Abstract
Objective: To explore the effects of baroreflex activation therapy (BAT) on hypertension in patients with treatment resistant or refractory hypertension.
Methods: This investigator-initiated randomized, double-blind, 1:1 parallel-design clinical trial will include 100 patients with refractory hypertension from 6 tertiary referral hypertension centers in the Nordic countries. A Barostim Neo System will be implanted and after 1 month patients will be randomized to either BAT for 16 months or continuous pharmacotherapy (BAT off) for 8 months followed by BAT for 8 months. A second randomization will take place after 16 months to BAT or BAT off for 3 months. Eligible patients have a daytime systolic ambulatory blood pressure (ABPM) of ≥145 mm Hg, and/or a daytime diastolic ABPM of ≥95 mm Hg after witnessed drug intake (including ≥3 antihypertensive drugs, preferably including a diuretic).
Results: The primary end point is the reduction in 24-hour systolic ABPM by BAT at 8 months, as compared to pharmacotherapy. Secondary and tertiary endpoints are effects of BAT on home and office blood pressures, measures of indices of cardiac and vascular structure and function during follow-up, and safety.
Conclusions: This academic initiative will increase the understanding of mechanisms and role of BAT in the refractory hypertension.
Introduction
Refractory hypertension (RH) is often defined as uncontrolled blood pressure (BP) despite therapy with ≥3 antihypertensive agents from at least 3 different classes including a diuretic. Some 10% to 15% of hypertensive subjects [Citation1–3], have RH, and there is an association to advanced age, obesity, diabetes mellitus, sleep apnea, and chronic kidney disease [Citation4–6]. These patients are at a clearly increased risk for end organ damage and mortality. Arterial hypertension is a multifactorial disease including genetic, lifestyle, dietary, metabolic, and sympato-adrenal factors. However, current treatment modalities have not been optimal in targeting compensatory functional changes in cardiovascular control by the autonomic nervous system, and new strategies have been warranted.
Baroreflex activation therapy (BAT) is a treatment option for patients with RH modulating the autonomic nervous system to restore sympatho-vagal balance. Electrical stimulation of the carotid baroreceptors (pressure sensors) results in centrally mediated reduction of sympathetic nervous outflow and increased parasympathetic activity [Citation7,Citation8]. Carotid baroreceptor activation was originally implemented via pulsatile electrical stimulation of the baroreceptors in dogs [Citation9], showing the potential of substantial BP reduction through prolonged baroreflex activation. These findings resulted in the development of a device for humans through which pulsatile baroreceptor stimulation aimed to achieve long-term BP control.
In the first randomized controlled trial engaging BAT in humans, patients with refractory hypertension underwent bilateral implantation of the Rheos device [Citation10]. At the 6-month follow-up, no difference was observed between the groups in the primary end point of the proportion of patients in whom systolic BP decreased by ≥10 mmHg. However, the number of patients achieving a BP of ≤140 mmHg at 6 months was higher in those treated with BAT. The pre-specified endpoints for safety were not achieved. Notably, in a post hoc analysis of the study, it was shown that after 6 months of BAT, the reduction in systolic BP was the greatest with unilateral right-sided carotid stimulation, as compared to unilateral left-sided or bilateral stimulation [Citation11].
A second-generation minimally invasive unilateral BAT system (Barostim Neo) has now been developed to address these limitations. One single-arm, open-label study of patients with RH treated with BAT have reported preliminary convincing results [Citation12]. Furthermore, it has recently been shown that BAT decreases ambulatory BP and arterial stiffness in patients with RH [Citation13,Citation14]. By using this second-generation device, the safety profile was comparable to a cardiac pacemaker [Citation12]. It has been suggested that the invasiveness of this methodology is ethically justified by the very high cardiovascular risk that characterizes the refractory hypertension condition [Citation15]. Thus, in the European Society of Hypertension/European Society of Cardiology (ESH/ESC) Guidelines for the management of arterial hypertension, carotid baroreceptor stimulation is mentioned as one of the options to treat RH [Citation15].
Based on these data the aim of this randomized controlled, double-blind study is to examine the effect of BAT compared to continuous pharmacotherapy on BP, sympathetic nerve function, and arterial and cardiac function and structure using non-invasive high technology methodology.
Methods
Study design
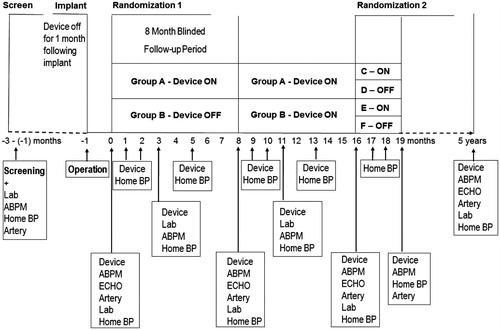
The design of this multinational randomized, double-blind, parallel-design clinical trial to assess the efficacy and safety of the Barostim Neo System in patients with RH is shown in . The study will include 100 patients with RH after informed written consent. The patients will be studied at well recognized tertiary referral Hypertension Centres at University Hospitals in the Nordic Countries (Helsinki, Malmö, Odense, Aarhus, Oslo, Gothenburg, other centres pending, see affiliations for each centre).
Figure 1. ABPM: 24-h ambulatory blood pressure measurement after witnessed drug intake; ECHO: echocardiography, Artery: measurements of arterial and autonomic function; Lab: blood and urine samples; Home BP: home blood pressure measurements (2 measurements every morning and evening for 1 week); Device: Titration (according to Home BP) and check of the BAT device.
Study objectives
The overall aim of this study is to explore the long-term effect of BAT in patients with RH.
The primary end point is:
The reduction in 24-hour systolic ambulatory BP at 8 months of follow-up by BAT, as compared to continuous pharmacotherapy.
Secondary end points are:
The response to turning off BAT for up to 3 months after 8 months of placebo treatment and 8 months of BAT or after 16 months of BAT, respectively, in 24-hour systolic ambulatory BP.
The reduction of BAT on home and office BP at 8 months of follow-up, as compared to continuous pharmacotherapy.
The response to turning off BAT for up to 3 months after 8 months of placebo and 8 months of BAT or after 16 months of BAT, respectively, in home/office BP within 3 months.
The effects of BAT on autonomic function measured as baroreflex sensitivity and heart rate variability) at 8 months of follow-up, and after turning off BAT for up to 3 months after 8 months of placebo and 8 months of BAT or after 16 months of BAT, respectively.
Tertiary end points are:
To find biomarkers at baseline (vascular, blood, urinary) in order to identify those patients who respond to BAT, by establishment of a biobank.
To elucidate the effect of BAT on cardiac structure and function by echocardiography during follow-up compared to continuous pharmacotherapy.
To assess whether BAT affects arterial stiffness, endothelial function, and intima-media thickness of the common carotid artery during follow-up, as compared to continuous pharmacotherapy.
To study the effect of BAT on BP and cardiovascular function and structure during long-term follow-up.
To study the effect of BAT on safety, including cardiovascular morbidity and mortality during long-term follow-up.
To assess the cost effectiveness of BAT, as compared to usual management of patients with RH.
To explore the effect of BAT on quality of life (QALY).
Eligibility
Inclusion criteria
Eligible patients are between 18 and 70 years old and have a daytime systolic ambulatory blood pressure of 145 mmHg or higher, and/or a daytime diastolic ambulatory blood pressure of 95 mmHg or higher, after witnessed intake of antihypertensive treatment (including at least 3 antihypertensive drugs preferably including a diuretic), with no changes in medication for a minimum of 4 weeks prior to enrolment (). A detailed description on office, home and ambulatory BP is given in the Supplement 1.
Table 1. Key eligibility criteria of the Nordic BAT study.
Exclusion criteria
Patients with secondary causes of hypertension will be excluded. Further exclusion criteria are renal insufficiency with an estimated glomerular filtration rate ≤30 ml/min/1.73 m2 (using the CKD-EPI formula) [Citation16], untreated sleep apnoea, pregnancy, type 1 diabetes, alcohol or substance abuse or psychiatric illnesses, other heart rhythm than sinus (well controlled atrial fibrillation is not a contraindication), uncontrolled systolic heart failure and/or a left ventricular ejection fraction of less than 40% by echocardiography), aortic stenosis with a mean gradient more than 25 mmHg by echocardiography). Myocardial infarction, hypertensive crisis, symptomatic orthostatic hypotension, unstable angina pectoris, syncope, or a cerebral vascular accident within the 3 months before enrolment will also be exclusion criteria. Furthermore, known or suspected baroreflex failure or autonomic neuropathy, carotid artery stenosis (>50%) as well as prior surgery, radiation, or endovascular stent placement in the carotid sinus region on both sides, as well as any complication that is a risk to the planned surgery are exclusion criteria ().
Screening and randomization
All screened patients eligible for the study who give their written informed consent to participate will be included in the study and have a Barostim Neo System implanted, and be subsequently randomized to either BAT (n = 50) for 16 months or continuous pharmacotherapy (BAT off) for 8 months, followed by the BAT for 8 months (n = 50) (, Randomization 1). Blood and urine samples will be collected, and measures of vascular and cardiac function and structure, as well as autonomic function will be performed at baseline after randomization, and after the 8 and 16 months. Thus, the time points for measurements will be 0 (after the BAT procedure), 3 months, 8 months, 11 months, 16 months, and 5 years after the BAT ().
Central remote randomization will take place at Karolinska Institutet by centre in blocks of 4, and will be stratified for diabetes (defined as treatment with any antidiabetic drugs), providing the centre their unique study patient ID after device implantation a blinded code for treatment allocation, only visible for the technician involved in the titration of the BAT device.
The titration of the device will be performed by a technician similarly in both groups as indicated in by a protocol-defined set of parameters shared across centers. In general, the therapy parameters (pulse amplitude [1–20 mA], pulse width [15–500 ms] and frequency 10–100 pulses/s) will be adjusted to aim a maximal controlled BP response during the testing up to the sensation limit in both treatment arms where after the device will be turned off (blinded to the investigators) in the BAT off group.
After 8 months of placebo therapy followed by 8 months of BAT or 16 months of BAT, respectively, all patients with BAT on are randomized (, Randomization 2) to either BAT on or off for 3 months and home BP after 1, 2 and 3 months and 24-hour ABPM at 3 months, or at early reactivation due to one of the following four safety situations: 1) Increase in home BP > 20%, 2) increase in home BP > 5% and home BP > 200/120 mmHg, 3) home BP > 220/130 mmHg, or 3) home BP > 180/110 mmHg with new symptoms of hypertensive encephalopathy.
After 19 months patients will be followed regularly (every 6–12 months) at the Hypertension Centres until the end of the follow-up period at 5 years. Thereafter, the BAT treatment continues at the treating physician’s discretion.
Ethics and safety
The study will be approved of by all relevant authorities at all participating centres before study start. All participating patients will be provided detailed information about the study and will have to give their informed written consent. They have the right to cancel their participation in the study at any time without consequences. In order to guarantee the ethical aspects a steering committee is founded to follow the patient investigations as well as data handling throughout the study.
The second-generation minimally invasive BAT system (Barostim Neo) showed a markedly better safety profile compared to the first generation Rheos system as number of patients who suffered from complications after the procedure decreased from 25% to 3% [Citation12]. However, the methodology involves an inherent risk of complications due to the invasive nature of the procedure, and long-term follow-up data (>12 months) are still lacking. In our study, only vascular surgeons with great experience of carotid surgery will perform the procedure. The procedure is minimally invasive compared to e.g. carotid endarterectomy for stroke prevention, and although implantation of a previous bilateral device has caused more cranial nerve injuries than expected, implantation of the second-generation device should overcome this problem. The measurements of arterial and cardiac function and structure are non-invasive and used routinely at the research centres involved.
The target BP in all patients during the study will follow the guidelines by ESH/ESC [Citation15]. By including only patients with clearly elevated BP despite maximal tolerated antihypertensive treatment at baseline, we hope in general to maintain baseline antihypertensive treatment throughout the study. However, if deemed necessary, the withdrawal of BP medication will mainly be done in the following order, at the treating physician’s discretion; vasodilators, central acting agonists, alpha blockers, mineralocorticoid receptor blockers, beta blockers, diuretics, calcium channel blockers, and lastly RAAS blockers.
Definition of events
CVD events are defined as an incident myocardial infarction (MI), a coronary artery procedure (by-pass surgery or angioplasty), stroke (ischemic or hemorrhagic), or a peripheral artery procedure (by-pass surgery or angioplasty), which will be verified on the basis of ICD discharge codes specifying the events. Coronary artery disease (CAD) is defined as a history of MI or a coronary artery procedure. Limb amputations will further be ascertained on the basis of ICD discharge codes specifying amputation, regardless of the presence or absence of documented peripheral vascular disease (PVD). Furthermore, hospitalization for heart failure, sudden death, aortic, carotid and/or iliac artery dissection, aneurysm, stenosis, thrombosis or vascular procedures (by-pass or angioplasty) are considered as CVD events.
Sample size and statistical considerations
A study population of 100 patients have an 80% power to detect a 6 mm Hg (SD 15 mmHg) difference between the groups at the two-sided 0.05 significance level. An interim analysis will be done after studying the first 50 patients.
Based on the anticipation that most covariates will be well-balanced between randomized groups, the primary efficacy analysis of the primary end point will be conducted according to intention to treat with no covariate adjustment using a log-rank test comparing 24-hour systolic ambulatory BP at 8 months of follow-up in BAT group compared to continuous pharmacotherapy [Citation17].
Pre-specified secondary analyses of the secondary and tertiary end points include comparisons of outcomes in the 2 treatment arms after covariate adjustment for baseline age, gender, baseline BP, eGFR, body mass index, and diabetes as covariates in a Cox proportional hazards model.
Baseline data and demographics of randomized population
All of the six patients included in the study so far, were men. The mean (±SD) age was 58 ± 10 years, and body mass index 30.3 ± 3.4 kg/m2. Two of the patients had type 2 diabetes, both were on oral antidiabetic medication, whereas none had insulin treatment. One of the patients was a current smoker and one a former smoker. Three patients had cardiovascular complications (stroke). The plasma mean (SD) creatinine was 89.7 ± 13.3 μmol/l, estimated glomerular filtration rate 81.3 ± 15.0 ml/min/1.73 m2, and urine albumin-to-creatinine ratio median (IQR) 7.3 (1–15) mg/mmol.
The mean office SBP was 174 ± 24 mmHg, DBP 106 ± 14 mmHg, HR 77 ± 17/min. The 24-hour ABPM values, after witnessed intake of antihypertensive medication, were as follows; SBP was 156 ± 10 mmHg, DBP 100 ± 5 mmHg, HR 78 ± 14/min. Furthermore, during the day SBP was 159 ± 10 mmHg, DBP 102 ± 5 mmHg, HR 78 ± 12/min, and during the night SBP was 146 ± 14 mmHg, DBP 92 ± 8 mmHg, HR 71 ± 14/min.
The number of antihypertensive drugs were median (range) 5 (4-8). 4 patients were on aldosterone blockers, 5 beta blockers, 6 calcium channel blockers, 6 diuretics, 6 ACEi or ATII receptor blockers, and 2 on other antihypertensive compounds.
Implantation procedure
BAT will be performed using the Barostim neo system, a second-generation system consisting of a pulse generator and a lead similar to a regular pacemaker system [Citation12]. It will be controlled with a laptop computer-based programming system via radiofrequency telemetry. The pulse generator is to be implanted unilaterally in the pectoral region ipsilateral to the stimulated carotid sinus. The electrode portion of the lead consists of a single platinum-iridium disc coated with iridium oxide attached concentrically to a circular insulative backer that is directly sutured to the carotid sinus via a small incision (ca 5 cm) placed over the carotid bifurcation that is first identified with ultrasound. Before final attachment of the electrode, optimal positioning is ensured with test stimulations and the site of attachment is recorded. A recorded decrease of at least 10 mmHg compared to baseline in BP is considered a true change caused by the baroreceptor stimulation. The miniaturized electrode and unilateral system design provide a minimally invasive implant procedure and as exploration of the external carotid artery or higher portion of the internal carotid artery is avoided the risk of cranial nerve damage should be minimal. In order to minimize the possible effect on the complex and variable nerve structure of the carotid sinus, damage to all visible nerve fibres is avoided upon opening the carotid sheath [Citation18]. Finally a subclavicular pouch is prepared and the wires are tunnelled carefully avoiding tension during neck movements.
The implantation procedures are performed under general anaesthesia. However, as conventional anaesthetic agents either weaken or eradicate the baroreceptor reflex, anaesthesia is accomplished with a combination of a sedative and a short-acting opioid during preparation and test stimulation [Citation19]. Sufficient depth of anaesthesia is ensured with entropy monitoring.
The BAT will be initiated 4 weeks after implantation. Programmed parameters (pulse amplitude, width, and frequency) will be individually titrated for optimal response. Device programming will be performed by trained physicians. The antihypertensive medication will be maintained unchanged during the study if clinically possible.
All adverse events and side effects will be reported from the time of enrolment onwards. A complication is defined as an adverse event resulting in death, permanent injury, or the need of invasive intervention to prevent death or permanent injury.
CVRx® received the CE mark in Europe for Barostim Neo™ for the treatment of RH in 2011 and for systolic heart failure in 2014 [Citation20,Citation21]. More than 1000 devices have been implanted in the world so far; approximately 800 for RH and approximately 200 for systolic heart failure.
Measurements of arterial and cardiac function and structure
In order to study the cardiovascular system throughout the study, the following comprehensive methodology will be used. 24-hour ambulatory BP (possibly with estimates of arterial stiffness) will be measured Arterial stiffness will be estimated by applanation tonometry at rest, and possibly also during 24 h with appropriate devices [Citation22]. Autonomic function is monitored by measuring continuous BP from the finger with a digital plethysmograph [Citation23]. Ultrasound of the heart by echocardiography will be performed. Moreover, common carotid artery intima-media thickness (IMT) is measured by an echotracking system, and endothelial function assessed by a peripheral arterial tonometer [Citation24,Citation25].
Biochemical analysis
Fasting blood samples are drawn for the determination of blood count, HbA1c, lipids, lipoproteins, sodium, potassium, ionized calcium, and serum creatinine. Urinary albumin excretion rate (as well as other urinary markers) are assessed from 24-h urine collections at time points; 0, 6 months and 5 years. Furthermore, blood samples characterizing possible pathophysiological mechanisms are further collected. These include markers of endothelial dysfunction, inflammation, oxidative stress, and kidney function markers.
Study management
The BAT study is managed exclusively by the executive and steering committees who are also responsible for the study design.
Current status
BAT study began enrolling patients in November 2015. As of January 2017, two centres have randomized six patients. The steering committeé is at this stage open to consider new centers to participate in this study.
Discussion
Despite the rather large number of antihypertensive pharmacological treatment options available, there is still a substantial proportion of patients suffering from refractory hypertension, and these patients are also at a markedly increased risk of end organ damage, cardiovascular morbidity and mortality. The current treatment modalities have not been optimal in targeting the compensatory changes in the sympathetic nervous system function and new strategies have therefore been warranted. To address these challenges, new treatment options like BAT have been developed.
Previous studies on BAT therapy in RH have resulted in promising preliminary results regarding BP reduction [Citation26]. Long-term (up to 6 years) blood pressure control using the Rheos system showed a substantial reduction of >30/15 mmHg in BP especially among patients who responded at the end of the randomized phase of the trial [Citation27,Citation28]. Furthermore, a propensity score matched cohort analysis of the first- and second generation devices suggested even superior BP reduction of the second-generation device compared to the control [Citation29]. Notably, the authors emphasized that a validated prospective randomized trial employing the second-generation Barostim Neo device should be performed. Therefore, the Nordic BAT study was designed to explore the magnitude of BP lowering effect of BAT therapy in a sham-controlled, randomized, double-blind study. Ambulatory 24-hour BP monitoring, the gold standard to measure BP, will be utilized to monitor BP changes in this study. In addition, effects of BAT therapy on autonomic nervous function will be evaluated and changes in cardiovascular structure and function will be monitored in order to gain insight into the possible mechanisms and predictors of BP lowering effects of BAT treatment. Of note, the design and conduction of the Nordic BAT study is an investigator-initiated initiative without industry involvement.
The patients hitherto randomized to BAT therapy in the Nordic BAT study were around 60 years old, had high BP levels confirmed by ABPM after witnessed intake of antihypertensive treatment. Importantly, secondary causes of hypertension were excluded. Renal function was not markedly lowered in any of the patients. All of the study participants were men, moderately obese, two were diabetic and were current or previous smokers. Half of them had had cardiovascular events, all of which were ischemic strokes, a serious condition closely associated to hypertension. Thus, these patients seem to be well representative of patients with RH [Citation3].
Poor drug adherence is an important cause for RH and needs to be considered in this study [Citation30]. Witnessed intake of antihypertensive medication prior to each ABPM on top of the double blind design of the study will be employed in order to minimize the risk of drug adherence to affect the results (). Moreover, blood and urine samples collected during the study will provide a possibility to measure concentrations of antihypertensive drugs. However, not even measuring plasma or urine metabolites of drugs can guarantee the intake of medications throughout the whole study period although non-drug adherent patients may be identified.
Fadl Elmula and coworkers have earlier shown that only one third of patients referred to hypertension centers as having RH has true RH [Citation30]. Thus, a considerable number of patients need to be screened for the study. To overcome this challenge additional centers may be included in the future to improve feasibility of the study.
Hypertension provides the greatest attributable risk for incident heart failure [Citation31]. In addition to hypertension, increased sympathetic and decreased parasympathetic nerve activity contributes to the development of heart failure. Of interest in this context, BAT therapy in patients with HF (NYHA class II-IV), has shown a positive influence on the autonomic function (assessed by muscle sympathetic nerve activity), NYHA class, and a six minutes walking test [Citation32]. Furthermore, a study comprising 145 patients with HF and reduced left ventricular ejection fraction (NYHA class III) randomized to BAT including best medical treatment or best medical treatment alone for six months showed that BAT improved NYHA class, six minutes walking test, and quality of life, and reduced plasma B-type N-terminal propeptide [Citation33]. These preliminary results are promising and the US Food and Drug Administration asked for more controlled studies. Thus, there is need for properly controlled randomized studies of BAT such as the Nordic BAT study in patients with uncontrolled hypertension
To summarize, one single-arm, open-label study of patients with RH treated with BAT have reported preliminary, promising results. However, blinded, sham-controlled, randomized studies are still required to demonstrate the BP lowering efficacy of BAT treatment. In addition, more studies have been demanded especially on the mechanisms behind the effect of BAT on the blood pressure. Furthermore, there is also an urgent need to identify those patients, that respond to the BAT treatment. The aim of this study is therefore to gain insight into the effect of BAT on blood pressure as well as the mechanisms of this treatment form.
Executive committeé: Daniel Gordin, Anders Albäck, Bert Andersson, Johan Elf, Fadl Elmula M. Fadl Elmula, Anders Gottsäter, Michael Hecht Olsen, and Ilkka Tikkanen.
Steering committeé: Kent Lodberg Christensen, Anders Gottsäter, Per-Henrik Groop, Thomas Kahan, and Sverre Kjeldsen.
BAT_Protocol_MS_Supplement_Blood_Pressure_Measurements.docx
Download MS Word (16.6 KB)Acknowledgements
The Nordic BAT Study is endorsed by the Nordic Hypertension Societies in Denmark, Finland, Norway, and Sweden. Mira Korolainen, Anita Makela, Leena Mantyla, Anna Sandelin, Jaana Tuomikangas, and Maikki Parkkonen are acknowledged for excellent technical assistance. Sources of funding: Folkhälsan Research Foundation, the Wilhelm and Else Stockmann Foundation, the Liv och Hälsa Foundation, the Finnish Medical Society (Finska Läkaresällskapet), the Finnish Medical Foundation, The Swedish Cultural Foundation in Finland, and the Dorothea Olivia, Karl Walter och Jarl Walter Perkléns Foundation.
Disclosure statement
DG has received consulting fees/lecture honoraria from Fresenius Medical Care and GE Healthcare, and support for travel to meetings from Sanofi and CVRx. JE has received lecture honoraria from Boehringer Ingelheim, Pfizer, and Leo Pharma. TK has received Research Grants to Karolinska Institutet from Medtronics and Recor. PV has received consulting fees from GE Healthcare, and support for travel to meetings from AL Gore medical and CVRx. LV has received consulting fees from GE Healthcare, and support for travel to meetings from CVRx. TBO has received consulting fees/lecture honoraria from AstraZeneca. P-H Groop has served on advisory boards for AbbVie, AstraZeneca, Boehringer Ingelheim, Cebix, Eli Lilly, Janssen, Medscape, MSD, Novartis, Novo Nordisk and Sanofi. P-H Groop has received lecture honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genzyme, MSD, Novartis, Novo Nordisk, and Sanofi. P-H Groop has received investigator-initiated grants from Eli Lilly and Roche. MHO has received personal research grant from Novo Nordisk Foundation. I.T. has received consulting fees/lecture honoraria from AstraZeneca, Boehringer Ingelheim, Merck Sharp and Dohme, Novartis, Orion Pharma and Servier and support for travel to meetings from Boehringer Ingelheim and CVRx. No other conflicts of interest were reported.
Additional information
Funding
References
- Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–1080.
- de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8,295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902.
- Holmqvist L, Bengtsson KB, Boström K, et al. Prevalence of treatment resistant hypertension, and important associated factors - results from the Swedish Primary Care Cardiovascular Database. J Am Soc Hypertens. 2016;10:836–846.
- Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52:1749–1757.
- Sander GE, Giles TD. Resistant hypertension: concepts and approach to management. Curr Hypertens Rep. 2011;13:347–355.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419.
- Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens. 2010;23:1052–1060.
- Georgakopoulos D, Little WC, Abraham WT, et al. Chronic baroreflex activation: a potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail. 2011;17:167–178.
- Lohmeier TE, Irwin ED, Rossing MA, et al. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311.
- Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol. 2011;58:765–773.
- de Leeuw PW, Alnima T, Lovett E, et al. Bilateral or unilateral stimulation for baroreflex activation therapy. Hypertension. 2015;65:187–192.
- Hoppe UC, Brandt MC, Wachter R, et al. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens. 2012;6:270–276.
- Wallbach M, Lehnig LY, Schroer C, et al. Effects of baroreflex activation therapy on ambulatory blood pressure in patients with resistant hypertension. Hypertension. 2016;67:701–709.
- Wallbach M, Lehnig LY, Schroer C, et al. Effects of baroreflex activation therapy on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Hypertens. 2015;33:181–186.
- Mancia G, Fagard R, Narkiewicz K, The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC), et al. ESH/ESC Guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281–1357.
- Levey AS, Stevens LA, Schmid CH, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Assmann SF, Pocock SJ, Enos LE, et al. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–1069.
- Toorop RJ, Scheltinga MR, Moll FL, et al. Anatomy of the carotid sinus nerve and surgical implications in carotid sinus syndrome. J Vasc Surg. 2009;50:177–182.
- Werner T, Lebar L, Wittmann S, (Anesthesia management in implantation of baroreceptor stimulators), et al. Anästhesiologisches Management bei Implantation von Barorezeptorenstimulatoren. Anaesthesist. 2015;64:683–688.
- CVRx®. CVRx® receives CE mark approval and introduces new implantable device for hypertension, the Barostim neo™, 2011 [Internet]. Available from: http://www.cvrx.com/intl_news/cvrx-receives-ce-mark-approval-and-introduces-new-implantable-device-for-hypertension-the-barostim-neo/
- CVRx®. CVRx® receives CE mark approval of the Barostim neo System™ for the treatment of heart failure, 2014 [Internet]. Available from: http://www.cvrx.com/usa_news/cvrx-receives-ce-mark-approval-barostim-neo-system-treatment-heart-failure/
- Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084.
- Rosengård-Bärlund M, Bernardi L, Fagerudd J, FinnDiane Study Group, et al. Early autonomic dysfunction in type 1 diabetes: a reversible disorder? Diabetologia. 2009;52:1164–1172.
- Paini A, Boutouyrie P, Calvet D, et al. Multiaxial mechanical characteristics of vulnerable carotid plaque: analysis by multiarray echotracking system. Stroke. 2007;38:117–1123.
- Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174.
- Heusser K, Tank J, Engeli S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626.
- Bakris GL, Nadim MK, Haller H, et al. Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the Rheos Pivotal Trial. J Am Soc Hypertens. 2012;6:152–158.
- de Leeuw PW, Bakris GL, Nadim MK, et al. Baroreflex activation therapy consistently maintains blood pressure reduction in a a large resistant hypertension cohort for at least 6 years. J Hypertens. 2015;33(Suppl1):e108.
- Wachter R, Halbach M, Bakris GL, et al. An exploratory propensity score matched comparison of second-generation and first-generation baroreflex activation therapy systems. J Am Soc Hypertens. 2017;11:81–91.
- Fadl Elmula FE, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension. 2014;63:991–999.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562.
- Gronda E, Seravalle G, Brambilla G, et al. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16:977–983.
- Abraham WT, Zile MR, Weaver FA, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail. 2015;3:487–496.
