Abstract
Objective. A fall in nocturnal blood pressure (BP) is generally observed in normotensive subjects as well as in those with mild to moderate essential hypertension, regardless of the level of daytime BP. Among elderly hypertensive subjects, extreme‐dippers with a marked nocturnal fall in BP as well as non‐dippers with nocturnal fall absence are at increased risk for cardiovascular and cerebrovascular complications. However, the relationship between these abnormal diurnal BP variation patterns in normotensive elderly subjects has not been investigated. Methods. We classified 45 healthy late middle‐aged and older adults into three groups according to the nocturnal systolic BP fall pattern examined by 24‐h ambulatory BP monitoring (dipper, non‐dipper and extreme‐dipper), and compared the parameters of initial atherosclerosis, endothelial function and autonomic function. As a parameter of atherosclerotic factors, the intima‐media thickness (IMT) of the carotid artery was examined, and as a parameter of endothelial function, brachial artery endothelium‐dependent flow‐mediated dilation (FMD) was ultrasonographyically measured. Autonomic function was assessed by power spectral analysis of heart rate variability (HRV). Results. No difference was observed in the severity of IMT between the three groups. The percent change of FMD in subjects in the extreme‐dipper group was significantly lower than that of subjects in the dipper group, indicating that extreme‐dippers in healthy elderly subjects may be associated with endothelial dysfunction. Also, HRV due to sympathetic modulation of subjects in the extreme‐dipper group was significantly higher than that of subjects in the dipper and non‐dipper groups, suggesting the activation of sympathetic tone. Conclusion. In healthy elderly subjects, the extreme‐dipper type may reflect a decrease in endothelial function, i.e. initial stage atherosclerosis, rather than the dipper type.
Introduction
The daily variation in blood pressure (BP) is generally characterized by a nocturnal fall and diurnal rise (with a nocturnal decrease in the mean BP between 10% and 20%) Citation[1]. In hypertensive patients, a variety of abnormal diurnal patterns have been described in which the nocturnal fall of BP may be >20% (extreme‐dippers) or <10% (non‐dippers) Citation[2]. These variations are of interest because they may be related to hypertensive target‐organ damage or to a poor cardiovascular prognosis. In several cross‐sectional studies, non‐dippers have been reported to show more clinical and subclinical target‐organ damage in the heart, brain and kidneys than do dippers Citation[3–5]. Besides non‐dipping, evidence suggests that extreme dipping (a marked nocturnal fall of BP) should be considered a type of abnormal diurnal BP variation in elderly patients with hypertension who are likely to have advanced silent cerebrovascular damage Citation[6]. The pathogenic significance of “extreme dipping” might be an “artificial” excess reduction in BP at night beyond the lower limit of BP in the autoregulation of cerebral blood flow. It is also possible that greater BP variation in extreme‐dippers itself accelerates hypertensive target‐organ damage. A fall in nocturnal BP is generally observed in normotensive subjects as well as in those with mild to moderate essential hypertension, regardless of the level of daytime BP Citation[7]. However, whether the abnormal diurnal BP variation in normotensives indicates a higher risk of target‐organ damage such as in hypertensive patients remains to be elucidated.
As measurements of atherosclerotic progression, we performed two ultrasound methods to assess brachial artery flow‐mediated dilation (FMD) and carotid intima‐media thickness (IMT). Also, the incidence of silent cerebral infarction (SCI) was assessed by subcortical hyperintensity on MRI. Brachial artery FMD is a non‐invasive assessment method to analyze endothelial function by quantitatively monitoring the vasodilation responses of vascular smooth muscle to nitric oxide produced by endothelial cells following hyperemia Citation[8], Citation[9]. Endothelial dysfunction is known to appear in the initiation of early atherosclerosis Citation[9]. The measurement of carotid IMT can detect atherosclerotic changes non‐invasively Citation[10], and its association with hypertension has also been suggested Citation[11], Citation[12]. Autonomic function was assessed by power spectral analysis of heart rate variability (HRV). Abnormalities of autonomic nervous function have been considered as one of the important factors in precipitating disrupted diurnal BP variation Citation[13], Citation[14]. The aim of the present study was therefore to investigate whether an abnormal diurnal BP variation pattern (examined by 24‐h ambulatory BP monitoring) in healthy elderly subjects is associated with the parameters of carotid atherosclerosis, endothelial dysfunction, sympathovagal imbalance and the incidence of SCI, and clarify the pathogenesis and clinical significance of abnormal diurnal BP variations in healthy elderly subjects.
Methods
Subjects
Forty‐five healthy late middle‐aged and older adults aged 51–69 years (mean±SD: 60.2±4.3 years) were enrolled in this study. These subjects were recruited from general inhabitants using a brochure that described the following exclusions: history of major atherosclerotic risk factors (such as hypertension, hypercholesterolemia, diabetes mellitus, coronary artery disease and hemodynamically significant valvular disease), chronic alcoholism, smoking, obesity with a body mass index (BMI) above 26, and continuous administration of drugs. All subjects gave written informed consent. The protocol of this study was approved by the ethics committee of our university.
Office BP and 24‐h BP monitoring
After a 15‐min rest in the supine position, office BP was measured using a standard mercury sphygmomanometer. Twenty‐four‐hour BP monitoring was performed using an FM‐200 (Fukuda Densi Co., Tokyo, Japan), which recorded BP by the oscillometric method. This ambulatory BP device has undergone validation testing as recommended by the Association for the Advancement of Medical Instrumentation Citation[15]. An appropriate cuff size, placed on the non‐dominant arm, was used in accordance with recommendations of the British Hypertension Society Citation[16]. The asleep BP was defined as the mean BP from the time at which the subjects went to bed until the time of awakening, and the awake BP was defined as the mean BP during the remaining portion of the day. Measurements were performed at 30‐min intervals for awake BP and at 1‐h intervals for asleep BP. According to the method of Kario et al. Citation[2], the degree of nocturnal systolic BP (SBP) fall was calculated by dividing the difference between awake SBP and sleep SBP by awake SBP. Nocturnal SBP fall patterns were classified into three types according to the degree of fall into the non‐dipper type showing a fall of <10%, dipper type showing a fall of ⩾10% but <20%, and the extreme‐dipper type showing a fall of ⩾20%.
Biochemical determinations
All subjects underwent venous blood sampling from an antecubital vein in the right arm before noon without having eaten breakfast. Serum concentrations of total cholesterol (TC), triglycerides, high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) were measured by enzyme determination. Glycosylated hemoglobin A1c (HbA1c) was determined by means of high‐pressure liquid chromatography. Platelet activation was examined by the plasma concentration of platelet factor 4 (PF4) and β‐thromboglobulin (β‐TG). PF4 and β‐TG were determined by enzyme immunoassay.
Brain MRI
Brain MRI was performed using a 1.5‐tesla Signa system (General Electric Medical Systems, USA). T2‐weighted images (TR = 3000 ms, TE = 80 ms) and proton density‐weighted images (TR = 3000 ms, TE = 20 ms) were obtained with a slice thickness of 5 mm parallel to the orbitomeatal line. Hyperintense lesions of ⩾3 mm in diameter demonstrated on both images were evaluated according to the modified Fazekas 4‐point (0−3) criteria Citation[17]. SCI was considered present when a lesion with a score of 2 or 3 was observed in deep white matter or subcortical gray matter. When lesions with a score of 0 or 1 were observed in both areas, SCI was considered to be absent.
Carotid B‐mode ultrasonography
Carotid B‐mode ultrasonography was performed using a SONOS 5500 (Philips Medical Systems, Andover, MA, USA), and was examined using an 11‐MHz linear array transducer. The IMT was measured as the distance between the lumen‐intima interface and the media‐adventitia interface on the B‐mode image, and an IMT of >1.0 mm was considered to indicate atherosclerotic lesions according to the methods of Handa et al. Citation[18]. The plaque score was computed by summing the maximal IMT (plaque thickness >1.0 mm) measured in millimeters on the near and far walls at each of the four divisions on both sides of the carotid arteries.
Brachial artery FMD and dilation response to GTN
Ultrasound imaging of the brachial artery was examined in the supine position with an upper arm cuff position using a SONOS 5500 system with an 11‐MHz linear array transducer. Hyperemia was induced by inflating a BP cuff on the proximal portion of the arm to occlude arterial flow (⩾200 mmHg) for 5 min. After releasing the cuff, brachial artery images were obtained at 30‐s intervals for a total of 3 min. FMD was calculated as the maximal postocclusion diameter relative to the averaged preocclusion diameters (recorded under the resting baseline condition two times). At least a 10‐min rest period was given after the reactive hyperemia before another image was acquired to re‐establish baseline conditions. Then, after the administration of sublingual glyceryl trinitrate (GTN) spray (0.3 mg), brachial artery images were obtained at 30‐s intervals for a total of 3 min. The %FMD and %GTN were each calculated as the percentage change in diameter compared with the resting baseline condition.
Power spectral analysis of HRV
In a quiet and comfortable environment, the subjects were instructed to lie in the supine position on a bed. After allowing 10 min for stabilization, the ECG was recorded for 15 min. During ECG, the subjects maintained their breathing at a fixed rate of 0.25 Hz in tempo with the sound of a metronome, because the respiratory frequency influences the HRV Citation[19]. Holter ECG monitoring (FM‐300, Fukuda Densi Co.) was performed to record RR intervals on a flash memory card. The RR intervals recorded on the card were converted to digital signals at 125 samples per sec (DMW‐9000H, Fukuda Densi Co.). The power spectral densities were computed with a commercially available program (HPS‐RRLOP version 1.02, Fukuda Densi Co.) using the fast‐Fourier transform method. The power spectral densities of rhythmic oscillations over a frequency range of 0.4 Hz or less were obtained during 512 beats to analyze the low‐frequency power (LF: 0.04–0.15 Hz) as an index of both sympathetic and parasympathetic nervous system activities, and high‐frequency power (HF: 0.15–0.40 Hz) as an index of parasympathetic nervous system activity. The LF/HF ratio, an indirect index of sympathetic nervous system activity, was calculated for each data set.
Statistical analysis
Statistical analyses were carried out using computer software (SPSS for Windows ver. 9; SPSS Japan Inc., Tokyo, Japan). All data are presented as the mean±SD. The relationships among age, BMI, TC, HDL, LDL, FMD and LF/HF were explored by simple correlation analysis using Pearson's product‐moment correlation coefficient. One‐way analysis of variance (ANOVA) was performed to detect differences among groups, and Fisher's protected least significant difference test was used to compare the mean values between groups. Fisher's exact test was used to detect differences among groups in the prevalence of SCI.
Results
Of the 45 subjects consisting of 24 males aged 61.6±4.6 years and 21 females aged 59.2±4.1 years, 29 subjects (64.4%) displayed the dipper type, nine (20%) displayed the non‐dipper type and seven (15.6%) displayed the extreme‐dipper type (Table ).
Table I. Demographic characteristics and laboratory data of the study subjects.
The demographic characteristics, office BP, 24‐h mean BP (daytime and night‐time) and biochemical determinations in subjects showing the three nocturnal SBP fall patterns are presented in Table . The resting office SBP or DBP and the mean 24‐h BP did not significantly differ between the three types. Also, the triglycerides, TC, PF4, β‐TG concentration and HbA1c were not significantly different between the three types.
SCI was observed in five (11.1%) of the 45 subjects (dipper type, 13.8%; non‐dipper type, 0%; extreme‐dipper type, 14.3%), with no significant difference between the three types. Furthermore, neither of the parameters of carotid atherosclerosis (mean‐IMT and plaque score) significantly differed between the three types (Table ).
Table II. Association of SCI complication and the severity of carotid atherosclerosis with the nocturnal systolic blood pressure pattern.
Simple correlation analysis showed no significant association between age, BMI, TC, HDL, LDL and FMD or LF/HF in the subjects (data not shown).
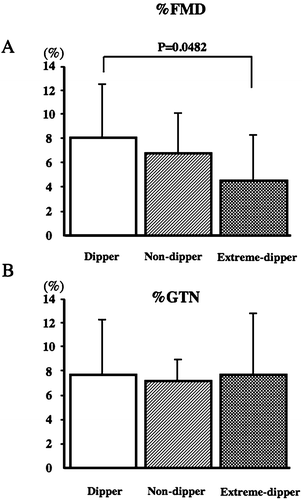
The %FMD (8.05% in the dipper type, 6.39% in the non‐dipper type, and 4.49% in the extreme‐dipper type) was significantly lower in the extreme‐dipper type than the dipper type (p<0.05; Figure ). On the other hand, the value for %GTN (7.22% in the dipper type, 7.51% in the non‐dipper type, and 7.62% in the extreme‐dipper type) was not significantly different between the three types (Figure ).
Figure 1 Comparison of percentage flow‐mediated dilation (%FMD) and percentage glyceryl trinitrate (%GTN) between the three groups (dipper, non‐dipper and extreme‐dipper) classified according to the degree of nocturnal systolic blood pressure (SBP) fall. Values are the mean±SD.
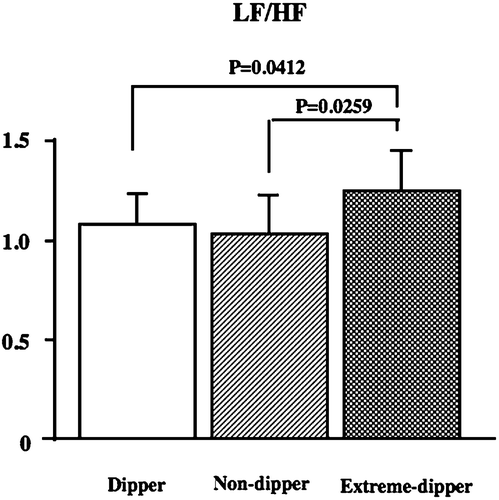
The LF/HF ratio (1.04 in the dipper type, 1.01 in the non‐dipper type and 1.21 in the extreme‐dipper type) was significantly higher in the extreme‐dipper type than in the other types (p<0.05, Figure ), but no significant difference in the HF component (2.12 in the dipper type, 2.10 in the non‐dipper type and 2.23 in the extreme‐dipper type) was noted between the three types.
Discussion
In this study, an abnormal nocturnal SBP fall pattern was observed in 35.6% (non‐dipper type: 20%, and extreme‐dipper type: 15.6%) of the healthy elderly subjects. However, the clinical significance of these abnormal diurnal BP variations shown in healthy elderly subjects has not been adequately evaluated. Also, in normotensives, there are a few studies regarding the relationship between diurnal BP variation and a risk of target‐organ damage Citation[20]. We classified elderly subjects into three groups according to the nocturnal SBP fall pattern (dippers, non‐dippers and extreme‐dippers) and compared the parameters of carotid atherosclerosis, endothelial and autonomic functions.
No difference was observed in biochemical determinations, resting office BP, or mean 24‐h BP between the three groups. Previous work in hypertensive patients has shown that silent cerebrovascular damage was significantly higher in non‐ and extreme‐dippers than in dippers Citation[2]. In a previous study, reverse‐dippers showed the highest incidence of strokes and had twice as many total cardiovascular events (cardiac and stroke events) as the other dipping groups Citation[21]. However, the reverse dipper type did not exist in this study. The incidence of SCI did not significantly differ between the three groups, and this finding was consistent with that of the previous study by Shinkawa et al. Citation[22] in a healthy population. Accordingly, there may be no association between the incidence of SCI and an abnormal diurnal BP variation pattern in healthy elderly subjects.
Carotid atherosclerosis is an important risk factor for the complication of hypertension. IMT has been proposed as a quantitative index of atherosclerosis in monitoring cardiovascular disease (CVD) progression. Previous studies have also shown that BP levels are a main determinant of the mean IMT, while the interaction of BP with aging and plasma lipids is more relevant for advanced IMT thickening such as plaques Citation[23], Citation[24]. Zakopoulos et al. Citation[12] reported that the rate of BP fluctuations is greater in hypertensive patients and is correlated independently with increased carotid artery IMT. However, in this study, no difference was observed in IMT thickness and the plaque score between the three groups. Hence, these carotid artery measures may not be parameters that influence abnormal diurnal BP variation patterns in healthy elderly subjects.
Endothelial dysfunction has been reported to be the initial step in atherosclerosis Citation[25]. Brachial artery FMD is a non‐invasive assessment method of endothelial function that differs from conventional methods such as the infusion of acetylcholine or other muscarinic receptor agonists Citation[26], Citation[27]. Also, brachial artery FMD has been reported to reflect early atherosclerosis more sensitively than the response to sublingual GTN, an endothelium‐independent dilator acting directly on vascular smooth muscle Citation[9]. In this study, %FMD in the subjects of the extreme‐dipper group was significantly lower than that of subjects in the dipper group. In addition, %GTN showed no significant difference between the three groups. It is possible that the extreme‐dipper phenomenon in healthy elderly subjects may be associated with endothelial dysfunction. Several investigators postulated that brachial artery FMD reflects earlier stages of atherosclerosis than carotid IMT Citation[28–30]. On this assumption, it is possible that brachial artery FMD has the capacity to reflect endothelial dysfunction associated with the extreme‐dipper type before morphological atherosclerotic progression in the carotid artery detected by carotid IMT. Additionally, our present study showed that the LF/HF in the subjects of the extreme‐dipper group was significantly higher than that in subjects of dipper and non‐dipper groups, suggesting the activation of sympathetic tone. Predominant sympathetic activity has been shown to be associated with abnormalities of hemodynamics, immune regulation and lipid metabolism Citation[31–33]. These chronic stress conditions in daily life are reportedly involved in the mechanism by which the enhancement of sympathetic modulation induces endothelial dysfunction, leading to a reduction in brachial artery FMD. Accordingly, it is possible that continuous sympathetic hyperactivity in daily life, resulting from cardiac autonomic system abnormalities in extreme‐dipper type subjects, might impair endothelial cells and lead to a decrease in brachial artery FMD.
Kario et al. Citation[2], Citation[34] recently identified that hypertensive extreme‐dippers, characterized by a marked nocturnal BP fall, showed an increased prevalence of SCI and advanced deep white matter ischemic lesions, raising the possibility that nocturnal cerebral hypoperfusion might be a risk factor for CVD. The pathogenic significance of “extreme dipping” might be an “artificial” excess reduction in BP at night beyond the lower limit of BP in the autoregulation of cerebral blood flow. It is also possible that greater BP variation in extreme‐dippers, even if they are normotensive, might induce endothelial dysfunction as a precipitating factor to the initial atherosclerotic target‐organ damage.
Cross‐sectional and prospective data have shown that non‐dippers have more target‐organ damage than dippers in hypertensive subjects Citation[5], Citation[35], Citation[36]. However, healthy elderly subjects with a non‐dipper circadian pattern in this study are not at a higher risk for cardiovascular and cerebrovascular complications than individuals with a dipper circadian rhythm. This finding may provide evidence that a higher BP at night in the non‐dippers is a critical prognostic value for the “non‐dipping” phenomenon. However, it is worth noting that the results might be controversial, partly because of the inability to reproduce correctly, over time, the classification of patients into dippers and non‐dippers Citation[37]. Moreover, the non‐dipping status has been frequently related to an increase in nocturnal activity and/or differences in the quality of sleep Citation[37], Citation[38]. Thus, the clinical significance of the dippers/non‐dippers/extreme‐dippers classification in the stratification of healthy elderly subjects with different levels of initial cardiovascular risk remains unclarified and needs further investigation.
The major limitation of this study was its small sample size. Furthermore, this study had a cross‐sectional design, so we could not demonstrate a causal relationship between diurnal BP variation, endothelial dysfunction and sympathovagal imbalance. Therefore, the findings of this study are only preliminary data, and further prospective studies with a large sample size are needed. Another limitation of this study is that the enrolled subjects were not strictly healthy (e.g. some of the extreme‐dippers actually showed systolic hypertension on mean ABPM as shown in Table ), because inhabitants with atherosclerotic risk factors or cardiovascular disease were excluded not by medical examination but based on self‐reported health information using a brochure. Large surveys with sampling of subjects by strict medical examination will be necessary in the future.
In conclusion, in healthy elderly subjects, the extreme‐dipper type may reflect a decrease in endothelial function, i.e. initial stage atherosclerosis, rather than the dipper‐type.
References
- Imai Y., Abe K., Munakata M., Sakuma H., Hashimoto J., Imai K., et al. Circadian blood pressure variations under different pathophysiological conditions. J Hypertens Suppl 1990; 8: S125–S132
- Kario K., Matsuo T., Kobayashi H., Imiya M., Matsuo M., Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension 1996; 27: 130–5
- Cuspidi C., Macca G., Sampieri L., Fusi V., Severgnini B., Michev I., et al. Target organ damage and non‐dipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. Hypertension 2001; 19: 1539–45
- Kobrin I., Oigman W., Kumar A., Ventura H. O., Messerli F. H., Frohlich E. D., et al. Diurnal variation of blood pressure in elderly patients with essential hypertension. J Am Geriatr Soc 1984; 2: 896–9
- Shimada K., Kawamoto A., Matsubayashi K., Nishinaga A., Kimura S., Ozawa T. Diurnal blood pressure variation and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens 1992; 10: 875–8
- Shimada K., Kario K. Altered circadian rhythm of blood pressure and cerebrovascular damage. Blood Press Monit 1997; 2: 333–8
- Imai Y., Abe K., Sasaki S., Minami N., Nihei M., Munakata M., et al. Influence of age on the nocturnal fall of blood pressure and its modulation by long‐acting calcium antagonists. Clin Exp Hypertens 1990; A12: 1077–94
- Nabel E. G., Selwyn A. P., Ganz P. Large coronary arteries in humans are responsive to changing blood flow: An endothelium‐dependent mechanism that fails in patients with atherosclerosis. J Am Coll Cardiol 1990; 16: 349–56
- Neunteufl T., Katzenschlager R., Hassan A., Klaar U., Schwarzacher S., Glogar D., et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis 1997; 129: 111–8
- O'Leary D. H., Polak J. F., Kronmal R. A., Savage P. J., Borhani N. O., Kittner S. J., et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke 1996; 27: 224–31
- Lakka T. A., Salonen R., Kaplan G. A., Salonen J. T. Blood pressure and the progression of carotid atherosclerosis in middle‐aged men. Hypertension 1999; 34: 51–6
- Zakopoulos N. A., Tsivgoulis G., Barlas G., Papamichael C., Spengos K., Manios E., et al. Time rate of blood pressure variation is associated with increased common carotid artery intima‐media thickness. Hypertension 2005; 45: 505–12
- Kario K., Eguchi K., Nakagawa Y., Motai K., Shimada K. Relationship between extreme‐dippers and orthostatic hypertension in elderly hypertensive patients. Hypertension 1998; 31: 77–82
- Kario K., Motai K., Mitsuhashi T., Suzuki T., Nakagawa Y., Ikeda U., et al. Autonomic nervous dysfunction in elderly hypertensive patients with abnormal diurnal blood pressure variation: Relation to silent cerebrovascular disease. Hypertension 1997; 30: 1504–10
- Association for the Advancement of Medical Instrumentation. American national standard. Electronic or automated sphygmomanometers. ANSI/AAMI SP 10‐1992. AAMI, Arlington, VA 1993
- O'Brien E., Coats A., Owens P., Petrie J., Padfield P. L., Littler W. A., et al. Use and interpretation blood pressure monitoring: Recommendations of the British Hypertension Society. BMJ 2000; 320: 1128–34
- Fazekas F., Kleinert R., Offenbacher H., Payer F., Schmidt R., Kleinert G., et al. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol 1991; 12: 915–21
- Handa N., Matsumoto M., Maeda H., Hougaku H., Ogawa S., Fukunaga R., et al. Ultrasonic evaluation of early carotid atherosclerosis. Stroke 1990; 21: 1567–72
- Sakakibara M., Takeuchi S., Hayano J. Effect of relaxation training on cardiac parasympathetic tone. Psychophysiology 1994; 31: 223–8
- Hoshide S., Kario K., Hoshide Y., Umeda Y., Hashimoto T., Kunii O., et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community‐dwelling normotensives. Am J Hypertens 2003; 16: 434–8
- Verdecchia P., Schillaci G., Borgioni C., Ciucci A., Gattobigio R., Guerrieri M., et al. Altered circadian blood pressure profile and prognosis. Blood Press Monit 1997; 2: 347–52
- Shinkawa A., Ueda K., Kiyohara Y., Kato I., Sueishi K., Tsuneyoshi M., et al. Silent cerebral infarction in a community‐based autopsy series in Japan. The Hisayama Study. Stroke 1995; 26: 380–5
- Pauletto P., Palatini P., Da Ros S., Pagliara V., Santipolo N., Baccillieri S., et al. Factors underlying the increase in carotid intima‐media thickness in borderline hypertensives. Arterioscler Thromb Vasc Biol 1999; 19: 1231–7
- Psaty B. M., Furberg C. D., Kuller L. H., Borhani N. O., Rautaharju P. M., O'Leary D. H., et al. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the cardiovascular health study. JAMA 1993; 269: 1287–91
- Ross R. Atherosclerosis – An inflammatory disease. N Engl J Med 1999; 340: 115–26
- Lind L., Sarabi M., Millgard J. Methodological aspects of the evaluation of endothelium‐dependent vasodilatation in the human forearm. Clin Physiol 1998; 18: 81–7
- Taddel S., Virdis A., Mattei P., Salvetti A. Vasodilatation to acetylcholine in primary and secondary forms of human hypertension. Hypretension 1993; 21: 929–33
- Anderson T. J., Roberts A. C., Hildebrand K., Conradson H. E., Jones C., Bridge P., et al. The fate of endothelial function testing: Rationale and design of the Firefighters And Their Endothelium (FATE) study. Can J Cardiol 2003; 19: 61–6
- Hashimoto M., Eto M., Akishita M., Kozaki K., Ako J., Iijima K., et al. Correlation between flow‐mediated vasodilatation of the brachial artery and intima‐media thickness in the carotid artery in men. Arterioscler Thromb Vasc Biol 1999; 19: 2795–800
- Lekakis J. P., Papamichael C. M., Vemmos C. N., Voutsas A. A., Stamatelopoulos S. F., Moulopoulos S. D. Peripheral vascular endothelial dysfunction in patients with angina pectoris and normal coronary arteriograms. J Am Coll Cardiol 1998; 31: 541–6
- Dzau V. J., Sacks F. M. Regulation of lipoprotein metabolism by adrenergic mechanisms. J Cardiovasc Pharmacol 1987; 10(Suppl 9)S2–S6
- Elenkov I. J., Wilder R. L., Chrousos G. P., Vizi E. S. The sympathetic nerve – An integrative interface between two supersystems: The brain and the immune system. Pharmacol Rev 2000; 52: 595–638
- Lucini D., Norbiato G., Clerici M., Pagani M. Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension 2002; 39: 184–8
- Kario K., Pickering T. G., Matuo T., Hoshide S., Schwartz J. E., Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38: 852–7
- Izzedine H., Launay‐Vacher V., Deray G. Abnormal blood pressure circadian rhythm: A target organ damage?. Int J Cardiol 2006; 107: 343–9
- Verdecchia P., Porcellati C., Schillaci G., Borgioni C., Ciucci A., Battistelli M., et al. Ambulatory blood pressure: An independent predictor of prognosis in essential hypertension. Hypertension 1994; 24: 793–801
- Hermida R. C., Calvo C., Ayala D. E., Mojón A., López J. E. Relationship between physical activity and blood pressure in dipper and non‐dipper hypertensive patients. J Hypertens 2002; 20: 1097–104
- Portaluppi F., Cortelli P., Provini F., Plazzi G., Manfredini R., Lugaresi E. Alterations of sleep and circadian blood pressure profile. Blood Press Monit 1997; 2: 301–13