disease in middle aged Norwegian men. Br Med J 1987; 294: 671–673
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L Jr., , Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, et al. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252
- Wang Y., Wang Q. J. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: New challenges of the old problem. Arch Intern Med 2004; 164: 2126–2134
- Qureshi A. I., Suri M. F., Kirmani J. F., Divani A. A., Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases?. Stroke 2005; 36: 1859–1863
- Mainous A. G 3rd., Everett C. J., Liszka H., King D. E., Egan B. M. Prehypertension and mortality in a nationally representative cohort. Am J Cardiol 2004; 94: 1496–1500
- Nesbitt S. D., Julius S., Leonard D., Egan B. M., Grozinski M. Is low‐risk hypertension fact or fiction? Cardiovascular risk profile in the TROPHY study. Am J Hypertens 2005; 18: 980–985
- Cameron J. D., Jennings G. L., Dart A. M. Systemic arterial compliance is decreased in newly‐diagnosed patients with coronary heart disease: Implications for prediction of risk. J Cardiovasc Risk 1996; 3: 495–500
- Oliver J. J., Webb D. J. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol 2003; 23: 554–566
- McGill H. C Jr., McMahan C. A., Malcom G. T., Oalmann M. C., Strong J. P. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 1997; 17: 95–106
- Lang R. M., Bierig M., Devereux R. B., Flachskampf F. A., Foster E., Pellikka P. A., , American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108
- Erdogan D., Caliskan M., Gullu H., Yildirim I., Ozer I., Ozgul A., et al. Aortic elastic properties and left ventricular diastolic function in white‐coat hypertensive individuals. Blood Press Monit 2006; 11: 191–198
- Devereux R. B., Alonso D. R., Lutas E. M., Gottlieb G. J., Campo E., Sachs I., et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458
- Vasan R. S., Benjamin E. J., Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: An epidemiologic perspective. J Am Coll Cardiol 1995; 26: 1565–1574
- Slama M., Susic D., Varagic J., Frohlich E. D. Diastolic dysfunction in hypertension. Curr Opin Cardiol 2002; 17: 368–373
- Appleton C. P., Hatle L. K., Popp R. L. Relation of transmitral flow velocity patterns to left ventricular diastolic function: New insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 1988; 12: 426–440
- Shimizu Y., Uematsu M., Shimizu H., Nakamura K., Yamagishi M., Miyatake K. Peak negative myocardial velocity gradient in early diastole as a noninvasive indicator of left ventricular diastolic function: Comparison with transmitral flow velocity indices. J Am Coll Cardiol 1998; 32: 1418–1425
- Naqvi T. Z., Neyman G., Broyde A., Mustafa J., Siegel R. J. Comparison of myocardial tissue Doppler with transmitral flow Doppler in left ventricular hypertrophy. J Am Soc Echocardiogr 2001; 14: 1153–1160
- O'Rourke M. F. Isolated systolic hypertension, pulse pressure, and arterial stiffness as risk factors for cardiovascular disease. Curr Hypertens Rep 1999; 1: 204–211
- Urschel C. W., Covell J. W., Sonnenblick E. H., Ross J Jr., Braunwald E. Effects of decreased aortic compliance on performance of the left ventricle. Am J Physiol 1968; 214: 298–304
- The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 1997; 157: 2413–2446
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure: The Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157: 657–667
- Vasan R. S., Larson M. G., Leip E. P., Kannel W. B., Levy D. Assessment of frequency of progression to hypertension in non‐hypertensive participants in the Framingham Heart Study: A cohort study. Lancet 2001; 358: 1682–1686
- Vasan R. S., Larson M. G., Leip E. P., Evans J. C., O'Donnell C. J., Kannel W. B., et al. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 345: 1291–1297
- Mainous A. G III., Everett C. J., Liszka H., King D. E., Egan B. M. Prehypertension and mortality in a nationally representative cohort. Am J Cardiol 2004; 94: 1496–1500
- Knight E. L., Kramer H. M., Curhan G. C. High‐normal blood pressure and microalbuminuria. Am J Kidney Dis 2003; 41: 588–595
- Washio M., Tokunaga S., Yoshimasu K., Kodama H., Liu Y., Sasazuki S., et al. Role of prehypertension in the development of coronary atherosclerosis in Japan. J Epidemiol 2004; 14: 57–62
- Toikka J. O., Laine H., Ahotupa M., Haapanen A., Viikari J. S., Hartiala J. J., et al. Increased arterial intima‐media thickness and in vivo LDL oxidation in young men with borderline hypertension. Hypertension 2000; 36: 929–933