Abstract
Background: Elevated heart rate (HR) increases cardiovascular morbidity and mortality in hypertension. The impact of beta-blockers on patient prognosis in hypertension is controversial. This study examined the age-related effects of betaxolol on HR, muscle sympathetic nerve activity (MSNA), blood pressure (BP) and sympathovagal balance in untreated males with hypertension and tachycardia.
Methods: Ten young (age 26 ± 1 years) and seven older (age 50 ± 4 years) males underwent measurement of BP, HR, HR variability (Poincare plot) and MSNA before and after 8 weeks treatment with betaxolol at the initial starting dose of 10 mg/day, which was increased to 20 mg/day once daily after 4 weeks in all subjects.
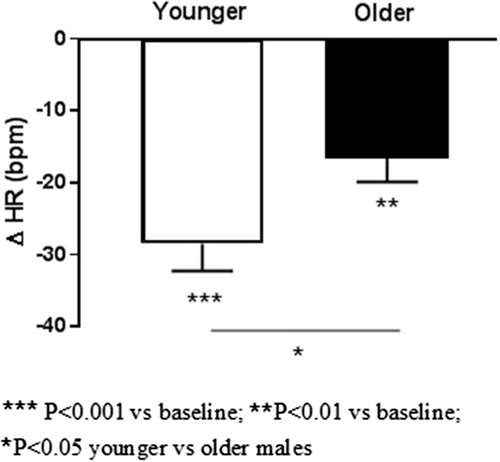
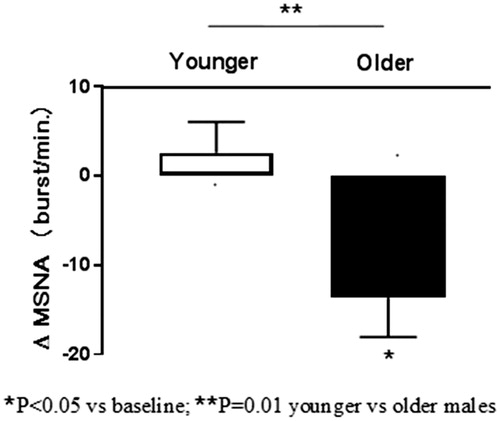
Results: In younger subjects, betaxolol decreased systolic BP (−13 ± 4 mm Hg, p = .01) and HR (−29 ± 4 bpm, p < .001) but not MSNA (3 ± 3 burst/min., p = 0.47) after 8 weeks. In older subjects a pronounced reduction in BP (−27 ± 7, p = .007) was accompanied by a significant decrease in MSNA (−13 ± 5 burst/min., p < .05) and HR (−17 ± 4 bpm, p = .002). SD1/SD2 ratio of Poincare plot increased in younger (0.36 ± 0.03 vs 0.51 ± 0.05, p = .004), but not in older (0.43 ± 0.08 vs 0.54 ± 0.12, p = .50) subjects.
Conclusion: Autonomic neural responses to betaxolol are age-dependent in hypertension-related tachycardia. Betaxolol reduces sympathetic drive to the heart, but not to the peripheral vessels confirming the contribution of augmented cardiac sympathetic activity to disease pathophysiology in younger adults. In older hypertensives, the sympathovagal balance is not influenced by betaxolol. The paradoxical reduction in MSNA despite lowering of BP and HR in older patients may suggest age-related functional decrements in autonomic control and/or inhibitory effects of betaxolol on the central nervous system.
Introduction
Tachycardia is an independent long-term predictor of all-cause cardiovascular (CV) morbidity and mortality in the general population, subjects with prehypertension, hypertension, CV disease and heart failure [Citation1–5]. The prognostic value of fast heart rate (HR) has been demonstrated in the Valsartan Antihypertensive Long-Term Use Evaluation trial which included patients with high-risk hypertension followed over a 5 year period [Citation6]. Patients in the highest HR quintile had a greater risk for cardiac events when compared to patients in the lowest HR quintile [Citation6]. The negative impact of elevated HR on patient prognosis was unrelated to BP control indicating that even patients with reasonably well-controlled hypertension but the presence of tachycardia display high risk for CV events. Another long-term study found that individuals with coexisting hypertension and elevated HR had increased risk of stroke and coronary heart disease [Citation7]. Very recently, elevated nighttime HR predicted future major CV events and all-cause death in patients with untreated hypertension indicating its important independent role for risk stratification [Citation8].
Neurochemical, neurophysiological and hemodynamic studies established the contribution of heightened sympathetic neural activity to the pathophysiology of essential hypertension [Citation9–12]. The mechanisms underlying tachycardia are complex and multifactorial involving numerous contributing factors to the elevation of resting HR (i.e. hyperinsulinemia, insulin resistance, dyslipidaemia, weight gain, etc.), associated blood pressure (BP) and increased sympathetic drive [Citation13]. Despite the mechanistic rationale for the use of beta-blockers in the treatment of hypertension-related tachycardia, their therapeutic impact on patient prognosis in hypertension remains controversial. A recent meta-analysis has demonstrated the benefits of beta-blocker use in reducing CV endpoints in younger hypertensive subjects, but not in older patients suggesting that age or non-atenolol beta-blocker might be the important determinant of outcomes in response to beta-blockers in hypertension [Citation14]. Considering the prognostic significance of elevated HR and unknown mechanisms underlying therapy with β1-selective receptor blocking agent in hypertension-induced tachycardia, we chose the cardioselective beta-blocker betaxolol due to its powerful effect on HR reduction with a single dose, its long biological half-life and penetration of the blood-brain barrier. This study sought for the first time to determine the effects of betaxolol on sympathetic nerve activity to the skeletal muscle, hemodynamics and HR variability in untreated males with essential hypertension and elevated HR.
Methods
Patients
This study involved 26 males with newly diagnosed essential hypertension. All patients were recruited from our Hypertension Unit Clinic at the Department of Hypertension and Diabetology of Gdansk. The recruitment criteria included: age 20–60 years, new onset untreated hypertension based on the mean of three readings taken during two separate visits with systolic BP >140 mm Hg and resting HR >70 beat per minute (bpm). Essential hypertension was diagnosed according to guidelines for the management of arterial hypertension [Citation15]. Secondary hypertension was excluded. No subject had tachycardia-related anemia or thyroid disease. All patients with confirmed hypertension and elevated HR by ambulatory BP and HR monitoring were included in the final study. Patients with white coat tachycardia were not recruited and therefore were considered not eligible for beta-blockade. Therefore, only 17 out of 26 non-smoker males fulfilled the study criteria. All subjects have never been treated for hypertension and were free of any other medication, and known chronic diseases.
Written, informed consent was obtained from all subjects. The study was approved by the Institutional review Board on Human investigation.
Study protocol
All subjects were asked to refrain from alcoholic beverages for at least 48 hours prior to study enrollment and were examined in a quiet room in a comfortable position. All patients entered an observation period of 2 weeks for the office BP and HR assessment. All measurements were obtained at baseline and after 8 weeks of treatment with betaxolol.
Office-seated and ambulatory blood pressure and heart rate
Office-seated systolic (S) and diastolic (D) BP was measured after a 5-min of rest on both arms and was averaged from three consecutive oscillometric measurements within a 1-min interval with a validated Omron 705 IT device (OMRON EUROPE B.V., Wegalaan, The Netherlands). The arm with a higher BP values was used for the final assessment at baseline and at follow-up.
To exclude white-coat hypertension and white coat-tachycardia 24-hour ambulatory BP and HR monitoring (ABPM) was performed with the Spacelabs 90207 recorder (Spacelabs Healthcare, Issaquah Washington, USA) 1 day prior to the laboratory data collection, and 1 day directly following 8 weeks of therapy with betaxolol, as described previously [Citation16].
Muscle sympathetic nerve activity
Subjects were studied in the morning after a standard light breakfast. After 15 minutes of rest, resting measurements of muscle sympathetic nerve activity (MSNA), BP, HR and respiration were recorded concurrently and continuously in a supine position in the undisturbed room over the period of 20 minutes and the final 5 minutes was analyzed. HR was registered from surface electrodes using a 12 lead switch box system (Dual Bio Amp). BP was measured continuously by the Finometer Medical System (FMS, Amsterdam, the Netherlands). Sympathetic activation was recorded (Nerve Tracking Analyzer system-model 662C-3) continuously by obtaining multiunit recordings of postganglionic sympathetic nerve activity to the skeletal muscle, measured from a muscle fascicle of the peroneal nerve posterior to the fibular head. The neural signals were amplified, filtered, rectified, and integrated to obtain a voltage display of sympathetic nerve activity as described previously [Citation17]. The amplitude of each burst was determined and sympathetic activity was calculated as bursts frequency (bursts per minute) and sympathetic neural burst amplitude distribution (%).
All measurements were performed at baseline and following directly an 8-week therapy with betaxolol.
Analysis of heart rate variability
Heart rate variability (HRV) was analysed using the Kubios HRV software as described previously [Citation18] which allows for the assessment of a wide variety of time-domain, frequency-domain and nonlinear analysis options. Data was analysed over a ten minute period of continuous stable HR recordings during microneurography. The following time-domain were analysed: RR interval, RMSSD (square root of the mean squared differences between successive RR intervals), NN50 (number of successive RR interval pairs that differ more than 50 ms), pNN50 (NN 50 divided by the total number of RR intervals), TINN (baseline width of the RR interval histogram). The analysed bands in the frequency-domain included: VLF (very low-frequency) with frequency domain 0–0.04 Hz, LF (low-frequency) between 0.04–0.15 HZ and HF (high-frequency) with 0.15–0.45 HZ ranges. Nonlinear parameters of the Poincare plot including the standard deviations of SD1 (short-term variability) and SD2 (long-term variability) were also analysed.
Betaxolol therapy
After baseline measurements, all patients were assigned to an 8-week treatment period with betaxolol hydrochloride, at the initial starting dose of 10 mg/day once daily in the morning, which was increased to 20 mg/day once daily in the morning after four weeks in all subjects. All patients were receiving the same dose of betaxolol throughout the study protocol.
Statistical methods
Results are expressed as mean ± SEM or percentage (%) distribution. The comparisons in parameters in response to betaxolol between the two visits were analysed using a paired t-test. Associations between variables were evaluated by Pearson’s rank correlations. Data was corrected for baseline values (HR and MSNA levels) as delta changes between baseline and 8 weeks follow-up were compared between both age groups using an unpaired t-test ( and ). The sample size analysis indicated that 7 subjects would have 80% power for a paired t-test to detect a decrease in office HR of 20 bpm at the level of significance 0.05 and an estimated standard deviation of 15. Our study revealed that seven older patients with a standard deviation of 9 and a reduction in HR of 17 bpm had 98% power. Additional analysis revealed that younger subgroup of 10 patients had 100% power for a paired t-test to detect a decrease in HR of 29 bpm at the level of significance of 0.05 and an estimated standard deviation of 13. Statistical analysis was performed using SigmaStat Version 3.5 (Systat Software, Point Richmond, CA). A value of p < .05 was considered significant.
Results
The final study cohort included younger males with a mean age of 26 ± 1 (from 21 to 31) years and body mass index (BMI) of 25 ± 1 kg/m2, and older males with a mean age of 50 ± 4 (from 43 to 60) years and BMI of 30 ± 2 kg/m2. Baseline resting BP, HR and MSNA for the two study groups are demonstrated in .
Table 1. Blood pressure, heart rate and muscle sympathetic nerve activity at baseline in younger and older males.
Younger patients had higher office and ambulatory HR levels, and significantly lower office, and ambulatory both SBP, and DBP at baseline. Despite comparable mean median burst amplitude distribution, older patients had significantly greater levels of burst MSNA than younger males ().
In the entire cohort, MSNA-related changes to betaxolol were significantly related to baseline burst MSNA (r = −0.69, p = .002) and age (r = −0.74, p = .0007). Median burst amplitude distribution strongly correlated with its change during therapy (r = −0.70, p = .0016).
Baseline resting MSNA was unrelated to the change in SBP (r = −0.27, p = .29) after 8 weeks of betaxolol therapy.
In younger subjects, betaxolol decreased systolic SBP (−13 ± 4 mm Hg, p = .01) and HR (−29 ± 4 bpm, p < .001) but MSNA remained unchanged (3 ± 3 burst/min., p = .47) after 8 weeks.
In contrast, in older subjects a pronounced reduction in SBP (−27 ± 7, p = .007) was accompanied by a significant decrease in MSNA (−13 ± 5 burst/min., p < .05) and HR (−17 ± 4 bpm, p = .002). Resting HR was unrelated to baseline levels of MSNA (r = −0.50, p = .25), and a higher resting HR was associated with a lower reduction in MSNA (r = 0.77, p < .05). A reduction in HR was unrelated to MSNA changes (r = −0.39, p = .39).
Age-related changes in HR and MSNA from baseline to follow-up are illustrated in and , respectively.
There was a comparable DBP decrease in young (−14 ± 3 mm Hg, p < .001) and older (−14 ± 3 mm Hg, p = .003) males after 8 weeks.
Effects of betaxolol on HRV
In younger males, treatment with betaxolol resulted in a significant increase in RMSSD, NN50, pNN50 and a reduction in TINN, and LF/HF ratio (). Betaxolol therapy was associated with an increase in SD1 and SD1/SD2 ratio of Poincare plot, but no significant changes were found in SD2 ().
Table 2. Effects of betaxolol on heart rate variability at 8 weeks follow-up in younger and older males.
In older males, TINN significantly decreased but no significant changes were observed in RMSSD, NN50, pNN50, LF/HF or Poincare components at 8 weeks follow-up ().
Discussion
This study is the first to demonstrate the effects of highly β1 cardioselective beta-adrenergic receptor antagonist betaxolol on sympathetic CV profile in males with untreated hypertension and ambulatory tachycardia. The major novel findings are that (1) the autonomic neural responses to betaxolol are age-dependent in untreated hypertension-related tachycardia; (2) chronic treatment with betaxolol reduces sympathetic drive to the heart but not to the peripheral vessels in younger adults; and (3) the paradoxical reduction in MSNA achieved with betaxolol occurs despite lowering of HR and BP in older males. Our study also revealed three other new findings to indicate (1) age-related difference in hemodynamic and sympathetic profile in hypertension-related tachycardia; (2) lowering HR with betaxolol bore no association with an inhibition of MSNA; and (3) betaxolol improves the dynamics of HRV in younger, but not in older subjects with untreated hypertension and tachycardia. Betaxolol was well tolerated and no drug-related side effects were noted in the study cohort.
The benefits associated with beta-blocker-induced HR lowering have been favorably documented in patients with heart disease, myocardial infarction (MI) and heart failure [Citation19]. On the contrary, the usefulness of beta-blockers as first line therapy for hypertension has been questioned based on concerns raised over less protective CV effects from meta-analyses of randomized controlled trials [Citation20–22]. Further unfavourable impact of beta-blockers on HR-related prognosis was documented in patients with resistant hypertension [Citation23]. While fast HR was associated with the adverse outcomes in patients receiving beta-blockers, slow HR was a significant predictor in those not using beta-blockers suggesting an overall U-shaped phenomenon between the levels of HR and prognosis [Citation23]. Very recently, masked tachycardia was associated with the increased risk of excess major adverse CV events and mortality in patients with hypertension [Citation8]. Notably, this prognostic significance in predicting future adverse outcomes was independent of beta-blocker use [Citation8].
In contrast to previous studies that found the link between beta-blocker therapy and worse outcomes in hypertension, more recent meta-analysis of 21 hypertensive trials indicated that beta-blockers resulted in a significant reduction in CV morbidity and mortality in younger patients but not in older subjects [Citation14]. The exact factors attributable to worse prognosis remain unclear. However, there is some evidence to suggest that smoking status, alterations in central BP and/or mechanisms of action (i.e. the effects on HR and/or BP reduction, and/or peripheral resistance, etc.) which depend on diverse pharmacokinetic and pharmacodynamic properties including lipo- and hydrophilicity, β1 selectivity, plasma half-life, intrinsic sympathomimetic activity, vasodilation, metabolism related to gene polymorphism, and metabolic effects could have contributed to the negative impact of beta-blockers on outcomes in managing hypertension [Citation24].
Previous studies evaluating the effects of beta-blockers on peripheral sympathetic nerve activity in essential hypertension have shown conflicting results. Atenolol therapy has been found to have no effects on plasma norepinephrine levels or total body NE spillover in essential hypertension [Citation25]. Microneurography studies have also shown inconsistent results with an increase in MSNA following a short-term therapy with metoprolol in patients with untreated essential hypertension [Citation26] or increase in MSNA after acute administration of atenolol in healthy subjects [Citation27]. Other studies reported no changes in MSNA in response to chronic therapy with metoprolol [Citation28] and atenolol [Citation29].
Betaxolol is an effective long acting highly selective β1-blocking agent (half-life ∼19 hours) with several advantages that are likely to overcome certain limitations of other beta-blockers [Citation30]. Betaxolol provides a steady plasma concentration, less fluctuation and intersubject, and intrasubject variability producing a more consistent therapeutic response and more dependable dosage adjustment when compared to atenolol [Citation31].
Hemodynamic characteristics of the initial phase of primary hypertension are not unequivocal, either induced by increased peripheral resistance or raised cardiac output [Citation32]. Approximately 30–40% of younger adults with borderline and/or mild hypertension demonstrate hyperkinetic state with elevated HR, cardiac output, forearm blood flow and plasma noradrenaline (NA) levels [Citation33]. The hyperkinetic state caused by excessive autonomic drive is likely to be induced by augmented sympathetic activity to the heart and the kidney. Elevated NA release from renal and cardiac sympathetic nerves is evident in essential hypertension, predominantly in males under the age of 40 [Citation10]. Other abnormalities commonly present in hyperkinetic borderline hypertension are increased renal blood flow and plasma renin activity [Citation34]. In comparison to younger hypertensives, NA release from sympathetic nerves has been found to be lower as a result of an age-dependent fall in NA plasma clearance in essential hypertension [Citation10]. With aging and disease progression, cardiac output generally becomes normal in uncomplicated hypertension, however a shift toward increased vascular resistance potentiates sympathetic activation which is a hallmark of established hypertension, leading to vascular remodeling, organ damage and adverse CV complications [Citation35].
Our study demonstrates age-related differences in sympathetic CV profile indicating a more pronounced elevation in HR and borderline hypertension in younger subjects and the higher levels of BP and MSNA in older males with hypertension and tachycardia (). These age-dependent hemodynamic features are likely to influence cardiac, pressor and sympathetic responses to β1-selective beta blocking agent (Figures). In the present study, betaxolol produced a greater reduction in HR in younger subjects but had no impact on peripheral sympathetic nerve activity ( and ) which supports a causative role for the selective increase in cardiac sympathetic activity to the pathogenesis of essential hypertension.
In older hypertensives, therapy with betaxolol was associated with a significant reduction in MSNA accompanied by lowering of HR and BP. However, resting HR was unrelated to the levels of MSNA. Likewise, changes in MSNA bore no association with BP or HR decrease in response to betaxolol. It seems that older subjects with faster baseline resting HR exhibited a lower reduction in MSNA. Notably, our results are in line with a previous study documenting that HR is not a reliable marker of the overall sympathetic activation in hypertension [Citation36]. In fact, the age, but not HR, was a major determinant of betaxolol-induced sympathetic inhibition in the treatment of essential hypertension and tachycardia.
While the exact mechanism through which betaxolol produced a paradoxical reduction in MSNA despite HR and BP lowering effects is not completely understood, there is evidence to suggest the age-related changes in autonomic neural control [Citation37]. Process of aging is associated with an increase tonic sympathetic nervous system activity and deterioration of regulatory mechanisms including loss of baroreflex sensitivity, atherosclerosis, and reductions in arterial distensibility, renin activity, and β1 adrenoreceptor sensitivity in the heart, increased peripheral resistance, low cardiac output and altered drug pharmacodynamics which independently and synergistically are likely to affect the treatment of hypertension and responses to beta-blockers [Citation37]. Persistent elevations in sympathetic activity and NA release with human aging per se causes cardiac β1-adrenergic receptor desensitization and impaired systemic α1-adrenergic vasoconstrictor responsiveness [Citation38] which influence sympathetic CV control and drug-effects.
Further major advantage of betaxolol is that it penetrates the blood-brain barrier and has the ability to antagonize β1 receptor that are expressed in several regions and tracts in the central nervous system [Citation30] including the locus coerules (LC) and its projections. In addition, the LC is the major noradrenergic brain nuclei and the largest source of NA production, thereby playing an integral role in autonomic neural activity. Furthermore, sympathetic activation accompanying aging influence augemented NA release from subcortical suprabulbar brain regions as estimated from measurements of NA turnover from the cerebrovascular circulation, in a company with activation of the sympathetic outflows to the heart [Citation39]. Noradrenergic neuronal projections to the suprabulbar subcortical areas result in sympathetic activation in healthy aging, and are an underlying contributor to the pathogenesis of essential hypertension [Citation40]. In this context, a higher affinity of betaxolol to blockade the central β1-adrenoreceptors in the brainstem and prejunctional beta-receptors in the periphery is likely to suppress the release of NA, leading to a reduction in MSNA. Results of our study indicate that lowering of HR and BP with betaxolol is associated with a decrease in MSNA in the elderly which is not opposed by the baroreflex. These findings support the concept that betaxolol treatment may result is a significant improvement of CV autonomic control in patients with uncomplicated essential hypertension and tachycardia.
Unlike other beta-blockers and selective serotonin reuptake inhibitor, betaxolol has no affinity to serotonin receptors and its psychotrophic benefits have been also successfully demonstrated in the treatment of anxiety disorders, obsessive-compulsive personality disorder or posttraumatic stress [Citation30], highlighting another neuroprotective effect of betaxolol.
Hyperkinetic circulation is associated with disturbed autonomic balance and reduced HRV [Citation41] and vagal inhibition [Citation42]. The present study shows that betaxolol increases vagal activity to the heart in younger but not in older males. Whether an improvement in sympathovagal balance is a contributing mechanism to better outcomes in response to beta-blocker therapy in younger subjects needs to be determined.
Strengths of our study include the first clinical and pathophysiological evaluations of the effects of β1 highly cardioselective beta-blocker in untreated essential hypertension and tachycardia. The steady-state effects of betaxolol on sympathetic CV profile were determined in newly diagnosed younger and older males with essential hypertension and tachycardia confirmed by ambulatory BP and HR monitoring, none of whom had ever been treated with antihypertensive medication, thereby eliminating potential confounding effects on HR, BP, MSNA and HRV. The specific pharmacokinetics properties of betaxolol and findings derived from this study appear to be of clinical relevance. However, the long-term CV outcomes associated with betaxolol warrants further studies.
Study limitations
The relatively modest number of patients and the lack of a control group are study limitations. Despite this, all subjects underwent comprehensive clinical investigation and included only males with hyperkinetic hypertension confirmed by ambulatory BP monitoring. This allowed for adequate determination of intra-subject and age-dependent therapeutic effectiveness of betaxolol on HR, BP, MSNA and HRV. Controlled studies aimed to compare the effects of different beta-blockers and/or required with placebo on HR and are subject to their own limitations including different impacts on BP and/or HR lowering, not allowing discrimination between hemodynamic parameters and study outcomes as indicated in the recent Expert Consensus Position Statement [Citation43]. Plasma and/or urine drug concentration measurements were not directly tested in the present study. The medication adherence to beta-blocking agent betaxolol was assured via its direct pronounced effect on HR. Given that the essential hyperkinetic hypertension [Citation33] affects mainly male subjects, females were not included in this study. A lack of data adjustment for baseline BMI could be viewed as a study limitation, however BMI was unrelated to both baseline resting HR and MSNA levels for the entire study cohort. Determination of baroreflex control of MSNA and HR responses to acute activation and deactivation of arterial baroreceptors before and after betaxolol therapy could provide a better understanding of the peripheral and central mechanisms with aging. However, a substantial reduction in BP achieved with betaxolol in older subjects, acting via the baroreflexes, may result in sympathetic activation rather than MSNA decrease. Therefore, other mechanisms of action specific to betaxolol including the contribution of genetic factors [Citation44] cannot be ruled out. Finally, data on cardiac output was not collected in the present study, which would be relevant in the context of the underlying pathophysiology and its association with MSNA [Citation45].
Perspectives
We describe the effect of lowering of HR and BP with betaxolol on MSNA in untreated males with hypertension and tachycardia, which has been previously shown to be a strong independent predictor of adverse CV events and death in hypertensive patients. We have demonstrated that betaxolol results in significant reductions in sympathetic activity to the heart in younger adults and central sympathetic outflow in older subjects. Whether the sympathetic responses to betaxolol may impact on patient outcomes in hypertension tachycardia need to be determined.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kannel WB, Kannel C, Paffenbarger RS Jr, et al. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494.
- Palatini P, Julius S. Association of tachycardia with morbidity and mortality: pathophysiological considerations. J Hum Hypertens. 1997;11(Suppl 1):S19–S27.
- Jouven X, Empana JP, Schwartz PJ, et al. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958.
- Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75.
- Hozawa A, Inoue R, Ohkubo T, et al. Predictive value of ambulatory heart rate in the Japanese general population: the Ohasama study. J Hypertens. 2008;26:1571–1576.
- Julius S, Palatini P, Kjeldsen SE, et al. Usefulness of heart rate to predict cardiac events in treated patients with high-risk systemic hypertension. Am J Cardiol. 2012;109:685–692.
- Zhong C, Zhong X, Xu T, et al. Combined effects of hypertension and heart rate on the risk of stroke and coronary heart disease: a population-based prospective cohort study among Inner Mongolians in China. Hypertens Res. 2015;38:883–888.
- Palatini P, Reboldi G, Beilin LJ, et al. Masked tachycardia. A predictor of adverse outcome in hypertension. J Hypertens. 2017;35:487–492.
- Esler MD, Julius S, Randall OS, et al. Relation of renin status to neurogenic vascular resistance in borderline hypertension. Am J Cardiol. 1975;36:708–715.
- Esler M, Jennings G, Biviano B, et al. Mechanism of elevated plasma noradrenaline in the course of essential hypertension. J Cardiovasc Pharmacol. 1986;8:S39–S43.
- Anderson EA, Sinkey CA, Lawton WJ, et al. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183.
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987.
- Grassi G, Arenare F, Quarti-Trevano F, et al. Heart rate, sympathetic cardiovascular influences, and the metabolic syndrome. Prog Cardiovasc Dis. 2009;52:31–37.
- Kuyper LM, Khan NA. Atenolol vs nonatenolol β-blockers for the treatment of hypertension: a meta-analysis. Can J Cardiol. 2014;30:S47–S53.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219.
- Hering D, Kucharska W, Kara T, et al. Resting sympathetic outflow does not predict the morning blood pressure surge in hypertension. J Hypertens. 2011;29:2381–2386.
- Hering D, Kara T, Kucharska W, et al. High-normal blood pressure is associated with increased resting sympathetic activity but normal responses to stress tests. Blood Press. 2013;22:183–187.
- Tarvainen MP, Niskanen JP, Lipponen JA, et al. Kubios HRV-heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113:210–220.
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847.
- Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA. 1998;279:1903–1907.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–1553.
- Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol. 2008;52:1482–1489.
- Salles GF, Cardoso CR, Fonseca LL, et al. Prognostic significance of baseline heart rate and its interaction with beta-blocker use in resistant hypertension: a cohort study. Am J Hypertens. 2013;26:218–226.
- Larochelle P, Tobe SW, Lacourciere Y. β-Blockers in hypertension: studies and meta-analyses over the years. Can J Cardiol. 2014;30:S16–S22.
- Jacobs MC, Lenders JW, Smits P, et al. Long-term beta 1-adrenergic blockade restores adrenomedullary activity in primary hypertension. J Cardiovasc Pharmacol. 1997;30:338–342.
- Sundlof G, Wallin BG, Stromgren E, et al. Acute effects of metoprolol on muscle sympathetic activity in hypertensive humans. Hypertension. 1983;5:749–756.
- Cogliati C, Colombo S, Ruscone TG, et al. Acute beta-blockade increases muscle sympathetic activity and modifies its frequency distribution. Circulation. 2004;110:2786–2791.
- Wallin BG, Sundlof G, Stromgren E, et al. Sympathetic outflow to muscles during treatment of hypertension with metoprolol. Hypertension. 1984;6:557–562.
- Burns J, Mary DA, Mackintosh AF, et al. Arterial pressure lowering effect of chronic atenolol therapy in hypertension and vasoconstrictor sympathetic drive. Hypertension. 2004;44:454–458.
- Swartz CM. Betaxolol in anxiety disorders. Ann Clin Psychiatry. 1998;10:9–14.
- Kunka RL, Wong YY, Andersen RL, et al. Steady-state fluctuation and variability of betaxolol and atenolol plasma levels. Ther Drug Monit. 1989;11:523–527.
- Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504.
- Julius S, Krause L, Schork NJ, et al. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens. 1991;9:77–84.
- Messerli FH, Ventura HO, Reisin E, et al. Borderline hypertension and obesity: two prehypertensive states with elevated cardiac output. Circulation. 1982;66:55–60.
- Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. Am J Hypertens. 1996;9:113S–120S.
- Grassi G, Vailati S, Bertinieri G, et al. Heart rate as marker of sympathetic activity. J Hypertens. 1998;16:1635–1639.
- Collins KJ. Age-related changes in autonomic control: the use of beta blockers in the treatment of hypertension. Cardiovasc Drug Ther. 1991;4(Suppl 6):1257–1262.
- Seals DR, Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–H1905.
- Esler M, Hastings J, Lambert G, et al. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. 2002;282:R909–R916.
- Ferrier C, Jennings GL, Eisenhofer G, et al. Evidence for increased noradrenaline release from subcortical brain regions in essential hypertension. J Hypertens. 1993;11:1217–1227.
- Guzzetti S, Piccaluga E, Casati R, et al. Sympathetic predominance in essential hypertension: a study employing spectral analysis of heart rate variability. J Hypertens. 1988;6:711–717.
- Julius S, Pascual AV, Sannerstedt R, et al. Relationship between cardiac output and peripheral resistance in borderline hypertension. Circulation. 1971;43:382–390.
- Palatini P, Rosei EA, Casiglia E, et al. Management of the hypertensive patient with elevated heart rate: Statement of the Second Consensus Conference endorsed by the European Society of Hypertension. J Hypertens. 2016;34:813–821.
- Zateyshchikov DA, Minushkina LO, Brovkin AN, et al. Association of CYP2D6 and ADRB1 genes with hypotensive and antichronotropic action of betaxolol in patients with arterial hypertension. Fundam Clin Pharmacol. 2007;21:437–443.
- Charkoudian N, Joyner MJ, Johnson CP, et al. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol (Lond). 2005;568:315–321.