Abstract
Aims: There may exist an effect modification of hypertension on the relation of vitamin D deficiency with cardiovascular disease. The aim of this study was to investigate this interaction on coronary heart disease.
Methods: We investigated 348 consecutive patients (mean age 62.4 ± 10.5 years; 56.3% male) who underwent coronary angiography because of chest discomfort at our heart center. Serum 25-OH vitamin D was also detected by ELISA method in these patients. Multivariable logistic regression models were used to estimate odd ratios (ORs) of CHD across vitamin D levels in hypertensives and normotensives, respectively.
Results: We found the multivariable-adjusted ORs of CHD in the bottom(≤8.5 ng/ml) and middle tertiles (8.5–13 ng/ml) of 25-OH vitamin D were 2.86 (95% confidence interval [CI]: 1.38, 5.92) and 1.63 (0.83, 3.20), respectively, compared with those in top tertiles (>13ng/ml) among hypertensives (Ptrend=0.005). In contrast, the corresponding ORs of the above two groups were 0.88 (0.28, 2.74) and 1.23 (0.42, 4.00), respectively, in the normotensives (Ptrend = 0.800; Peffect modification = 0.020). The multivariable-adjusted OR of CHD in patients with severe hypovitaminosis D (<10 ng/ml) versus those with higher vitamin D (≧10 ng/ml) was also greater in hypertensives (2.76; 95% CI: 1.51, 5.04) than that in normotensives (0.92; 95% CI: 0.37, 2.33; Peffect modification=0.013). Similar results were observed when Gensini Score was treated as a dependent variable.
Conclusion: Our finding suggests the presence of hypertension may modify the association of vitamin D deficiency with severity of coronary stenosis.
Introduction
A large body of prior evidence has shown that vitamin D deficiency is significantly associated with cardiovascular disease [Citation1–7]. Of note, in a few subgroup analysis of these studies, hypertension was found to modify the association between vitamin D and cardiovascular disease. In the Framingham Offspring Study [Citation2], vitamin D deficiency was found to be associated with incident cardiovascular disease in participants with hypertension but not in those without hypertension. In a recent cross-sectional study [Citation1], lower vitamin D levels were also identified to be significantly associated with ischemic stroke in hypertensives but not in normotensives. Thus, hypertension may modify the relation between vitamin D and cardiovascular disease.
In regarding to effect modification, however, as Farin Kamangar [Citation8] has warned, many interactions from subgroup analyses may be chance findings that result from increased type I error; unless such findings are repeated in other studies, often the results of subgroup analyses are not taken seriously. Given studies with regard to the effect modification of hypertension on the association of vitamin D with cardiovascular disease are limited and particularly no studies, to the best of our knowledge, have specifically focused on such an effect modification on the association of vitamin D with coronary heart disease (CHD), we have therefore investigated whether the association of vitamin D deficiency with significant coronary artery stenosis detected via coronary angiography was modified by hypertension in patients suspected of CHD in present study.
Materials and methods
Study population
From September 2014 to May 2015, a total of 371 consecutive patients for elective coronary angiography due to suspected CHD at the Heart Center of our Hospital were enrolled. Of these patients, 23 cases with missing vitamin D values were excluded. Thus, a total of 348 patients were left to be analyzed in present study.
The inclusion and exclusion criteria as well as the methods of collecting relevant clinical data have been reported in detail previously [Citation9]. Briefly, we included symptomatic patients who were suspected of suffering from CHD due to their chest discomfort and/or ischemia evidence by a noninvasive test such as a cardiac stress test or a dynamic change of an electrocardiogram. We excluded the patients who suffered from severe liver or kidney diseases, acute or chronic inflammation, or malignancy in this study. Patients who were taking vitamin D at admission, or refused to give an informed consent were also ruled out. The study was approved by the ethics committee of our Hospital and all patients provided written informed consent.
Blood pressure was measured after resting at least 10 minutes after admission by nurses. Measurements were performed twice with 10-minute intervals and averaged, which obtained one’s blood pressure value. Hypertension was defined as a systolic blood pressure of 140 mmHg or more or a diastolic blood pressure of 90 mmHg or more (or both) or current treatment for hypertension.
Angiographic analysis
The detailed method of performing angiographic analysis has also been reported previously [Citation9]. Angiographic CHD was defined as great than 50% or more in left main artery or a 70% or more in other major coronary artery or major branches. Gensini score system was used for assessment of severity and extent of CHD in present study as in literature [Citation10–12].
Laboratory examinations
Blood was collected for routine laboratory examinations in the early morning after overnight fast on admission and analyzed shortly after sampling. Fasting plasma glucose (FPG) was measured using the glucose oxidase method. Direct enzymatic methods were used to determine serum cholesterol. Serum 25-OH vitamin D was measured using ELISA method and was reported in ng/ml with coefficient of variations of intra and inter assay being 3.1% and 9.7%, respectively.
Statistical analysis
Descriptive statistics were shown as mean ± standard deviation or median (interquartile range) for continuous variables, and count variables were shown as percent (%). The trends of baseline characteristics across tertiles of 25 (OH) vitamin D were assessed using “nptrend” command.
We first modeled tertiles of 25 (OH) vitamin D, i.e. < =8.5, 8.5–13, and >13(ng/ml), to estimate odds ratios (ORs) of CHD in hypertensives and non-hypertensives, respectively, by using multivariable logistic regression models. Moreover, we also divided Gensini Score into tertiles, i.e. <6, 6–28, and > =28, and used an ordered logistic regress model with the tertiles as a dependent variable to estimate ORs of the extent of CHD across the vitamin D subgroups. The models were first adjusted for age and sex, and further were adjusted for diabetes, smoking, systolic blood pressure, and total cholesterol. Given the fact that there was a significant trend for higher usage of calcium antagonist therapy across tertiles of vitamin D in a previous study [Citation13] and a higher proportion (41%) of hypertensives were treated with calcium antagonists in present study, which is of unclear relevance for vitamin D metabolism, we also adjusted for use of calcium antagonist in our second multivariate model.
Also, given a value of 25-OH vitamin D < 10 ng/ml was considered as severe hypovitaminosis D and to have pathological implications for both the musculoskeletal and cardiovascular systems [Citation5,Citation14,Citation15], we also categorized the patients to two groups based on 25-OH vitamin D levels, i.e. 25-OH vitamin D < 10 ng/ml and 25-OH vitamin D > = 10 ng/ml, and estimated multivariate-adjusted ORs of CHD and higher Genisini Score in hypertensives and non-hypertensives, respectively. We set a multiplicative interaction term of hypertension (hypertension versus normotension) and tertiles of 25-OH vitamin D in a logistic regression model and tested its effect on the risks of CHD, independent of hypertension, 25-OH vitamin D levels and other confounding factors. We also set another multiplicative interaction term of hypertension and 25-OH vitamin D levels (<10 ng/ml and > = 10 ng/ml) in a multivariable logistic regression model. All p values were 2-tailed, and a significance of 0.05 was used. All statistical analyses were conducted using STATA 12.0 (StataCorp LP, College Station, Texas, USA).
Results
Of the total 348 patients, 56.3% were male and the mean age was 62.4 ± 10.5 years. CHD was identified in 212 patients (60.9%).
shows baseline characteristics of the study population. Lower levels of 25 (OH) vitamin D were correlated with older age, fewer proportions of men and ever-smokers. There were no significant differences across tertiles of vitamin D with regard to other risk factors, such as hypertension, diabetes and serum concentration of cholesterol. The Gensini Score was also not significantly different across tertiles of vitamin D.
Table 1. Base characteristics of study patients according to tertile of 25(OH) vitamin D.
presents base characteristics of hypertensive vs. normotensive participants.
Table 2. Base characteristics of hypertensive vs. normotensive participants.
shows the ORs of CHD according to presence of hypertension and 25-OH vitamin D levels (tertiles of vitamin D). In patients with hypertension, the ORs of CHD appeared to increase in a graded fashion with declining levels of 25-OH vitamin D (p for trend = 0.005 in the multivariable-adjusted model). In contrast, we did not observe such a trend in patients without hypertension (p for trend = 0.800 in the multivariable-adjusted model). A significant interaction between hypertension and 25-OH vitamin D level on presence of CHD was disclosed (Peffect modification = 0.020 in the multivariable-adjusted model).
Table 3. Odds Ratio (ORs) of significant CHD according to presence of hypertension/25-OH Vitamin D levels.
shows, similar to the ORs of CHD, the ORs of higher Gensini Score seemed to increase with 25-OH vitamin D levels decreasing in hypertensives (p for trend = 0.003 in the multivariable- adjusted model); however, this trend disappeared in non-hypertensives (p for trend = 0.823; for interaction= 0.045 in multivariable- adjusted model).
Table 4. Odds ratio for higher gensini score according to presence of hypertension/25-OH vitamin D levels.
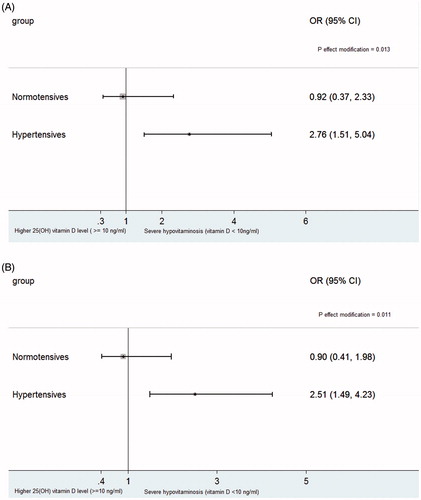
displays the multivariable-adjusted OR of CHD for patients with severe hypovitaminosis D, relative to those with 25-OH vitamin D ≧10 ng/ml, was greater in hypertensives than that in non-hypertensives. A similar results were also observed when Gensini Score (tertiles) was treated as a dependent variable ().
Figure 1. Odd ratios (ORs) and 95% confidence intervals (CIs) of coronary heart disease (CHD) (A) and higher Gensini Score (B) for patients with severe hypovitaminosis D, relative to those with higher 25-OH vitamin D levels (≧10 ng/ml), among hypertensives and normotensives, respectively. Results were adjusted for age, sex, diabetes, calcium antagonist, smoking, systolic blood pressure, total cholesterol.
Discussion
In this study in patients referred for coronary angiography, we found the association between vitamin D deficiency and presence of CHD as well as extent of coronary stenosis was much stronger among patients with hypertension than those without hypertension; a significant interaction was detected between presence of hypertension and 25-OH vitamin D level on severity of coronary stenosis.
The present observation extends and corroborates the findings of previous studies that have examined the effect modification of hypertension on association of vitamin D with cardiovascular disease. A Framingham Offspring Study [Citation2] showed vitamin D deficiency was associated with incident cardiovascular disease in participants with hypertension but not in those without hypertension in the subgroup analyses. In this Framingham Study, the cardiovascular events of interest included myocardial infarction, coronary insufficiency, angina, stroke, transient ischemic attack, peripheral claudication, or heart failure. It is not specifically focused on CHD. Moreover, a test for interaction is of borderline significance (p = 0.08) in that study. In subgroup analyses of a recent cross-sectional study [Citation1], Majumdar V et al. found lower vitamin D levels were associated with a higher risk of ischemic stroke in hypertensives, but not in non-hypertensives (Peffect modification = 0.04). In another subgroup analysis of a cross-sectional study [Citation16], Reis JP et al. showed 25(OH) vitamin D was inversely associated with carotid artery intima-media wall thickness (IMT) only in those with hypertension (Peffect modification = 0.036). While not particularly focusing on CHD, these findings, to some extent, support our observation.
Also of note is that all of the aforementioned findings come from post hoc analyses and the p values of effect modification in these studies close to 0.05, which means these studies may suffer from a higher risk of identifying a false-positive association. In contrast, the p values for interaction in present study were lower (Peffect modification close to 0.01) when we compared subjects with severe hypovitaminosis to those with higher vitamin D (>10 ng/ml). In general, it is hard to dismiss a lower P value as being due to chance, particularly when there exist a higher prior probability of hypothesis, which is mentioned in the following section. The reasons for the discrepancy between P values for interaction in this study and that in the aforementioned studies may be, at least partly, due to the different endpoints of interest across these studies.
There are a few possible mechanisms whereby hypertension could magnify the adverse effects of vitamin D deficiency on the coronary artery. Hypertension has been proven to be associated with endothelial dysfunction and inflammation [Citation17,Citation18]. Also, it plays a key role in development of vascular remodeling [Citation19]. Because vitamin D deficiency may also influence endothelial function [Citation20,Citation21], vascular remodeling or stiffness [Citation20,Citation22], and increase vascular inflammation [Citation7], hypertension could have an interaction effects on association of vitamin D deficiency with coronary artery. Additionally, experimental and clinical data have suggested that vitamin D deficiency could promote the development of hypertension [Citation23–25]; hypertension could increase the risk of CHD as we know. Thus, hypertension maybe a mediator in the relationship between vitamin D deficiency and CHD. Of course, not all those with vitamin D deficiency will develop hypertension. In this investigation, those with hypertension and without have similar levels of vitamin D. The reasons of why parts of vitamin D deficiency do not develop hypertension may be due to gene or other factors. If this is true, the relation between vitamin D deficiency and CHD will disappear among non-hypertensives. This speculation need confirmation in future studies.
Although amounting epidemiological studies have reported the association between vitamin D deficiency and cardiovascular risk including CHD, to date, this finding from observational studies has not been replicated in interventional studies [Citation3,Citation26]. The message from present study, if confirmed, may be of great importance from a research perspective. Performing an interventional study focusing on hypertensives with vitamin D deficiency instead of a general population may be a reasonable design to explore the effect of vitamin D supplementation on CHD risk in the future.
A major strengthen of our study is that we not only examined the interaction between hypertension and 25-OH vitamin D on the presence of CHD, but also explored their interaction effect on the extent of coronary stenosis assessed by Gensini Score. And we found a consistent result.
There are also some limitations that should be mentioned. First, the cross-sectional design of the study is a major limitation. Thus, a causality can not be drawn from present study and the results should be treated conservatively. It needs to be confirmed in a future prospective study.
Second, the sample size in this study is relatively small, thus the effect modification identified in this study may be a chance finding that result from increased type I error. Given the possibility that unless the findings are repeated in other studies, often the effect modification found in subgroup analyses, like aforementioned studies, are not taken seriously, our findings can be believed to corroborate the previous studies.
Third, one’s vitamin D values may change in different seasons. We only measure it one time in our manuscript. However, to the best of our knowledge, the majority of exiting literature focusing on relation of vitamin D with disease risk also have only measured vitamin D one time. Major JM et al. [Citation27] measured 25(OH) vitamin D in 538 individuals in a prospective, nationwide study at two time points within a 1-yr period, most measured in different seasons and found the intra-class correlation coefficient (ICC), which was calculated to measure the fraction of total variance that is from the between-subject variance component, was 0.72. This suggests an individual’s 25(OH) vitamin D level is relatively stable over a 1-yr period and a single blood sample may provide a reasonable average for 25(OH) vitamin D levels over a 1-yr period. In one study by Lange NE et al. [Citation28], vitamin D was measured in a cross-sectional design and then three times in a longitudinal design; the finding from the cross-sectional multivariable model using vitamin D measured one time is same as that from the longitudinal multivariable model using repeated vitamin D levels. Even so, we believe a future study measuring vitamin D levels repeatedly is needed to conform our findings.
Lastly, this is a single-center study and the participants were not sampled from the general population, limiting its generalization. Thus, a prospective cohort study in a general population is warranted to confirm our findings.
In conclusion, we found that the association between vitamin D and severity of coronary artery stenosis was modified by hypertension; the association between vitamin D deficiency and severity of coronary artery was identified in hypertensives but not in normotensives. This finding adds to the evidence that hypertension may modify the association of vitamin D with cardiovascular disease.
Disclosure statement
The Authors declare that there is no conflict of interest.
Additional information
Funding
References
- Majumdar V, Prabhakar P, Kulkarni GB, et al. Vitamin D status, hypertension and ischemic stroke: a clinical perspective. J Hum Hypertens. 2015;29:669–674.
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511.
- Pilz S, Verheyen N, Grubler MR, et al. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13:404–417.
- Liew JY, Sasha SR, Ngu PJ, et al. Circulating vitamin D levels are associated with the presence and severity of coronary artery disease but not peripheral arterial disease in patients undergoing coronary angiography. NMCD. 2015;25:274–279.
- Verdoia M, Schaffer A, Barbieri L, et al. Impact of gender difference on vitamin d status and its relationship with the extent of coronary artery disease. NMCD. 2015;25:464–470.
- Chen WR, Chen YD, Shi Y, et al. Vitamin D, parathyroid hormone and risk factors for coronary artery disease in an elderly chinese population. J Cardiovascular Med (Hagerstown, Md.). 2015;16:59–68.
- Kunadian V, Ford GA, Bawamia B, et al. Vitamin D deficiency and coronary artery disease: a review of the evidence. Am Heart J. 2014;167:283–291.
- Kamangar F. Effect modification in epidemiology and medicine. Arch Iran Med. 2012;15:575–582.
- Li K, Yang X, Wang L, et al. Modification of the association between smoking status and severity of coronary stenosis by vitamin D in patients suspected of coronary heart disease. Medicine (Baltimore). 2016;95:e4817.
- Gensini GG. Coronary arteriography: role in myocardial revascularization. Postgraduate Med. 1978;63:121–128, 133–128.
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606.
- Neeland IJ, Patel RS, Eshtehardi P, et al. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J. 2012;164:547–552.
- Verdoia M, Schaffer A, Sartori C, et al. Vitamin D deficiency is independently associated with the extent of coronary artery disease. Eur J Clin Invest. 2014;44:634–642.
- Vierucci F, Del Pistoia M, Fanos M, et al. Vitamin D status and predictors of hypovitaminosis d in italian children and adolescents: a cross-sectional study. Eur J Pediatr. 2013;172:1607–1617.
- Lavie CJ, Dinicolantonio JJ, Milani RV, et al. Vitamin D and cardiovascular health. Circulation. 2013;128:2404–2406.
- Reis JP, von Muhlen D, Michos ED, et al. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207:585–590.
- Mordi I, Mordi N, Delles C, et al. Endothelial dysfunction in human essential hypertension. J Hypertens. 2016;34:1464–1472.
- Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep. 2016;18:21.
- Belo VA, Guimaraes DA, Castro MM. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J Vasc Res. 2015;52:221–231.
- Al Mheid I, Patel R, Murrow J, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–192.
- Liu ZM, Woo J, Wu SH, et al. The role of vitamin D in blood pressure, endothelial and renal function in postmenopausal women. Nutrients. 2013;5:2590–2610.
- Rebsamen MC, Sun J, Norman AW, et al. 1alpha,25-dihydroxyvitamin d3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ Res. 2002;91:17–24.
- Chen S, Sun Y, Agrawal DK. Vitamin D deficiency and essential hypertension. J Am Soc Hypertens. 2015;9:885–901.
- Li YC, Kong J, Wei M, et al. 1,25-dihydroxyvitamin d(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238.
- Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin d levels and risk of incident hypertension. Hypertension (Dallas, TX: 1979) 2007;49:1063–1069.
- Bolland MJ, Grey A, Gamble GD, et al. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:307–320.
- Major JM, Graubard BI, Dodd KW, et al. Variability and reproducibility of circulating vitamin D in a nationwide U.S. Population. J Clin Endocrinol Metab. 2013;98:97–104.
- Lange NE, Sparrow D, Vokonas P, et al. Vitamin D deficiency, smoking, and lung function in the normative aging study. Am J Respir Crit Care Med. 2012;186:616–621.
