Abstract
Tumor necrosis factor alpha (TNF-α) inhibitors are increasingly being used in inflammatory rheumatic diseases (IRD). The risk of cardiovascular disease is elevated in patients with IRD and TNF-α inhibitors reduce this risk. We assessed whether the beneficial effect of TNF-α inhibitors on cardiovascular risk is mediated by blood pressure reduction. We measured blood pressure levels with 24-h ambulatory blood pressure measurements device in patients with IRD before and 3 months after treatment with TNF-α inhibitors. The study population consisted of 15 subjects (6 men; mean age 45.9 ± 14.1 years). Most patients had either rheumatoid arthritis or psoriatic arthritis and adalimumab was the most common TNF-α inhibitor used. Mean 24-h systolic and diastolic blood pressure levels remained the same after treatment (121 ± 12/66 ± 7 before and 123 ± 11/67 ± 10 mm Hg after; p = 0.88 and 0.66, respectively). The study demonstrates that TNF-α inhibitors have no effect on blood pressure levels.
Introduction
Patients with inflammatory rheumatic diseases (IRD) carry a high risk for cardiovascular (CV) diseases. This risk remains high after adjustment for traditional CV risk factors, and is mainly attributed to accelerated atherosclerosis. This excess risk has been shown mainly for rheumatoid arthritis (RA),[Citation1–3] but has also been demonstrated in several other rheumatic diseases, such as systemic lupus erythematosus (SLE),[Citation4,Citation5] psoriatic arthritis (PsA) [Citation6] and ankylosing spondylitis (AS).[Citation7,Citation8] Tumor necrosis factor alpha (TNF-α) is an inflammatory cytokine produced by macrophages which is involved in the pathogenesis of several rheumatic diseases.[Citation9,Citation10] Serum TNF-α is elevated in hypertensive patients,[Citation11] and a correlation between blood pressure (BP) and serum TNF-α levels has been reported.[Citation12] Inhibitors of TNF-α are being used increasingly for the treatment of IRD.[Citation13–20] There is accumulating evidence that these drugs reduce CV risk among patients with IRD.[Citation21–25] The effect of TNF-α inhibitors on BP is not well established. In patients with RA, the TNF-α receptor blocker etanercept has been found to reduce left ventricular mass index but with no change in BP.[Citation26] Among patients with recent-onset RA, the TNF-α monoclonal antibody infliximab decreased BP irrespective of the reduction in disease activity.[Citation27] In another recent study which assessed BP response by 24-h ambulatory blood pressure monitoring (ABPM) infliximab reduced BP significantly in patients with RA, particularly during daytime.[Citation28] Most studies which evaluated the effect of TNF-α inhibitors on BP were done in patients with RA and did not use 24-h ABPM. We therefore evaluated the effect of treatment with TNF-α inhibitors on BP, assessed by 24-h ABPM, in various IRD.
Materials and methods
Study population
We included subjects examined in the rheumatology clinic between June 2013 and May 2015. Hypertensive patients were recruited only if they were treated with the same drug regimen at least 3 months before the study and their BP levels were well controlled. Twenty three subjects who were intended to initiate treatment with TNF-α inhibitors agreed to perform a 24-h ABPM before and after treatment. Of this group, three patients were excluded because their 24-h ABPM showed elevated BP levels which required BP lowering drugs. Four patients refused to perform a second 24-h ABPM and one additional patient died shortly after he performed the first 24-h ABPM. Thus, eventually 15 patients constituted our study group.
Study design
Blood pressure levels were assessed with 24-h ABPM before and 3 months after initiation of TNF-α inhibitors. During the follow-up, non biological disease-modifying anti-rheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAID) and BP lowering drugs remained unchanged.
The research protocol was approved by the local Helsinki committee and all patients gave their consent to participate in the study.
Twenty four-hour ambulatory blood pressure monitoring
24-h ABPM was performed by the Oscar2 24-HR ABP (SunTech Medical Inc., Morrisville, NC). The monitor was mounted on the left arm. A mercury sphygmomanometer was initially attached to the monitor through a Y connector to ensure conformity between the two modes of measurements. BP was measured every 20 min during the day and evening (from 6:00 to 22:00) and every 30 min at night (from 22:00 to 6:00). Measurements were not acceptable when pulse pressure was <20 mmHg, diastolic BP was <40 mmHg and systolic BP was <80 mmHg or when a single value differed greatly from the preceding and subsequent values. An acceptable 24-h BPM recording for our study should have had at least 50 acceptable measurements. The average systolic and diastolic BP levels were calculated for 24 h and separately for day and night. Systolic BP load was defined as percentage of systolic readings above 140 mmHg during the day and above 120 mmHg during night time. Diastolic BP load was defined as percentage of diastolic readings above 90 mmHg during the day and above 80 mmHg during the night.
Statistical analysis
Data were presented as mean and standard deviation for continuous variables and as frequency and percentage for categorical variables. The differences between values pre- and post-treatment with TNF-α blockers were compared by independent t-tests. A p value of <0.05 was considered significant.
Results
Patients’ characteristics
The study population consisted of 15 subjects (6 men; mean age 45.9 ± 14.1 years). Most patients had either RA or PsA (). Adalimumab was the most common TNF-α inhibitor used (n = 11). Two patients were prescribed etanercept and two patients were prescribed infliximab. Several patients were treated with non-biological DMARDs, or NSAID (). Two patients had hypertension and were treated with BP lowering drugs.
Table 1. Baseline characteristics of study population.
Changes in BP following treatment with TNF-α inhibitors
Mean baseline BP levels were normal (). Changes in BP and other parameters are presented in . The 24-h, awake and night systolic and diastolic BP did not change post treatment with TNF-α inhibitors (, ). The 24-h, awake and night systolic and diastolic BP load was the same before and after treatment ().
Figure 1. Hourly means of ambulatory systolic BP and diastolic BP levels before and after 3 months of treatment with TNF-α inhibitors.
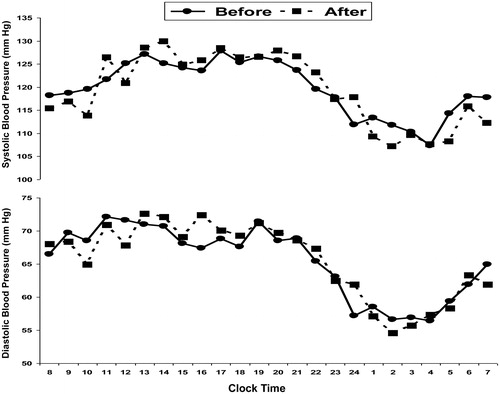
Table 2. Ambulatory blood pressure values pre- and post-TNF-α inhibitor treatment.
Discussion
In the present study, we showed by employing 24-h ABPM that treatment with TNF-α inhibitors does not affect BP levels. To our knowledge, this is the first study that assessed the effect of prolonged treatment with TNF-α inhibitors on BP levels using 24-h ABPM. The use of TNF-α inhibitors in recent years has revolutionized the treatment of several inflammatory rheumatic diseases, mainly RA, PsA and AS.[Citation13–20] Given the high CV risk associated with IRD,[Citation1–8] the effect of DMARDs on CV risk is of great significance. There is accumulating evidence that TNF-α inhibitors reduce CV risk among patient with IRD.[Citation21–25] This beneficial effect may be mediated through several mechanisms such as flow mediated vasodilation,[Citation29] reduction of aortic inflammation and stiffness,[Citation30] improvement in endothelial function [Citation31] and improvement in insulin resistance.[Citation32] Additional possible mechanism for lowering CV risk with DMARDs may be through lowering BP. Drugs used for the treatment of IRD have different effects on BP levels. Continuous use of NSAIDs has been found to increase BP.[Citation33] In patients with RA, leflunomide – a non-biological DMARD and cyclosporine increased BP.[Citation34,Citation35] Conversely, methotrexate [Citation36] and mycophenolate mofetil [Citation37] were reported to lower BP. The effect of TNF-α inhibitors on BP is controversial. TNF-α inhibitors have been reported to reduce BP in mice.[Citation38] Several studies have shown that TNF-α inhibitors have a beneficial effect on arterial stiffness, but do not reduce BP.[Citation39,Citation40] Daien et al. have shown that etanercept reduces left ventricular mass with no effect on BP in patients with RA.[Citation26] Klarenbeek and colleagues have shown in RA patients that lowering disease activity with various DMARDs is associated with reduction in BP levels. Treatment with infliximab was associated with a larger decrease in BP irrespective of the change in disease activity.[Citation27] In most studies, BP was assessed by a single measurement without a standardized protocol. A single BP measurement may not reflect the true BP levels, and 24-h ABPM more accurately reflects BP levels.[Citation41–45] We studied a relatively small group of patients with various IRD, but we assessed the effect of TNF-α inhibitors on BP by 24-h ABPM. Unlike Klarenbeek et al. [Citation27] we did not observe any effect of TNF-α inhibitors on BP. Use of DMARDs improves disease activity and thereby enables discontinuation of NSAIDs. Since NSAIDs increase BP levels, the observed effect of DMARDs may be related to discontinuation of NSAID rather than a direct effect. In previous studies, the use of NSAIDs during the study was not reported. In our study only two patients were treated with NSAIDs occasionally at the beginning of the trial and continued to use them in the same frequency and dose. Yoshida et al. used 24-h ABPM to assess the influence of Infliximab on BP levels in patients with RA.[Citation28] They found that infliximab significantly reduced the 24-h systolic BP levels, particularly during the morning. The decrease in BP was associated with a significant fall in plasma levels of norepinephrine and plasma renin activity. Moreover, the reduction in morning systolic BP levels correlated with the reduction of norepinephrine levels, but not with inflammatory markers related to RA. Their findings suggest that infliximab reduces BP through suppression of the sympathetic nervous system. However, it should be noted that infliximab improved clinical symptoms, as reflected by the marked reduction of disease activity score, and it is still possible that the improvement in inflammatory markers, was partially responsible for the reduction in sympathetic activity and BP levels. Therefore, a direct effect of TNF-α inhibitors on norepinephrine and BP levels cannot be determined conclusively. The study of Yoshida et al. included only patients with RA treated with Infliximab, and therefore the conclusions of the study may not be applicable to the diverse patients with IRD treated with various types of TNF-α inhibitors. In addition, the patients were followed for a short period, and the influence of long-term administration of TNF-α inhibitors was not investigated. Our study included patients with various IRD treated with different TNF-α inhibitors agents. In our study, we evaluated patients after a period of 3 months, which is a sufficient time to assess the influence of TNF-α inhibitors on BP levels. Unlike Yoshida et al. [Citation28] we did not observe a difference in 24-h systolic and diastolic BP levels after treatment with TNF-α inhibitors.
Our study has several limitations. First, it is a small single-arm trial, without a control group, and without a comparison to other DMARDs. However, since we used 24-h ABPM before and after treatment, we were able to see that TNF-α inhibitors have no effect on BP levels. Second, we studied patients with various IRD and therefore could not assess properly improvement in clinical parameters. However, since we did not observe a decrease in BP levels, the degree of clinical improvement is less relevant.
In conclusion, in this study we showed that treatment with TNF-α inhibitors does affect BP levels, as assessed by 24-h ABPM. According to our results, the favorable effects of TNF-α inhibitors on CV risk are not mediated through BP reduction.
Disclosure statement
All the authors declare no financial or other relationships that might lead to a conflict of interest.
References
- Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697.
- del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745.
- Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348–353.
- Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337.
- Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol. 1997;145:408–415.
- Gladman DD, Ang M, Su L, et al. Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis. 2009;68:1131–1135.
- Peters MJ, van Eijk IC, Smulders YM, et al. Signs of accelerated preclinical atherosclerosis in patients with ankylosing spondylitis. J Rheumatol. 2010;37:161–166.
- Peters MJ, van der Horst-Bruinsma IE, Dijkmans BA, et al. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheu. 2004;34:585–592.
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916.
- Rahman P, Siannis F, Butt C, et al. TNFalpha polymorphisms and risk of psoriatic arthritis. Ann Rheum. Dis. 2006;65:919–923.
- Dorffel Y, Latsch C, Stuhlmuller B, et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117.
- Zinman B, Hanley AJ, Harris SB, et al. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocr Metab. 1999;84:272–278.
- Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563.
- Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–681.
- Lethaby A, Lopez-Olivo MA. Maxwell, et al. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD004525.
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147.
- Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–486.
- Callhoff J, Sieper J, Weiss A, et al. Efficacy of TNFα blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis. 2015;74:1241–1248.
- McLeod C, Bagust A, Boland A, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–158.
- Sterry W, Ortonne JP, Kirkham B, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ. 2010;340:c147.
- Jacobsson LT, Turesson C, Gulfe A, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1213–1218.
- Dixon WG, Watson KD, Lunt M, et al. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British society for rheumatology biologics register. Arthritis Rheum. 2007;56:2905–2912.
- Greenberg JD, Kremer JM, Curtis JR, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2010;70:576–82.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197–204.
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244–1250.
- Daien CI, Fesler P, du Cailar G, et al. Etanercept normalises left ventricular mass in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:881–887.
- Klarenbeek NB, van der Kooij SM, Huizinga TJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: a post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342–1345.
- Yoshida S, Takeuchi T, Kotani T, et al. Infliximab, a TNF-α inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens. 2014;28:165–169.
- Szekanecz Z, Kerekes G, Soltesz P. Vascular effects of biologic agents in RA and spondyloarthropathies. Nat Rev Rheumatol. 2009;5:677–684.
- Maki-Petaja KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126:2473–2480.
- Spinelli FR, Metere A, Barbati C, et al. Effect of therapeutic inhibition of TNF on circulating endothelial progenitor cells in patients with rheumatoid arthritis. Mediators Inflamm. 2013;2013:537539.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Morrison A, Ramey DR. van Adelsberg, et al. Systematic review of trials of the effect of continued use of oral non-selective NSAIDs on blood pressure and hypertension. Curr Med Res Opin. 2007;23:2395–2404.
- Rozman B, Praprotnik S, Logar D, et al. Leflunomide and hypertension. Ann Rheum Dis. 2002;61:567–569.
- Kvien TK, Zeidler HK, Hannonen P, et al. Long term efficacy and safety of cyclosporin versus parenteral gold in early rheumatoid arthritis: a three year study of radiographic progression, renal function, and arterial hypertension. Ann Rheum Dis. 2002;61:511–516.
- Choi HK, Hernan MA, Seeger JD, et al. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177.
- Herrera J, Ferrebuz A, MacGregor EG, et al. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225.
- Elmarakby AA, Quigley JE, Pollock DM, et al. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–562.
- Angel K, Provan SA, Fagerhol MK, et al. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. 2012;25:644–650.
- Dulai R, Perry M, Twycross-Lewis R, et al. The effect of tumor necrosis factor-alpha antagonists on arterial stiffness in rheumatoid arthritis: a literature review. Semin Arthritis Rheum. 2012;42:1–8.
- Grossman E. Ambulatory blood pressure monitoring in the diagnosis and management of hypertension. Diabetes Care. 2013;36:S307–S311.
- Hara A, Tanaka K, Ohkubo T, et al. Ambulatory versus home versus clinic blood pressure: the association with subclinical cerebrovascular diseases: the Ohasama study. Hypertension. 2012;59:22–28.
- Hansen TW, Kikuya M, Thijs L, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25: 1554–1564.
- Ohkubo T, Hozawa A, Nagai K, et al. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens. 2000;18:847–854.
- Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic hypertension in Europe trial investigators. JAMA. 1999;282:539–546.
