Abstract
Purpose: Inhibition of the renin-angiotensin system may have effects on vascular structure and function beyond the effects on blood pressure (BP) reduction. We studied the ability of a single arm cuff oscillometric method (Arteriograph, TensioMed, Hungary) to assess effects of antihypertensive treatment on BP and arterial stiffness. Furthermore, this technique was compared to pulse wave analysis and applanation tonometry (SphygmoCor, AtCor Medical, Australia).
Materials and methods: Brachial and aortic BP, augmentation index (AIx), and carotid-femoral pulse wave velocity (PWV) was simultaneously assessed by both techniques in 71 untreated hypertensive patients. Thereafter, 58 completed double-blind randomized treatment for 12 weeks with ramipril or doxazosin.
Results: Treatment (assessed by the Arteriograph) reduced aortic more than brachial systolic BP (−13.2 vs. −11.2 mm Hg; p = .002) and improved all indices of arterial stiffness. This greater reduction in aortic to brachial systolic BP was more marked by ramipril than by doxazosin (−20.9 and −17.1 vs. −4.3 and −4.2 mm Hg; p = .006), with a similar trend for AIx (−6.2 vs. −2.2%; p = .058). Both devices showed correlations for aortic and brachial systolic and diastolic BP and AIx (r = 0.75–0.86, all p < .001), while agreement for PWV was weaker (r = 0.28; p = .043). The Arteriograph generally recorded higher values for aortic BP and AIx than the SphygmoCor.
Conclusions: Antihypertensive treatment reduced aortic systolic BP more than brachial BP and improved arterial stiffness. Blocking the renin-angiotensin system may have additional effects beyond BP reduction. We demonstrate the feasibility of the Arteriograph to monitor changes in BP and arterial stiffness by treatment.
Introduction
Hypertension causes subclinical organ damage, including structural vascular changes. Compared to brachial blood pressure (BP) assessment of central aortic BP gives additional prognostic information [Citation1]. Also pulse wave analysis for the assessment of the stiffening of the aorta and conduit arteries by pulse wave velocity (PWV), and pulse pressure amplification by the augmentation index (AIx) representing predominantly peripheral arterial resistance (but to some extent also arterial stiffness and endothelial function), provide independent prognostic information [Citation2,Citation3]. Several non-invasive methods are available to assess indices of arterial stiffness. However, they are often time consuming and require special training and skills. Thus, more simple reliable methods are desirable. The Arteriograph (AG; TensioMed, Hungary) is an easy to use single arm cuff oscillometric device, providing information on brachial and central aortic BP, and on AIx and PWV, all assessed within the same measurement cycle [Citation4]. The AG has been validated against invasive measurements with good agreement [Citation5]. However, some comparisons to other non-invasive methods have given conflicting results [Citation6–8]. Moreover, the AG has not yet been evaluated for the study of effects of antihypertensive drug treatment on BP and indices of arterial stiffness.
The renin-angiotensin system and the sympathetic nervous system are both important for BP regulation and vascular control. In hypertension, an increased activation of the sympathetic nervous system with an increased noradrenergic sympathetic vascular tone is present. Furthermore, formation of angiotensin II by activation of the renin-angiotensin system induces vasoconstriction, vascular remodelling, and promotes arterial stiffness. Blocking the renin-angiotensin system by angiotensin converting enzyme (ACE) inhibitors seems to reduce arterial stiffness and wave reflection [Citation9], and it has been proposed that the renin-angiotensin system may have effects on vascular structure and function beyond the effects of BP alone [Citation10].
By use of pulse wave analysis with applanation tonometry (Sphygmocor, SC; AtCor Medical, Australia) we have recently shown that antihypertensive treatment by blocking the renin-angiotensin system with the ACE inhibitor ramipril reduced aortic BP and indices of arterial stiffness to a greater extent than reducing blood pressure with the alpha 1-adrenoceptor antagonist doxazosin to blocking sympathetic vascular control [Citation11]. In the current ancillary study, we report on the results obtained with the AG, which was simultaneously used throughout that study [Citation11]. The first aim of this report was to study the ability of the AG to monitor changes in brachial and aortic BP and indices of arterial stiffness by antihypertensive treatment. Second, we aimed to study whether inhibition of the renin–angiotensin system has effects on vascular structure and function beyond the effects on BP reduction alone. Finally, we wanted to compare the AG to the widely used method with pulse wave analysis and applanation tonometry.
Methods
Study design and subjects
In the single centre Doxazosin-Ramipril Study women and men 18 years of age or older with mild-to-moderate primary hypertension (>140 mm Hg systolic and/or >90 mm Hg diastolic with no previous antihypertensive drug treatment for at least 4 weeks) were randomized to double-blind treatment (stratified by sex) with doxazosin 4 mg od or ramipril 5 mg od for 2 weeks, with forced titration to doxazosin 8 mg od and ramipril 10 mg od for additional 10 weeks (Supplementary material Figure S1). For details, see elsewhere [Citation11]. We included 71 patients (63 were previously never treated for hypertension). During the study, 10 patients (5 women and 5 men) discontinued due to reported side-effects (8 on doxazosin and 2 on ramipril), and another 3 patients were excluded due to incomplete data on vascular function, This provides 65 patients (60 previously never treated) with complete measurements by the AG device at week 0, and 58 patients (53 previously never treated) with complete information at both weeks 0 and 12. Results obtained by the SC device have been presented in detail elsewhere [Citation11]. The study was approved of by the Regional Ethics Committee in Stockholm. All participants gave their oral and written informed consent.
Patients arrived for the investigations in the morning after fasting overnight. The study drug was taken approximately 2h before the investigations during the treatment period to achieve peak concentrations. All examinations were performed in the supine position, following at least 20 min of rest. Brachial BP was obtained by an oscillometric device (OMRON 705IT, OMRON Healthcare Co Ltd, Kyoto, Japan) on the right arm with an appropriately sized cuff as a mean of three readings 1 min apart. These values were used for the SC to calculate aortic BP and AIx.
Pulse pressure was calculated as systolic minus diastolic BP. Mean arterial pressure was calculated as diastolic BP +1/3 x pulse pressure. Body mass index (in kg/m2) was calculated as weight/height2. Routine biochemistry was analysed by standard procedures from fasting blood samples. Estimated glomerular filtration rate was calculated by the CKD-EPI formula [Citation12].
Pulse wave analysis and assessment of central aortic blood pressure
The AG (Arteriograph, TensioMed Kft, Budapest, Hungary) is an oscillometric single arm cuff device [Citation4,Citation13]. First, by automatic inflation of the cuff the brachial systolic and diastolic BP is measured. Second, pressure changes during suprasystolic BP (35 mm Hg above peak systolic value) are driven to the device by the hose from the cuff and recorded by a high fidelity piezoelectric sensor. The time difference of the first systolic peak (the ejection of blood from the left ventricle to the aorta) and the second peak (the retrograde reflected pulse wave from the iliac bifurcation) represents the return time. Aortic PWV was calculated by dividing the travelled distance (the tapered measured distance by from the jugulum to the symphysis) by the return time/2. AIx was calculated as 100 x (second – first systolic wave)/pulse pressure. Central aortic BP was achieved by an algorithm in the software, based on calculations from invasive aortic BP measurements [Citation5].
Pulse wave analysis was also performed by applanation tonometry (Millar Instruments, Houston, TX, USA) and SC equipment and software (SphygmoCor, AtCor Medical Pty Ltd, West Ryde, NSW, Australia) [Citation14]. The radial pressure wave form was calibrated using brachial systolic and diastolic BP measured in the same arm (see above), the central aortic waveform was calculated by the device software by a generalized transfer function, and central BP values were derived. AIx was measured through the software. Recordings were then repeated at the level of the common carotid artery and the femoral artery, and carotid-femoral PWV was calculated as carotid–femoral distance (where the carotid–suprasternal notch distance was subtracted from the suprasternal notch–femoral distance), divided by the pulse transit time difference between carotid and femoral pulse wave registrations.
Statistics
The co-primary outcomes in the Doxazosin–Ramipril Study were changes in endothelial function, assessed by flow mediated vasodilatation, and in haemostatic function measured by the generation of thrombin–antithrombin complex, and have been reported elsewhere [Citation11,Citation15]. To determine the size of the study population, assuming 2 alpha 0.05 and beta 0.80, we a priori calculated 2 × 24 subjects sufficient to detect a 0.6% difference between the two study groups in flow mediated vasodilatation by treatment (with SD 0.72% in our laboratory); and 2 × 26 subjects sufficient to detect a 0.4 µg/L difference in thrombin–antithrombin complex by treatment (with SD 0.5 µg/L in our laboratory) between the two groups [Citation11,Citation15].
Data are presented as mean values ± SD (or SEM for calculated differences). We used Pearson’s correlation coefficients and Bland-Altman agreement analysis to study the relationship between the AG and SC devices. Group comparisons were made by analysis of variance or by multivariate analysis of variance. To account for potential confounders concerning PWV and AIx, the initial multivariate model included baseline mean arterial pressure, heart rate, age and gender. Replacing mean arterial pressure by changes in BP from week 0 to 12 did not influence the statistical evaluation. All statistical tests were 2-sided and carried out to a significance level (p) of .05. The statistical program used was JMP version 13.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
General
The entire study population of 71 patients (26 women) had a mean age of 55 ± 13 years, height 175 ± 9 cm, body mass index 26.8 ± 4.7 kg/m2, outpatient office BP 151 ± 15/89 ± 10 mm Hg, and resting heart rate 61 ± 8 bpm. Mean fasting plasma value of total cholesterol was 5.4 ± 1.1 mmol/L, glucose 5.4 ± 0.6 mmol/L, creatinine 76 ± 14 μmol/L, and estimated glomerular filtration rate was 90.4 ± 14.5 mL/min/1.73 m2.
Comparison of the cuff based oscillometric technique and pulse wave analysis by applanation tonometry
Aortic and brachial systolic and diastolic BP and pulse pressures measured at week 0 by the AG and by the SC were closely related (, ). Whereas absolute values of brachial systolic BP were in agreement with the two methods, the AG recorded higher absolute values than the SC for aortic systolic BP. The AG also recorded higher absolute values for both brachial and aortic diastolic BP and mean arterial pressure, as compared to SC (). Thus, aortic pulse pressure was higher and brachial pulse pressure was lower when recorded by the AG, as compared to the SC ().
Figure 1. Comparison of the single cuff oscillometric cuff method (Arteriograph) and pulse wave analysis with applanation tonometry (SphygmoCor) concerning systolic aortic BP (SBP) (a), diastolic aortic BP (DBP) (b), aortic pulse pressure (PP) (c), aortic mean arterial pressure (MAP) (d), augmentation index (AIx) (e), and pulse wave velocity (PWV) (f).
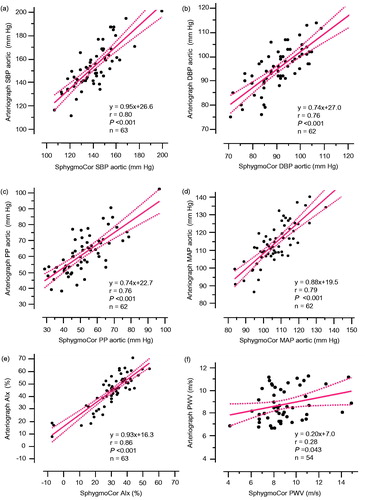
Table 1. Comparison of measurements by applanation tonometry with SphygmoCor and by oscillometric cuff method with Arteriograph.
AIx values measured at week 0 by the AG and the SC were closely related but values were consistently higher with the AG (, ). Mean PWV values obtained by the AG and the SC were similar, and related weakly but significantly (, ).
Figure 2. Bland-Altman analyses of the difference in mean values obtained by the single cuff oscillometric cuff method (Arteriograph) and by pulse wave analysis with applanation tonometry (SphygmoCor). Systolic aortic BP (SBP) (a), diastolic aortic BP (DBP) (b), aortic pulse pressure (PP) (c), aortic mean arterial pressure (MAP) (d), augmentation index (AIx) (e), and pulse wave velocity (PWV) (f).
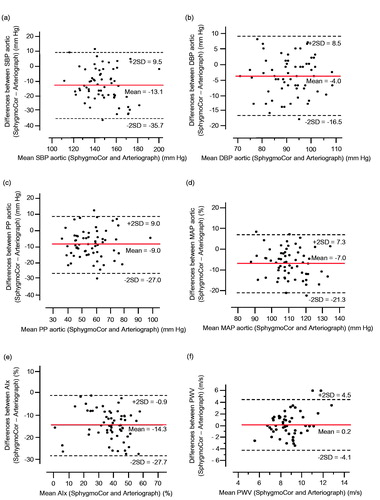
At week 12, the agreement between the AG and the SC for absolute values and for relations of aortic and brachial systolic and diastolic BP, pulse pressures, and AIx was very similar to the observations at week 0 (Supplementary material Table S1). PWV values obtained by the AG and the SC were similar but did not relate significantly.
Effects of treatment on blood pressure and vascular function assessed by the cuff based oscillometric technique
In all, 58 patients completed the 12 week drug intervention study. All patients achieved their target doses of the study drugs. The two study groups were comparable and their background information is presented in . Outpatient office BP values on study inclusion were 151 ± 8/93 ± 10 and 155 ± 9/93 ± 7 mm Hg in the doxazosin and ramipril groups, respectively. Baseline aortic and brachial BP and heart rate values (recorded in the laboratory at the time of the investigation), and indices of aortic stiffness measured with the oscillometric cuff technique were also similar (). The effects of treatment assessed by the SC device have been reported elsewhere [Citation11].
Table 2. Baseline data for 58 patients treated with doxazosin or ramipril.
Table 3. Treatment effects on blood pressure and vascular function measures by the cuff based oscillometric technique; univariate and multivariate analyses.
Drug treatment reduced both central aortic and brachial BP assessed by the AG (). The mean differences (±SEM) induced by treatment were −11.2 ± 2.0 and −7.4 ± 1.2 mm Hg in brachial systolic and diastolic BP, −13.5 ± 2.4 and −7.2 ± 1.2 mm Hg in aortic systolic and diastolic BP, −3.8 ± 1.3 mm Hg in brachial pulse pressure, and −6.4 ± 1.5 mm Hg in aortic pulse pressure (all p < .01). The absolute and relative changes in BP by treatment group are presented in and . These reductions were greater by ramipril than by doxazosin. Also the relative reductions (±SEM) in brachial pulse pressure (−10 ± 3 vs. 0 ± 4%; p = .019), and aortic pulse pressure (−16 ± 2 vs. −2 ± 4%; p = .004) were greater by ramipril than by doxazosin. Drug treatment reduced aortic systolic BP more than brachial systolic BP, (−13.2 ± 2.4 vs. −11.2 ± 2.0 mm Hg; p = .002) (Supplemental material Table S2). This greater reduction in aortic to brachial systolic BP was more marked by ramipril than by doxazosin (p = .006). The reductions in aortic and brachial diastolic BP were similar, with no differences between the two groups.
Figure 3. Relative changes in BP and vascular function by treatment, presented as mean values ± SEM. SBP: systolic blood pressure; DBP: diastolic blood pressure; AIx: augmentation index; PWV: pulse wave velocity. Absolute changes for doxazosin and ramipril were −4.2 ± 3.0 and −17.1 ± 2.3 mm Hg for brachial SBP, −4.3 ± 3.4 and −20.9 ± 2.7 mm Hg for aortic SBP, −3.4 ± 1.4 and −10.7 ± 1.6 mm Hg for brachial DBP, −2.7 ± 1.5 and −10.8 ± 1.5 mm Hg for aortic DBP, −2.2 ± 1.4 and −6.2 ± 1.6% for AIx, −0.6 ± 0.2 and −0.4 ± 0.2 m/s for PWV, and 3.2 ± 1.5 and 4.2 ± 1.6 ms for transit time, respectively. Significance values for univariate analyses are denoted as *p < .05,**p < .01, and ***p < .001. Multivariate analyses are presented in .
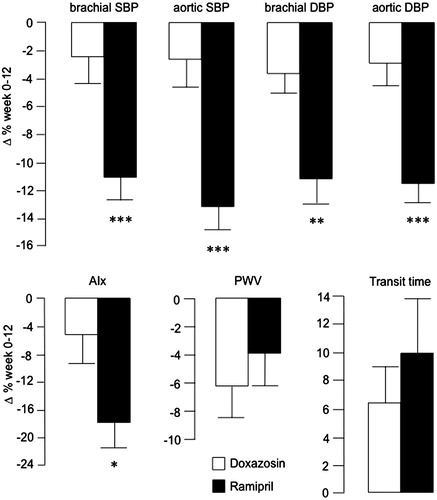
Treatment improved AIx, transit time, and PWV (). The mean differences (±SEM) by treatment were −4.4 ± 1.1% (p < .001), 3.7 ± 1.1 ms (p = .002), and −0.5 ± 0.1 m/s (p = .001), respectively. By univariate analyses, the changes in AIx were greater by ramipril than by doxazosin (). However, when adjusting for potentially confounding factors (mean arterial pressure, heart rate, height, age, and gender), this difference in AIx between the two study groups was attenuated (p = .075; ). The drug induced changes in transit time and PWV were not different between the study groups (, ).
The drug induced relative changes by treatment in aortic systolic and diastolic BP, and AIx recorded by the AG and the SC were directionally similar (Supplemental material Table S2) and related (r =0.52, 0.43, and 0.44, respectively, n = 55–56, all p < .001); while changes for PWV did not relate significantly (r = 0.05, n = 46). Of note, the reductions by ramipril, as compared to doxazosin, were greater when evaluated by the AG than by the SC (Supplemental material Table S2).
Discussion
This appears to be the first study with the AG single arm cuff oscillometric method to examine effects of antihypertensive drug treatment on BP and indices of aortic stiffness. The study provides three major findings. First, there was a greater reduction in central aortic systolic BP and AIx by the ACE inhibitor ramipril than by the alpha 1-adrenoceptor doxazosin. Second, the results with the AG and the SC were closely related for aortic BP and AIx values, whereas agreement for PWV between the two methods was weaker. However, the AG generally recorded higher values for aortic BP and Aix than the SC. Third, this ancillary study with a single cuff oscillometric method suggests the ability of the AG to monitor changes in brachial and aortic BP and indices of arterial stiffness by antihypertensive treatment.
We demonstrated with the AG that antihypertensive treatment with ramipril or doxazosin reduced BP, with greater reductions in aortic systolic BP than in brachial systolic BP. These ancillary study results with the AG support our previous findings with the SC [Citation11], and demonstrate for the first time the ability of the AG single cuff oscillometric technique to evaluate drug induced changes in aortic and brachial BP. Whereas PWV reflects aortic stiffness, pulse pressure amplification (i.e., central pulse pressure, as compared with brachial pulse pressure) and AIx reflect pulsatile flow and give information about central hemodynamic (large artery stiffness) and microvascular (increased arterial resistance and endothelial dysfunction) damage. AIx may be a more sensitive early marker of increased arterial resistance in younger individuals than PWV [Citation16,Citation17]. In this study, antihypertensive treatment improved all indices of vascular function, suggesting (at least some) restoration of central and peripheral arterial vascular dysfunction within 12 weeks of treatment. Furthermore, we observed greater reductions (i.e., improvement) in AIx and central pulse pressure by ramipril than by doxazosin (although somewhat attenuated when potential confounders were accounted for by multivariate analyses). The reduction in PWV by treatment was modest, with numerically greater improvement by ramipril than by doxazosin (see ). These results, directionally similar to our findings obtained with the SC [Citation11], are in agreement with other reports [Citation18–20]. In addition, the treatment induced changes of aortic BP and AIx recorded by the AG were in good agreement with our earlier reported findings with the SC [Citation11]. Thus, our results suggest that ramipril reduces arterial stiffness beyond the effects on BP alone. Furthermore, the AG appears well suited to monitor changes in BP and indices of arterial stiffness by antihypertensive drug treatment.
Brachial BP readings with the AG and the SC were in good agreement, and consistent over time (i.e. at weeks 0 and 12), confirming previous validation of the AG to non-invasive measurements of peripheral BP [Citation21]. The AG has also been successfully validated to non-invasive and invasive aortic BP recordings [Citation5,Citation22,Citation23]. Also for aortic BP, the comparison of the AG and the SC showed close relations, in agreement with other studies [Citation8,Citation23]. However, aortic systolic BP values were generally lower for the SC than for the AG, confirming other findings [Citation24,Citation25]. There are several tentative reasons for this. The AG measures brachial artery BP by an oscillometric cuff technique, and central aortic BP is calculated from pulsatile pressure changes at this same time point by an algorithm in the device software [Citation5]. The SC uses pulse wave analysis with applanation tonometry recorded from the radial artery, while blood pressure is measured in the brachial artery and not simultaneously, with a general transfer function to obtain aortic systolic BP [Citation26]. This general transfer function seems to underestimate aortic systolic BP and overestimate aortic diastolic BP, when compared with invasive BP recordings [Citation27–29]. Accordingly, the calibration procedure for the SC is important [Citation30], and changing the calibration procedure for the SC has been shown to diminish the differences in aortic BP values between the AG and SC devices [Citation24]. Thus, our results suggest that the AG can assess aortic systolic and diastolic BP well.
We furthermore observed a close and consistent relation between the AG and SG for AIx measurements. However, values were lower for the SC, in line with the observed lower aortic BP by the SC. This confirms previous results and suggests a systematic error in the general transfer function of the SC, resulting in lower central aortic pulse pressure augmentation and AIx [Citation8,Citation24].
This ancillary study with the AG, when compared to our results obtained with the SC [Citation11], suggests that the reductions in aortic systolic and diastolic BP and in AIx by antihypertensive treatment were similar, as assessed with both devices. However, at a closer look the changes with ramipril were greater, and those with doxazosin smaller, when evaluated by the AG, as compared to the SC. (Supplementary material Table S2). Differences in methods may be of importance to these findings. There is amplification of the central-to-peripheral pulse pressure due to haemodynamic characteristics. Heart rate, vascular function and peripheral pulse wave reflection are important, and brachial-to-radial pulse pressure contributes substantially [Citation31]. Thus, for the SC brachial BP measured by a sphygmomanometer and radial pulse wave recordings are used by the SC transfer function to calculate the aortic pressure waveform and aortic systolic BP, and will underestimate aortic BP [Citation30]. The AG records brachial BP and thereafter pulsatile flow in the aortic arch and large arteries during cuff occlusion, from which central aortic BP and aortic AIx are calculated. In addition, the AG calculates the central aortic systolic BP by use of the late (reflected) systolic peak from the peripheral pressure waveform, which shows good agreement and strong correlation with both estimated aortic systolic BP and invasively measured aortic systolic BP [Citation32]. If ACE inhibitors reduce aortic stiffness more than other drug classes [Citation9], our results may suggest that the differences between the two methods in the reductions of aortic BP and AIx by ramipril and doxazosin could be explained by the AG device able to detect treatment induced changes in arterial stiffness more accurately. However, these findings of our study remain to be fully understood and warrant further studies.
Mean PWV values in our study population obtained by the AG and the SC were similar (8.9 and 8.8 m/s, respectively), and are in good agreement with expected values from populations similar to ours [Citation5,Citation8,Citation33]. However, the agreement between the two methods was considerably weaker for PVW than for BP and AIx. Thus, in the individual subject there was some disagreement of PWV measurements between the two devices. PWV assessed by the AG has been validated to invasive aortic PWV with good agreement [Citation5]. Also PWV assessed by the SC has been validated with invasive PWV; however the results with the SC were critically dependent on how travel distance was measured, which has not been well standardized with that device [Citation34]. Differences in methodology for the two devices may help explaining these discrepancies in PWV in favour of the AG. First, the AG uses the transit time of oscillations of the reflecting pulse wave during a single registration, whereas the SC records transit time of pulse wave propagation with repeated ECG tracking as reference. However, the transit time of the femoral artery pulse wave varies beat-to-beat due to variations in systolic isovolumetric contraction time, which may introduce an error [Citation4]. Second, high quality recordings with applanation tonometry are highly user dependent and may be difficult to obtain in overweight people [Citation35]. Third, measurement of the actual travel distance of the pulse wave is based on somewhat different assumptions, which may introduce bias [Citation34,Citation36]. Fourth, compared to invasive PWV measurements, the limits of agreement for estimated PWV is greater for the SC than for the AG, and the range in mean values and the variance for repeat measures were higher for the SC than for the AG [Citation4,Citation5,Citation34]. In addition, we obtained fewer values for PWV than for aortic BP and Aix, which could attenuate the relation between the two methods. Taken together, PWV measurements with the AG and the SC are significantly related but may not be interchangeable in the individual subject.
There are some strengths to this study, which appears to be the first with the AG to examine effects of antihypertensive drug treatment on BP and indices of arterial stiffness. First, this study was performed in mostly never treated hypertensive patients. Second, the potential effects of blocking the renin-angiotensin system on arterial function beyond BP reduction was assessed against antihypertensive treatment with an alpha 1-adrenergic receptor blocker, in an attempt to account for confounding effects of changes in BP. Furthermore, we attempted to account for potential residual confounding effects of BP reduction in the statistical analyses.
However, this study also has important limitations besides the limited number of patients included. First, no invasive measurements were performed. The AG device has already been validated against invasive aortic BP with good agreement [Citation5]. However, that small study [Citation5] investigated subjects during coronary angiography, suggesting a higher prevalence of established atherosclerosis than representative for typical patients with mild-to-moderate hypertension. Also, reference values of central aortic BP and indices of arterial stiffness are not well established for the AG. Second, the duration of the study was only 12 weeks, which might be too short to evaluate treatment induced structural and functional vascular changes. However, improvement of arterial stiffness within this time has been reported [Citation37,Citation38].
In conclusion, antihypertensive treatment for 12 weeks improved indices of arterial stiffness, and suggest that blocking the renin-angiotensin system by ramipril have effects beyond BP reduction by doxazosin alone. The results of this ancillary study show that central aortic BP and AIx recorded by the AG single cuff oscillometric method were closely related to results obtained by pulse wave analysis with applanation tonometry [Citation11]. Thus, we demonstrate for the first time the feasibility of the AG to monitor changes in BP and indices of arterial stiffness by drug treatment in hypertensive subjects.
Supplementary.zip
Download Zip (117.9 KB)Acknowledgements
We thank Ms. E. Andersson, J. Rasck and E. Wallén Nielsen for expert technical assistance.
Disclosure statement
The authors report no conflicts of interest.
This study is registered at ClinicalTrials.gov (NCT02901977)
Additional information
Funding
References
- McEniery CM, Cockcroft JR, Roman MJ, et al. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719–1725.
- Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646.
- Vlachopoulos C, Aznaouridis K, O'Rourke MF, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871.
- Baulmann J, Schillings U, Rickert S, et al. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens. 2008;26:523–528.
- Horváth IG, Németh A, Lenkey Z, et al. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28:2068–2075.
- Boutouyrie P, Revera M, Parati G. Obtaining arterial stiffness indices from simple arm cuff measurements: the holy grail? J Hypertens. 2009;27:2159–2161.
- Mihalcea DJ, Florescu M, Suran BM, et al. Comparison of pulse wave velocity assessed by three different techniques: Arteriograph, Complior, and Echo-tracking. Heart Vessels. 2016;31:568–577.
- Jatoi NA, Mahmud A, Bennett K, et al. Assessment of arterial stiffness in hypertension: comparison of oscillometric (Arteriograph), piezoelectronic (Complior) and tonometric (SphygmoCor) techniques. J Hypertens. 2009;27:2186–2191.
- Shahin Y, Khan JA, Chetter I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis. 2012;221:18–33.
- Ong KT, Delerme S, Pannier B, et al. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: a meta-analysis of individual data in 294 patients. J Hypertens. 2011;29:1034–1042.
- Jekell A, Kalani M, Kahan T. The effects of alpha 1-adrenoceptor blockade and angiotensin converting enzyme inhibition on central and brachial blood pressure and vascular reactivity: the doxazosin-ramipril study. Heart Vessels. 2017;32:674–684.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Brinton TJ, Kailasam MT, Wu RA, et al. Arterial compliance by cuff sphygmomanometer. Application to hypertension and early changes in subjects at genetic risk. Hypertension. 1996;28:599–603.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605.
- Ekholm M, Jekell A, Wallén NH, et al. The effects of angiotensin converting enzyme inhibition and alpha 1-adrenoceptor blockade on inflammation and haemostasis in human hypertension. J Cardiovasc Pharmacol. Forthcoming.
- McEniery CM, Yasmin, Hall IR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760.
- Davies JE, Baksi J, Francis DP, et al. The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol. 2010;298:H580–H586.
- Jiang XJ, O'Rourke MF, Zhang YQ, et al. Superior effect of an angiotensin-converting enzyme inhibitor over a diuretic for reducing aortic systolic pressure. J Hypertens. 2007;25:1095–1099.
- Pannier BM, Guerin AP, Marchais SJ, et al. Different aortic reflection wave responses following long-term angiotensin-converting enzyme inhibition and beta-blocker in essential hypertension. Clin Exp Pharmacol Physiol. 2001;28:1074–1077.
- Mitchell GF, Dunlap ME, Warnica W, et al. Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49:1271–1277.
- Nemeth Z, Moczar K, Deak G. Evaluation of the tensioday ambulatory blood pressure monitor according to the protocols of the British Hypertension Society and the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 2002;7:191–197.
- Gunjaca G, Jeroncic A, Budimir D, et al. A complex pattern of agreement between oscillometric and tonometric measurement of arterial stiffness in a population-based sample. J Hypertens. 2012;30:1444–1452.
- Rossen NB, Laugesen E, Peters CD, et al. Invasive validation of arteriograph estimates of central blood pressure in patients with type 2 diabetes. Am J Hypertens. 2014;27:674–679.
- Rezai MR, Goudot G, Winters C, et al. Calibration mode influences central blood pressure differences between SphygmoCor and two newer devices, the Arteriograph and Omron HEM-9000. Hypertens Res. 2011;34:1046–1051.
- Ring M, Eriksson MJ, Zierath JR, et al. Arterial stiffness estimation in healthy subjects: a validation of oscillometric (Arteriograph) and tonometric (SphygmoCor) techniques. Hypertens Res. 2014;37:999–1007.
- Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937.
- Smulyan H, Siddiqui DS, Carlson RJ, et al. Clinical utility of aortic pulses and pressures calculated from applanated radial-artery pulses. Hypertension. 2003;42:150–155.
- Cloud GC, Rajkumar C, Kooner J, et al. Estimation of central aortic pressure by SphygmoCor requires intra-arterial peripheral pressures. Clin Sci. 2003;105:219–225.
- Ding FH, Fan WX, Zhang RY, et al. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens. 2011;24:1306–1311.
- Papaioannou TG, Lekakis JP, Karatzis EN, et al. Transmission of calibration errors (input) by general transfer functions to the aortic pressures (output) at different hemodynamic states. Int J Cardiol. 2006;110:46–52.
- Segers P, Mahiue D, Kipps J, et al. Amplification of the pressure pulse in the upper limb in healthy, middle-aged men and women. Hypertension. 2009;54:414–420.
- Hickson SS, Butlin M, Mir F, et al. The accuracy of central systolic BP determined from the second systolic peak of the peripheral pressure waveform. J Hypertens. 2009;27:1784–1788.
- Mattace-Raso F, Hofman A, Verwoert GC, et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31:2338–2350.
- Weber T, Ammer M, Rammer M, et al. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27:1624–1630.
- Van Bortel LM, Duprez D, Starmans-Kool MJ, et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002;15:445–452.
- Rajzer MW, Wojciechowska W, Klocek M, et al. Comparison of aortic pulse wave velocity measured by three techniques: complior, SphygmoCor and arteriograph. J Hypertens. 2008;26:2001–2007.
- Mackenzie IS, McEniery CM, Dhakam Z, et al. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–413.
- Rajzer M, Klocek M, Kawecka-Jaszcz K. Effect of amlodipine, quinapril, and losartan on pulse wave velocity and plasma collagen markers in patients with mild-to-moderate arterial hypertension. Am J Hypertens. 2003;16:439–444.
