Abstract
Ascending aortic blood pressure‐derived indices were shown to be related to coronary atherosclerosis. However, no study so far has analyzed the relation between ascending aortic pulsatility and the extent of coronary atherosclerosis in normotensives. Therefore, the aim of the present analysis was to assess the relation between central blood pressure‐derived indices and the presence and extent of coronary artery disease in patients with and without hypertension. The study group consisted of 821 patients (590 men and 231 women; mean age: 57.3±10.0 years) with preserved left ventricular function (ejection fraction>50%) undergoing coronary angiography. Hypertension was diagnosed in 639 (77.8%) patients. Ascending aortic blood pressure during catheterization was measured. After multivariate stepwise adjustment the odds ratio (OR) and confidence interval (CI) of coronary artery disease was: pulsatility per standard deviation (SD) OR 1.36 (95% CI 1.01–1.82) in hypertensives and OR 3.96 (1.95–8.07) in normotensives; pulsatility index per SD OR 1.36 (95% CI 1.01–1.85) in hypertensives and OR 4.41 (2.03–9.56) in normotensives. Stepwise linear regression analysis revealed that pulsatility is related to the mean stenosis in the coronary tree in hypertensives (β = 0.0862, SE = 0.0448, p<0.05) as well as in normotensives (β = 0.1704, SE = 0.0718, p<0.05). In conclusion, ascending aortic pulsatility is related to the extent of coronary atherosclerosis irrespectively of the presence of hypertension.
Introduction
Blood pressure (BP) is a well‐known cardiovascular risk factor. Since BP waveform differs along the arterial tree, ascending aortic BP is expected to be more relevant to investigate cardiovascular risk than brachial BP, being closer to the heart, coronary and carotid arteries, the sites of occurrence of major cardiovascular events. Recently, there has been more emphasis on aortic rather than peripheral BP to predict cardiovascular risk Citation[1].
Although traditionally pulse pressure (PP) was considered a pulsatile component of BP Citation[2], it seems that it can be more correctly to use pulsatility as a representative of the pulsatile component of BP. Indeed, it was shown that PP is quite well correlated with mean BP (MBP) suggesting that PP contains information about the absolute value of BP Citation[3], Citation[4]. On the other hand, pulsatility is not correlated with MBP at all, suggesting that pulsatility contains information only about changes of BP during the heart cycle Citation[4]. There is an increasing amount of data that suggest that cyclic tensile stress is a major BP‐related factor inducing development and progression of atherosclerosis Citation[4–6].
Ascending aortic BP‐derived indices were shown to be related to coronary atherosclerosis Citation[7–11]. Ascending aortic PP, fractional PP, fractional systolic pressure (SBP), fractional diastolic pressure (DBP) and pulsatility index are independently related to the presence and extent of coronary atherosclerosis Citation[7–11]. Recently, increased ascending aortic pulsatility and PP were shown to be related to the risk of major cardiovascular complications Citation[12–14].
The effect of the ascending aortic pressure waveform on the severity of coronary artery disease (CAD) in normotensive subjects has not previously been reported. Although the demonstration of the independent relationship between aortic BP‐derived indices and coronary atherosclerosis in subjects without hypertension can improve our understanding of atherosclerosis pathogenesis as well as having a profound influence on the management of normotensives, no study so far has analyzed the relation between ascending aortic pulsatility and the extent of coronary atherosclerosis in normotensives. Therefore, the aim of the present study was to assess the influence of hypertension on the relation between central BP‐derived indices and the presence and extent of coronary atherosclerosis.
Methods
Study population
Consecutive patients hospitalized in our department, suspected for having CAD and undergoing diagnostic coronary angiography were eligible for our study. We excluded from the analysis all patients with acute myocardial infarction within a month before angiography, patients after percutaneous coronary intervention, and those with hemodynamically significant valvular heart disease as assessed during catheterization or echocardiography and patients with atrial fibrillation/flutter at the time of examination. Patients with primary pulmonary hypertension and those after valve replacement operation were also excluded. Since it was shown that significant left ventricular function impairment disturbs BP‐derived indices – coronary atherosclerosis correlation Citation[15] – we also excluded patients with low ejection fraction (defined as ejection fraction ⩽50%).
All blood samples were taken before coronary angiography. Fasting blood samples were taken for the analysis of total cholesterol, high‐density lipoprotein cholesterol, triglycerides, and low‐density lipoprotein (LDL) cholesterol as well as glycemia. The presence of diabetes was defined as a fasting blood glucose of 7.0 mmol/l or more and/or the use of an antidiabetic drug. Because of the low number of patients with type 1 diabetes, we decided not to differentiate type 1 diabetes from type 2 in the present analysis. Hypercholesterolemia was defined as having LDL cholesterol level ⩾2.6 mmol/l and/or being prescribed a lipid‐lowering drug. Hypertension was defined as having high BP (brachial SBP⩾140 mmHg and/or brachial DBP⩾90 mmHg; both were measured in seated patients after at least 10 min of rest) and/or being prescribed a BP‐lowering drug for high BP. We defined current smokers as individuals who smoked any tobacco in the previous month and included those who had quit within the last month. Ejection fraction was determined using contrast ventriculography. CAD was defined as having >50% stenosis in the coronary tree and/or having myocardial infarction (pathologic Q wave on ECG and/or elevated necrosis markers) in the medical history. The institutional ethics committee approved the protocol of the study.
Measurement of hemodynamic variables
Hemodynamic measurements were obtained from the patient in the supine position. Aortic SBP and DBP were measured using low‐compliance fluid‐filled system at the ascending aorta. MBP was obtained by direct integration of the BP curve. To quantify relative PP, we normalized the PP to the MBP, and refer to this value as the pulsatility Citation[8]. We also used the ratio of PP to DBP (pulsatility index) for another index of aortic stiffness.
Measurement of angiographic variables
Cardiac catheterization was performed according to a standard technique. Optimal views of the arteries from all technically suitable angiograms were analyzed. Angiographic measurements were calibrated using the guiding catheter as the reference dimension. The absolute values for the minimal lumen diameter and the reference lumen diameter were assessed measured at end‐diastole.
Coronary angiograms were scored using four techniques:
Number of diseased arteries. The three major coronary vessels (the left anterior descending artery, the circumflex artery, and the right coronary artery) were considered for evaluation of the extent of coronary atherosclerosis. A diseased artery was defined as having >50% stenosis of at least one of its segments. Significant left main artery stenosis was coded as two‐vessel disease.
Mean stenosis. In this score, a maximum stenosis in each segment ]of 15 segments as defined by the American Heart Association Citation[16]] was measured, and then mean stenosis was calculated.
Severity score. In this score, a value of 1 was given to any of 15 arterial segments [as defined by the American Heart Association Citation[16]] that contained greater or equal to 70% stenosis, giving a potential score range from 0 to 15 Citation[17].
Gensini score. This scoring system assigns a different severity score depending on geometrically increasing severity of the lesion, the cumulative effects of multiple obstructions and the significance of their locations Citation[18]. The Gensini score was used widely in previous years to assess the severity and extent of coronary atherosclerosis Citation[19], Citation[20].
Statistical analysis
All data were analyzed using the Statistica 6.0 software. Categorical variables are reported as percentages and continuous variables as means±standard deviation (SD). The Pearson's chi‐squared test was applied to all categorical variables. Normally distributed continuous variables were compared using Student's t‐test. Mann–Whitney U‐test was used in case of variables without normal distribution. A two‐tailed p‐value of less than 0.05 was considered to indicate statistical significance. Adjusted (for age and gender) means of BP‐derived indices were compared using a general linear model procedure. Correlations between pulsatility/pulsatility index and the mean stenosis in the coronary tree/Gensini score were calculated using Pearson correlation. Correlations between the severity score and pulsatility/pulsatility index were established by a Spearman correlation. Logistic regression analysis was performed to evaluate the independent effects of hemodynamic variables on the risk of having CAD. We performed three sequential multivariable models. The first included aortic pulsatility or pulsatility index and age, gender and ejection fraction. The second included all the above factors plus MBP. The third model included all the previous variables plus main traditional risk factors (smoking, diabetes, body mass index and hypercholesterolemia), heart rate, creatinine and glucose levels, and cardiovascular drugs. The logistic regression analysis results are reported using odds ratios (95% confidence intervals). In order to compare the indices with respect to their ability to differentiate patients with CAD from those without CAD, we report the odds ratios for increase of SD of each variable. SDs were calculated separately for patients with and without hypertension. Multiple linear regression models for the mean stenosis in coronary tree and for the Gensini score were derived by using stepwise, backward regression analysis. All analyses are reported separately for patients with and without hypertension.
Results
The study group consisted of 821 patients (590 men and 231 women; mean age: 57.3±10.0 years). CAD was diagnosed in 652 (79.4%) subjects, whereas hypertension in 639 (77.8%). Clinical characteristics and classic risk factors are shown in Table . Hypertensive as well as normotensive patients with CAD were more likely to be men and more likely to have diabetes when compared with subjects without CAD. Mean creatinine level was higher and mean ejection fraction was lower in CAD patients in both groups. Normotensives with CAD were older when compared with normotensive subjects without CAD.
Table I. Clinical characteristics of the study population.
Unadjusted mean values of pulsatility and pulsatility index according to the number of stenosed (stenosis ⩾50%) coronary arteries are shown on Figure . Mean values of ascending aortic BP‐derived indices after adjustments for age and gender are given in Table . PP, pulsatility and pulsatility index were significantly higher in patients with CAD when compared to those without CAD, irrespectively of the presence of hypertension. Both pulsatility and pulsatility index were related to the presence of CAD in univariate logistic regression analysis as well as after adjustments for a number of potential confounders (Table ).
Figure 1 Pulsatility(a) and pulsatility index (b) mean values in patients with and without hypertension according to the number of diseased coronary vessels.
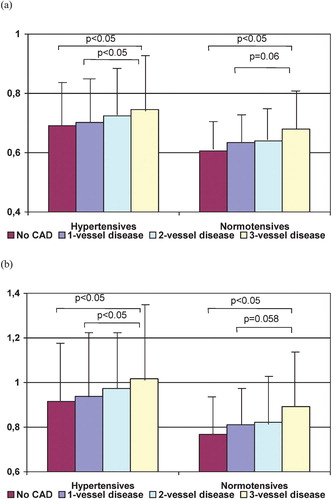
Table II. Mean values of blood pressure derived indices in patients with and without hypertension, according to the presence/absence of coronary artery disease (adjusted for age and gender).
Table III. Odds ratios (95% confidence intervals) for the association between pulsatility/pulsatility index and the presence of coronary artery disease (1 = CAD, 0 = no CAD).
Pulsatility and pulsatility index were correlated with the mean stenosis in the coronary tree, with the Gensini score as well as with the severity score (r = 0.16, p<0.001; r = 0.12, p<0.01; r = 0.14, p<0.01 for pulsatility and r = 0.16, p<0.001; r = 0.12, p<0.01; r = 0.14, p<0.01 for pulsatility index, respectively) in hypertensives. Similar correlations were found in normotensives (r = 0.19, p<0.05; r = 0.18, p<0.05; r = 0.15, p<0.05 for pulsatility and r = 0.20, p<0.05; r = 0.18, p<0.05; r = 0.15, p<0.05 for pulsatility index, respectively). Correlation coefficients calculated for normotensives did not vary significantly from those calculated for hypertensives. Stepwise linear regression analysis with the mean stenosis in the coronary tree (Table ) and with the Gensini score (Table ) as dependent variables was also performed. When we included pulsatility and pulsatility index into the initial models, only the former variable appeared to be a significant predictor of the mean stenosis in the coronary tree as well as of the Gensini score. Pulsatility, but not SBP, DBP, PP or MBP was a significant predictor of coronary atherosclerosis extent when pulsatility was included in initial models, together with SBP, DBP, PP or MBP. When pulsatility was replaced with pulsatility index, again the latter but not SBP, DBP, PP or MBP was significantly related to the extent of coronary atherosclerosis.
Table IV. Linear multiple regression analysis of the mean stenosis in the coronary tree as a dependent variable.
Table V. Linear multiple regression analysis of the Gensini score as a dependent variable.
We also performed the above analyses in subgroups of patients taking and not taking specific cardiovascular drugs (antiplatelets, beta‐blockers, etc.). These analyses produced very similar results (data not shown).
Discussion
BP varies throughout the arterial tree due to wave reflection and differences in vessel stiffness. It is now well established that the non‐invasive measurement of brachial BP is often inaccurate representation of central pressure and the latter is expected to be directly involved in atherosclerosis progression Citation[4], Citation[21]. Ascending aortic BP‐derived indices were shown to be related to the coronary atherosclerosis Citation[4], Citation[8–11]. To our best knowledge, this is first study showing the independent relation between the ascending aortic BP waveform (as measured invasively) and the presence of CAD in normotensive subjects. Since correlation coefficients, odds ratios (in logistic regression analysis) and regression coefficients (in linear regression analysis) are consistently higher (although the differences were not statistically significant) in normotensives when compared with hypertensives, the risk of false results is extremely low. Moreover, we also showed that pulsatility and pulsatility index are better predictors of coronary atherosclerosis compared to SBP, DBP, MBP or PP.
The present results suggest that pulsatile component of BP waveform may be of at least similar significance in the development of CAD in normotensives as compared to patients with hypertension. If this hypothesis is correct, improvement in arterial compliance and wave reflection would be of comparable importance in subjects with and without hypertension. Recent experiments with a novel class of agents, thiazolium derivatives, have demonstrated significant improvements in arterial compliance associated with reductions in non‐enzymatic cross‐links between vascular matrix proteins Citation[22], Citation[23]. Indeed, Kass showed that alagebrium, an agent from this class, significantly reduces arterial compliance, PP and pulsatility in patients with hypertension Citation[23]. The present results suggest that these agents could be beneficial also in normotensives. Actually, it seems that antipulsatile rather than hypotensive therapy is needed in normotensive subjects at risk of atherosclerosis development. Our results agree with the studies of Vaccarino et al. and Benetos et al., who showed that the relation of brachial PP with the incidence of CAD and cardiovascular mortality is even more pronounced in normotensive subjects when compared with hypertensives Citation[24], Citation[25].
One can argue that aortic pulsatility is just an indicator of the extent of atherosclerosis. Although the design of the present study does not allow for ultimately excluding or confirming this hypothesis, it seems that the pure pulsatile component of BP is an independent factor related to the increased cardiovascular risk. Indeed, aortic pulsatility was shown to be an independent predictor of major cardiovascular complications in patients undergoing coronary angiography Citation[13]. Moreover, it is known that bending of unstable plaques due to cyclic stress is the most important trigger of plaque rupture and the latter is the main mechanism of acute coronary syndromes. Plaque ruptures are also involved in the atherosclerosis progression. In recent years, much attention has been paid to mechanical forces in the development of atherosclerosis Citation[4–6], Citation[26]. A possible additional explanation for the relationship between aortic pulsatility and atherosclerosis is provided by the concept of bidirectionality, i.e. high pulsatility is both a cause and a consequence of atherosclerosis. The latter hypothesis does not play down the importance of our results.
Although pulsatility was not the most predictive variable in multiple regression analyses, it should be underlined that pulsatility was the only potentially reversible factor or almost the only (with the exception only for BMI and hypercholesterolemia when mean stenosis in the coronary tree is considered).
There is growing evidence that analysis of the ascending aortic BP waveform may provide useful information in patients undergoing catheterization and coronary angioplasty. In recent years, great progress has been made in predicting BP in the ascending aorta by pulse wave analysis Citation[21], Citation[27]. It seems that non‐invasive estimation of ascending aortic BP waveform, after further validation, might be a useful tool in the management of CAD patients Citation[28]. The present study suggests that aortic pulsatility (as assessed invasively, but probably also using non‐invasive methods) may complement the risk stratification provided by current guidelines. These data provide also additional insight into the pathogenesis of coronary atherosclerosis and offer additional evidence to suggest that new therapeutic agents designed to treat arterial stiffness and decrease pulsatile component of BP may be another effective treatment to forestall the progression of CAD.
There are several limitations to this study. First, although we analyzed a larger patient group when compared with the previous reports concerning the ascending aortic BP waveform–atherosclerosis relationship, it is still a limited number Citation[7–11]. Second, we used a fluid‐filled system to record the ascending aortic pressure. The use of a high‐fidelity pressure transducer would increase the accuracy of the recorded pressure waveform. Third, most participants of the study were prescribed cardiovascular drugs; some of these agents were shown to interfere with central SBP augmentation Citation[29–31]. However, inclusion of these drugs into the multivariate analyses did not change significantly odds ratios (in logistic regression analysis) or regression coefficients (in linear regression analysis). Moreover, we performed also analyses in subgroups of patients taking and not taking antiplatelets, beta‐blockers, angiotensin‐converting enzyme (ACE) inhibitors, etc., which suggested that cardiovascular medications do not mediate the association between aortic pulsatility and the extent of coronary atherosclerosis. Fourth, as our results were obtained from selected population (patients with preserved left ventricular function undergoing coronary angiography), it should be taken into account that these data may not be generalizable to other patients groups. Fifth, as PP is determined directly by stroke volume, not by ejection fraction, stroke volume measurement would increase the precision of our analysis.
Conclusion
Ascending aortic pulsatility is related to the extent of coronary atherosclerosis irrespectively of the presence of hypertension.
References
- Safar M. E., Blacher J., Pannier B., Guerin A. P., Marchais S. J., Guyonvarc'h P. M., et al. Central pulse pressure and mortality in end‐stage renal disease. Hypertension 2002; 39: 735–738
- Safar M. E., Levy B. I., Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003; 107: 2864–2869
- Dart A. M., Kingwell B. A. Pulse pressure – A review of mechanisms and clinical relevance. J Am Coll Cardiol 2001; 37: 975–984
- Jankowski P., Bilo G., Kawecka‐Jaszcz K. The pulsatile component of blood pressure – Its role in the pathogenesis of atherosclerosis. Blood Press 2007, in press
- Xu Q. Biomechanical‐stress‐induced signaling and gene expression in the development of atherosclerosis. Trends Cardiovasc Med 2000; 10: 35–41
- Yamamoto K., Ikeda U., Shimada K. Role of mechanical stress in monocytes/macrophages: Implications for atherosclerosis. Curr Vasc Pharmacol 2003; 1: 315–319
- Jankowski P., Kawecka‐Jaszcz K., Czarnecka D., Brzozowska‐Kiszka M., Styczkiewicz K., Styczkiewicz M., et al. Ascending aortic, but not brachial blood pressure‐derived indices are related to coronary atherosclerosis. Atherosclerosis 2004; 176: 151–155
- Guray Y., Guray U., Altay H., Cay S., Yilmaz M. B., Kisacik H. L., et al. Pulsatility of ascending aortic blood pressure waveform is associated with increased risk of coronary heart disease. Blood Press 2005; 14: 293–297
- Nakayama Y., Hayashi T., Yoshimaru K., Tsumura K., Ueda H. Low fractional diastolic pressure in the ascending aorta increased the risk of coronary heart disease. J Hum Hypertens 2002; 16: 837–841
- Philippe F., Chemaly E., Blacher J., Mourad J. J., Dibie A., Larrazet F., et al. Aortic pulse pressure and extent of coronary artery disease in percutaneous transluminal coronary angioplasty candidates. Am J Hypertens 2002; 15: 672–677
- Danchin N., Benetos A., Lopez‐Sublet M., Demicheli T., Safar M., Mourad J. J., on behalf of the ESCAPP Investigators. Aortic pulse pressure is related to the presence and extent of coronary artery disease in men undergoing diagnostic coronary angiography: A multicenter study. Am J Hypertens 2004; 17: 129–133
- Chirinos J. A., Zambrano J. P., Chakko S., Veerani A., Schob A., Perez G., et al. Relation between ascending aortic pressures and outcomes in patients with angiographically demonstrated coronary artery disease. Am J Cardiol 2005; 96: 645–458
- Jankowski P., Kawecka‐Jaszcz K., Czarnecka D., Brzozowska‐Kiszka M., Styczkiewicz K., Curyło A. M., et al. Ascending aortic pulsatility and pulsatility index are better predictors of event‐free survival compared to systolic, diastolic, mean and pulse pressure. The results from the ABPS Study. Eur Heart J 2006; 27 Suppl: 114 [abstract]
- Williams B., Lacy P. S., Thom S. M., Cruickshank K., Stanton A., Collier D., et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113: 1213–1225
- Jankowski P., Kawecka‐Jaszcz K., Czarnecka D., Brzozowska‐Kiszka M., Pośnik‐Urbańska A., Styczkiewicz K. Ascending aortic blood pressure‐derived indices are not correlated with the extent of coronary artery disease in patients with impaired left ventricular function. Atherosclerosis 2006; 184: 370–376
- Austen W. G., Edwards J. E., Frye R. L., Gensini G. G., Gott V. L., Griffith L. S., et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975; 51 Suppl 4: 5–40
- Zebrack J. S., Muhlestein J. B., Horne B. D., Anderson J. L. C‐reactive protein and angiographic coronary artery disease: Independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol 2002; 39: 632–637
- Gensini G. G. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51: 606
- Kaneda H., Taguchi J., Ogasawara K., Aizawa T., Ohno M. Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis 2002; 62: 221–225
- Al‐Fakhri N., Linhart R. E., Philipp M., Heidt M., Hehrlein F. W., Gardemann A., et al. Endotelin‐1 and vasopressin plasma levels are not associated with the insertion/deletion polymorphism of the human angiotensin I‐converting enzyme gene in patients with coronary artery disease. J Hum Hypertens 2003; 17: 133–138
- O'Rourke M. F., Adji A. An updated clinical primer on large artery mechanics: Implications of pulse waveform analysis and arterial tonometry. Curr Opin Cardiol 2005; 20: 275–281
- Bakris G. L., Bank A. J., Kass D. A., Neutel J. M., Preston R. A., Oparil S. Advanced glycation end‐product cross‐link breakers: A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens 2004; 17: 23S–30S
- Kass D. A. Ventricular arterial stiffening. Integrating the pathophysiology. Hypertension 2005; 46: 185–193
- Vaccarino V., Holford T. R., Krumholz H. M. Pulse pressure and the risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol 2000; 36: 130–138
- Benetos A., Rudnichi A., Safar M., Guize L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension 1998; 32: 560–564
- Kiefer C. R., McKenney J. B., Trainor J. F., Snyder L. M. Pulse pressure‐driven neutral lipid accumulation and correlative proinflammatory markers of accelerated atherogenesis. Atherosclerosis 2005; 183: 17–24
- Pauca A. L., O'Rourke M. F., Kon N. D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38: 932–937
- Weber T., Auer J., O'Rourke M. F., Kvas E., Lassnig E., Berent R., et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004; 109: 184–189
- Kelly R. P., Millasseau S. C., Ritter J. M., Chowienczyk P. J. Vasoactive drugs influence aortic augmentation index independently of pulse‐wave velocity in healthy men. Hypertension 2001; 37: 1429–1433
- Morgan T., Lauri J., Bertram D., Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens 2004; 17: 118–123
- Safar M. E., O'Rourke M. F. Pulse pressure and antihypertensive agents. Hypertension 2005; 46: e6