Abstract
Purpose
Sodium and water handling by the kidney and the sympathetic nervous system have been implicated in the development of obesity-related hypertension and kidney disease. They have seldom been studied together during stress conditions. The objective of this study was to compare the systemic, renal and hormonal responses to lower body negative pressure (LBNP) in adult healthy participants (H), obese normotensive (OBN) and obese hypertensive patients (OBH).
Materials and methods
This was a prospective case-control study. Participants from the three groups were exposed to one hour of LBNP. Systemic and renal haemodynamics, sodium and water excretion and hormones were measured before and after LBNP. Intergroup LBNP responses were tested using a Student t-test or a Wilcoxon rank-sum test. An extension of the Wilcoxon rank-sum test was used to test for a trend across the three groups.
Results
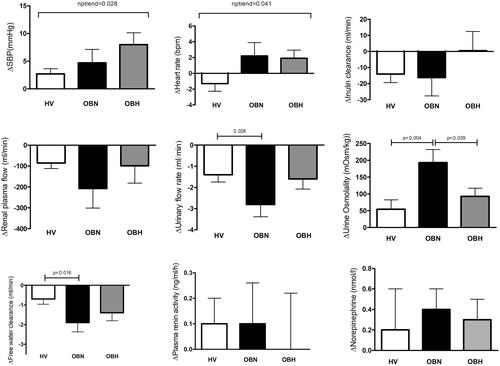
The study included 54 participants (H: 25, OBN: 16, OBH: 13). LBNP induced a stepwise increase in systolic blood pressure (+2.7 ± 4.7 mmHg (H) vs. +4.7 ± 8.8 mmHg (OBN) vs. +8.0 ± 8.6 mmHg (OBH, p = .028)) and heart rate (−1.3 ± 4.9 bpm (H) vs. 2.2 ± 6.1 bpm (OBN) vs. 1.9 ± 4.1 bpm (OBH, p = .041). Urinary output (−2.8 ± 2.1 ml/min vs. −1.4 ± 1.7 ml/min, p = .028) and free water clearance (−1.9 ± 1.7 mOsm/kg vs. −0.7 ± 1.3 mOsm/kg, p = .016) responses were more marked in OBN compared to H.
Conclusions
These results show that the systemic and the renal response to LBNP differ according to weight and to BP categories. Systolic BP and heart show a progressive increased response form healthy volunteers to OBN and then to obese hypertensive participants while urinary output and free water clearance responses are increased in OBN only, suggesting that the occurrence of hypertension in obese individuals modifies the early kidney responses to stress.
ClinicalTrial.gov Identifier
NCT01734096
Introduction
Epidemiological studies have shown that overweight and obesity are major factors predicting the development of hypertension [Citation1]. Their increasing prevalence worldwide [Citation2–4] is a worrisome public health threat. While the importance of obesity as a cause of hypertension is well established, the mechanisms by which excess weight increases blood pressure are complex and not yet fully elucidated. Studies have shown that the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous systems (SNS) are activated in obesity and may interact with leptin [Citation5,Citation6]. As far as systemic haemodynamics are concerned, increases in heart rate, cardiac output and arterial blood pressure (BP) are observed in parallel with increased sodium and water reabsorption [Citation7,Citation8]. However, the modification in renal sodium and water handling induced by the development of obesity with or without hypertension and their interactions with renal haemodynamics and neuro-endocrine responses remain poorly understood in humans [Citation9].
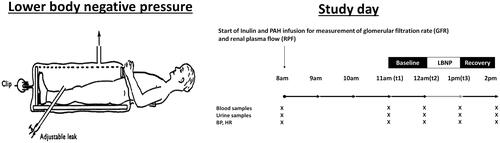
Lower body negative pressure (LBNP) has been used to investigate the cardiovascular and renal physiological effects of hypovolemia and thereupon simulate the SNS and the RAAS [Citation10–13]. The principle of his technique is to place the lower part of the participant’s body in a Plexiglas box with the application of negative pressure to the lower limbs (). The application of LBNP traps a portion of the circulating volume to the lower limbs, thus reducing the venous return to the heart and therefore decreasing the central venous pressure and cardiac output. This orthostatic stress contributes to progressive activation of arterial and cardiac baroreceptors, which stimulate efferent sympathetic nervous system through an afferent signalling pathway going to the brain. We have previously shown that increasing levels of sustained negative pressure progressively increases sympathetic nervous system activity, the renin-angiotensin system and finally induces sodium and water retention [Citation12]. At higher levels, renal vasoconstriction occurs in humans as well as in the dog [Citation14].
Figure 1. Illustration of LBNP investigation day. PAH: para-aminohippurate; BP: blood pressure; HR: heart rate.
We hypothesized that systemic hemodynamic, hormonal and renal responses to LBNP are modified in obese normotensive (OBN) compared to healthy normotensive volunteers (H) and in obese hypertensive (OBH) compared to OBN participants. The objectives were therefore to compare the blood pressure, the heart rate, the glomerular filtration rate (GFR), the renal plasma flow (RPF), the hormonal and the renal sodium and water excretion in response to 1 h LBNP in these three groups.
Methods
Patients and methods
This was a single centre prospetive case-control study and all participants were recruited either in the outpatient clinic of obesity of the Service of endocrinology or in the outpatient clinic of the Service of nephrology and hypertension at the University Hospital of Lausanne, Lausanne, Switzerland. Patients aged between 18 and 65 years old were eligible to the study. Healthy normotensive (H, office BP <140/90 mmHg, body mass index (BMI) <25 kg/m2) and obese participants (BMI ≥ 30 kg/m2) with hypertension (OBH, office BP ≥140/90 mmHg) or without hypertension (OBN, office BP <140/90 mmHg) were included in the study. Central obesity was defined as WHR ≥0.90 in men and ≥0.85 in women. We excluded pregnant patients or patients with a history of allergic reaction, orthostatic hypotension, diabetes, history of alcohol abuse, renal artery stenosis, cardiovascular and renal diseases or acute illness and smokers. After explaining the nature and purpose of the study, written informed consent was obtained from each patient. The protocol was approved by the local institutional review committee (Ethical Committee of the Canton de Vaud, Switzerland).
Complete medical history, physical examination including an active standing test (Schellong-test), blood sampling and urinalysis were performed at screening. After a 24-h ambulatory blood pressure monitoring (WatchBP03, Microlife AG, 9443 Widnau, Switzerland) with appropriate cuff size and 24-h urinary collection were performed one day before the investigation study day. Obese treated hypertensive patients stopped their antihypertensive drugs during two weeks before the investigation day to avoid confounding effect of antihypertensive drugs of the renal haemodynamics and the RAAS. To guarantee the security of the patient, we planned participants with symptomatic hypertension symptoms or BP >160/100 mmHg during this washout period were excluded. Caffeine and alcohol-containing beverage and smoking were prohibited 24 h before study days. The next morning, participants arrived at the investigation facility for the study day after an overnight fast. A standardized hydration protocol (5 ml/kg initial bolus followed by 150 ml each hour) was started and participants received intravenous inulin and para-aminohippurate (PAH) bolus followed by a constant infusion for the measurement of GFR and renal plasma flow (RPF), respectively. Hemodynamic parameters including blood pressure and heart rate were measured each 15 min. After an equilibration period of 2 h followed by two hours of baseline measurements, participants underwent LBNP during 1 h at −22.5 mmHg (−30 mbar) negative pressure. The chosen intensity of negative pressure corresponds to the level, which stimulates an anti-natriuresis response in healthy subjects [Citation12]. Urine and blood samplings to measure sodium, creatinine and endogenous lithium were performed hourly from the equilibration to the end of LBNP period. The design of the standard investigation day is illustrated in .
Analytical methods
Norepinephrine (NE), epinephrine (EPI) and urinary metanephrines (normetanephrine and metanephrine) were measured using ultra high-performance liquid chromatography-tandem mass spectrometry in plasma and urine [Citation15–17]. PRA was determined by a radioimmunoassay kit for the quantitative determination of Angiotensin I in human plasma (REN-CT2, Cisbio Bioassays, Codolet, France), and we measured aldosterone with a commercial RIA kit (Aldo-Riact; CIS Bio International, Yvette, Cedex, Saclay, France). Endogenous trace lithium, a marker of proximal sodium reabsorption, was measured by atomic absorption spectrophotometry [Citation18]. Plasma leptin was determined using a sensitive and specific sandwich ELISA for human leptin (EZHL-81K; Linco Research, St. Charles, MO). Total plasma adiponectin levels were determined using a sensitive and specific sandwich ELISA for human adiponectin (EZHADP-61K; Linco Research, St. Charles, MO).
The clearance of inulin, PAH or lithium (ml/min) was calculated using the standard formula Cx = Ux * V/Px, where Ux and Px are the urine and plasma concentrations of x and V is the urine flow rate in ml/min. Urinary electrolyte excretion rate was calculated as Ux * V. The fractional excretions were calculated as the clearance of x divided by the measured GFR. Renal blood flow (RBF) was calculated as RBF = RPF/(1 – Hematocrit) and renal vascular resistance (RVR) as RVR = mean blood pressure/RBF. Finally, the filtration fraction (FF) was calculated as inulin clearance/PAH clearance and free water clearance was calculated as ClH2O = (1 − urine osmolality/plasma osmolality) *urinary flow rate. Cumulated urinary normetanephrine and metanephrine were calculated as Cumulated x = (volume T1*UxT1) + (volume T2*UxT2) + (volume T3*UxT3), where T1, T2 and T3 are hourly timing before, during and after LBNP.
Statistical analyses
All results are expressed as mean ± standard deviation (SD) or median with interquartile range if variables were not normally distributed. A one-way ANOVA test was conducted to determine if baseline characteristics were different between the three groups (healthy participants [H], OBN or obese hypertensive participants). A Tukey post-hoc test was then conducted for intergroup comparisons. Intragroup comparisons between baseline characteristics and LBNP responses were analysed using Student t-test for repeated measures or Wilcoxon matched-pairs signed-rank test if variables were not normally distributed. Intergroup response (H vs. OBN and OBN vs. OBH) to LBNP was tested using a Student t-test or a Wilcoxon rank-sum test. In unadjusted analyses, we used a non-parametric test for a trend across groups (nptrend function in Stata developed by Cuzick, which is an extension of the Wilcoxon rank-sum test). Values with a p < .05 were considered statistically significant. All statistical analyses were conducted in Stata version 16.0 (Stata Corp, College Station, TX).
Results
Study population
Twenty-five healthy normotensive volunteers, 16 normotensive-obese and 13 hypertensive-obese patients were included in this study. Their baseline characteristics are shown in . Obese hypertensive was older than healthy volunteers. BMI and waist to hip ratio (WHR) were higher in the two obese groups. Central obesity was present in 69% OBN participants and 77% in OBH participants (p = .624). As expected, daytime, night-time and 24-h systolic and diastolic BP were higher in the obese hypertensive groups compared to the two other groups. There was no difference in 24-h sodium excretion between the three groups. The estimated GFR (CKD-EPI formula) was similar in the three groups in contrast to 24-h creatinine clearance, which was higher in obese patients. Compared to H, creatinine clearance was higher (+23.1 ± 8.9 ml/min)p = .033 in the OBN group and higher (+33.7 ± 9.5 ml/min (p = .002)) in the obese hypertensive group. All participants completed the LBNP procedure, which was well tolerated.
Table 1. Demographic characteristics.
Baseline (before LBNP) hemodynamic, renal and hormonal characteristics
SBP and DBP were higher in the OBN group than in the H group and higher in the OBH group than in the OBN group before the LBNP period (). RPF was higher (+378 ± 135 ml/min) in the OBN group compared to the H group. Leptin was higher in both obese groups compared to the H group as opposed to adiponectin, which was lower in the OBN group compared to the H group. There was no difference between groups in the other hormonal measurements.
Table 2. Clinical and biological parameters during baseline period (before LBNP).
Responses to LBNP
Systemic haemodynamic
LBNP induced an increase in systolic and mean blood pressure in all groups and an increase in DBP in the H and the OBH group (Supplementary Table 1). The magnitude of the systolic response increased progressively from healthy volunteers to OBN participants and further to obese hypertensive participants (nptrend, p = .028) (). Diastolic blood pressure increased in each group during the LBNP period. However, the magnitude of the response was not different between groups. Heart rate response to LBNP increased progressively from H to OBN and then to OBH (nptrend, p = .041) ().
Renal response
During orthostatic stress, water and sodium retention occurred leading to decreases in urinary output and free water clearance and an increase in urine osmolality in all groups. However, water retention was more marked in the OBN than in the H and OBH groups (). The increase in urine osmolality was also more pronounced in the OBN than in the H group during LBNP. Sodium excretion and lithium clearance decreased during the LBNP period in the H and the OBN group only with no difference in the magnitude of the response between groups.
LBNP induced a decrease in GFR in the H group only but RPF decreased both in the H and OBN groups but not in the OBH group. RVR increased in the H group only. An increase in the FF was noticed in the OBH group only. There was no difference in the magnitude of the GFR, RPF, FF and RVR responses between the three groups.
Hormones
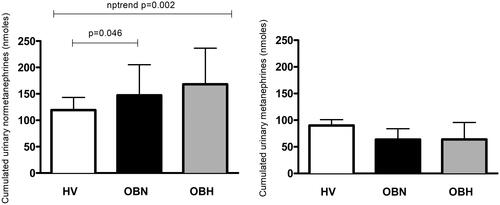
The hormonal response to LNBP was characterized by increases in NE and EPI in all groups. LBNP increased plasma renin activity in all groups except the OBH group. The magnitude of the response was however not different between groups () Cumulated urinary normetanephrine over a 3-h period including one hour of LBNP was higher in the OBN and the OBH group compared to the H group and progressively increased between groups (). This effect was not seen with cumulated urinary metanephrine.
Discussion
This study shows that 1 h of LBNP induces a gradual increase in systolic blood pressure and heart rate from healthy volunteers to OBN and then to obese hypertensive participants. OBN show an increased renal response to LBNP compared to healthy volunteers characterized by more severe drop in urine output and free water clearance resulting in an increased urine osmolality, which was not observed in obese hypertensive participants despite increased exposure to catecholamines as suggested by stepwise rise in urinary normetanephrine.
The association between obesity and increased sympathetic activity, such as muscle sympathetic nerve activity is well documented [Citation19]. The integrated systemic and renal response to stress has however not been studied as thoroughly and never simultaneously in obese participants with or without hypertension. So far, hemodynamic reactivity studies have used postural challenge, mental stress, front head cold pressor test to stress obese participants [Citation20–22]. In a cohort of adolescents, Barnes et al. showed that the upper tertile of waist-to-hip ratio, reflecting central adiposity, exhibited higher systolic and diastolic response to a postural challenge and a forehead cold pressure test [Citation21]. A greater systolic and diastolic BP and heart rate response was also demonstrated in older African American with the higher waist circumference [Citation20]. These two studies are in line with the graded response in systolic BP and heart rate response we observed across the H, the OBN and OBH groups. The results however differ from the study of Torres et al., which showed that moderate adiposity was associated with decreased systolic reactivity to a mental stress. Older age, shorter stimulation and a different stress test used in this latter study may explain the discordant findings.
As a key organ for sodium and water balance and a target of the sympathetic nervous system, renal function is essential to understand the pathogenic mechanisms of obesity-related hypertension. Our study is the first to report the effects of LBNP on renal haemodynamics and salt and water handling in obese participants with or without hypertension. Our results show that OBN patients decrease their urinary output and free water clearance and increase their urine osmolality significantly more than H, thereby suggesting that they are more sensitive to LBNP. The decrease in urinary output after one hour of −22.5 mmHg was expected. Indeed, we have shown in a previous study with the same design including healthy volunteers, that vasopressin (AVP) increases at this level of LBNP and is associated with lower urinary output, lower water clearance and higher urine osmolality [Citation12]. Thus, the stronger response observed in OBN participant suggests that either the AVP response is increased in this group or the sensitivity of renal V2 receptors is increased. A clue to the importance of the AVP system in obese patients is given by the prospective Malmö Diet and Cancer Study, which showed that the higher quartile of copeptin, the C-terminal fragment of AVP prohormone, were associated with incident abdominal adiposity [Citation23]. However, dynamic studies using insulin-induced hypoglycaemia, nicotine or metoclopramide to stimulate AVP have shown that AVP response to these stimuli is rather impaired in OBN patients [Citation24,Citation25]. Finally, Schinke et al., have shown recently that the association between presynaptic NE transporter availability with hypothalamic-pituitary-adrenal axis activation and AVP release is different in obese and non-obese participants, which suggests as central origin [Citation26]. Interestingly, new drugs targeting the brain renin-angiotensin system, such as brain aminopeptidase A inhibitors have shown to decrease vasopressin and to have some antihypertensive effect in obese hypertensive patients (OBH) [Citation27,Citation28]. The increased free water clearance response observed in OBN suggests that the blockade of the vasopressin 2 receptor could be an interesting early pharmacological target in the development of obesity-related hypertension.
We found that LBNP decreased sodium excretion in healthy volunteers and OBN participants, which is in line with our previous results in healthy volunteers [Citation12]. However, no antinatriuresis was detected in the obese hypertensive group. Several mechanisms may explain this difference. First, the obese hypertensive group had higher blood pressure and an increased blood pressure response to LBNP, which suggests a possible pressure natriuresis effect [Citation29]. Second, obese participants, whether hypertensive or not, have increased absolute GFR and RPF [Citation30,Citation31] that was also observed in this study. Thus, our obese participants had higher 24 h creatinine clearance (), higher GFR (+20.0 ± 9.6 ml/min, p = .042) and higher RPF (+273 ± 116 ml/min), p = .026). Exposure to LBNP induced a decrease in GFR only in the H group and a decrease RPF in the H and the OBN group without affecting the OBH group. This resulted in an increased FF on the OBH group. Therefore, one could postulate that the renal hemodynamic response to LBNP observed in OBH has offset the sodium retention classically found in healthy subjects. In our three groups, sodium reabsorption seemed to parallel changes in renal haemodynamics.
Limitations to our study include the sample size, which was however calculated to see 30% changes between groups. It is not excluded that more subtle changes might have been seen with a larger sample. Some hormones such AVP or copeptin were not measured in our study. Retrospectively, their measurement would have been useful to interpret the change in water clearance observed in our study. A fourth group of non-obese hypertensive patient could have been interesting to study and to compare to the obese hypertensive group. We did not include a fourth group (hypertensive non-obese) in our study. A posteriori, the inclusion of this group would have been interesting in the comparison between obese hypertensive and non-obese hypertensive. If some studies have compared the LBNP-response in healthy and hypertensive participants, we are not aware of a study focussing on renal electrolytes and water excretion [Citation32,Citation33]. Frank et al. have shown that the hypertensive participants respond similarly to healthy volunteers with an increase in heart and BP and a decrease in GFR and RPF during LBNP, which confirms our findings in healthy volunteers but sets them apart from obese who did not change their GFR during LBNP [Citation33].
In conclusion, our data show that there is a graded response in blood pressure and heart rate from healthy volunteers to OBN and then to obese hypertensive participants with increased exposure to NE as suggested by increasing levels of urinary normetanephrine. The renal response to LBNP however seems to be dependent on two factors, the presence or absence of obesity and the presence or absence of hypertension. OBN is clearly more sensitive to the sympathetic stress in terms of urinary output and free water clearance. These differences in renal responses might reflect the natural history towards hypertension and/or chronic kidney damage in obese patients. Whether weight loss reverse this increased sensitivity to orthostatic stress induced by LBNP remains to be determined.
Supplementary_table_1.docx
Download ()Acknowledgements
The authors wish to thank Professor Burnier for critical review of the manuscript.
Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Funding
References
- Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23(11):1170–1178.
- Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814.
- Johnson F, Cooke L, Croker H, et al. Changing perceptions of weight in Great Britain: comparison of two population surveys. BMJ. 2008; 337:a494.
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781.
- Haynes WG. Role of leptin in obesity-related hypertension. Exp Physiol. 2005;90(5):683–688.
- Vaz M, Jennings G, Turner A, et al. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96(10):3423–3429.
- Esler M, Straznicky N, Eikelis N, et al. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48(5):787–796.
- Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10(5 Pt 2):49S–55S.
- Schwotzer N, Burnier M, Maillard M, et al. Sex and body mass index modify the association between Leptin and sodium excretion: a cross-sectional study in an African population. Am J Hypertens. 2019;32(11):1101–1108.
- Convertino VA. Lower body negative pressure as a tool for research in aerospace physiology and military medicine. J Gravit Physiol. 2001;8(2):1–14.
- Wuerzner G, Chicléro A, Maillard M, et al. Angiotensin II receptor blockade prevents acute renal sodium retention induced by low levels of orthostatic stress. Kidney Int. 2004;65(1):238–244.
- Wurzner G, et al. Renal and neurohormonal responses to increasing levels of lower body negative pressure in men. Kidney Int. 2001;60(4):1469–1476.
- Wuerzner G, Chiolero A, Maillard M, et al. Metoprolol prevents sodium retention induced by lower body negative pressure in healthy men. Kidney Int. 2005;68(2):688–694.
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77(1):75–197.
- Grouzmann E, Drouard-Troalen L, Baudin E, et al. Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. Eur J Endocrinol. 2010;162(5):951–960.
- Kairisto V, Koskinen P, Mattila K, et al. Reference intervals for 24-h urinary normetanephrine, metanephrine, and 3-methoxy-4-hydroxymandelic acid in hypertensive patients. Clin Chem. 1992;38(3):416–420.
- Dunand M, et al. High-throughput and sensitive quantitation of plasma catecholamines by ultraperformance liquid chromatography-tandem mass spectrometry using a solid phase microwell extraction plate. Anal Chem. 2013;85:3539–3544.
- Magnin JL, Decosterd LA, Centeno C, et al. Determination of trace lithium in biological fluids using graphite furnace atomic absorption spectrophotometry: variability of urine matrices circumvented by cation exchange solid phase extraction. Pharm Acta Helv. 1996;71(4):237–246.
- Grassi G, Biffi A, Seravalle G, et al. Sympathetic neural overdrive in the obese and overweight state. Hypertension. 2019;74(2):349–358.
- Waldstein SR, Burns HO, Toth MJ, et al. Cardiovascular reactivity and central adiposity in older African Americans. Health Psychol. 1999;18(3):221–228.
- Barnes VA, Treiber FA, Davis H, et al. Central adiposity and hemodynamic functioning at rest and during stress in adolescents. Int J Obes. 1998;22(11):1079–1083.
- Torres SJ, Turner AI, Jayasinghe SU, et al. The effect of overweight/obesity on cardiovascular responses to acute psychological stress in men aged 50–70 years. Obes Facts. 2014;7(6):339–350.
- Enhörning S, Bankir L, Bouby N, et al. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: the prospective Malmö Diet and Cancer Study cardiovascular cohort. Int J Obes (Lond). 2013;37(4):598–603.
- Coiro V, Chiodera P. Effect of obesity and weight loss on the arginine vasopressin response to insulin-induced hypoglycaemia. Clin Endocrinol (Oxf)). 1987;27(2):253–258.
- Chiodera P, Capretti L, Davoli C, et al. Effect of obesity and weight loss on arginine vasopressin response to metoclopramide and nicotine from cigarette smoking. Metab Clin Exp. 1990;39(8):783–786.
- Schinke C, Hesse S, Rullmann M, et al. Central noradrenaline transporter availability is linked with HPA axis responsiveness and copeptin in human obesity and non-obese controls. Stress. 2019;22(1):93–102.
- Llorens-Cortes C, Touyz RM. Evolution of a new class of antihypertensive drugs: targeting the brain renin-angiotensin system. Hypertension. 2020;75(1):6–15.
- Ferdinand KC, Balavoine F, Besse B, et al. Efficacy and safety of firibastat, a first-in-class brain aminopeptidase A inhibitor, in hypertensive overweight patients of multiple ethnic origins. Circulation. 2019;140(2):138–146.
- Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41(3 Pt 2):625–633.
- Ribstein J, Du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995; 26(4):610–615.
- Wuerzner G, Pruijm M, Maillard M, et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am J Kidney Dis. 2010;56(2):303–312.
- Floras JS, Aylward PE, Mark AL, et al. Adrenaline facilitates neurogenic vasoconstriction in borderline hypertensive subjects. J Hypertens. 1990;8(5):443–448.
- Frank H, Schobel H-P, Vitkowsky J, et al. Effects of angiotensin II receptor antagonism on the renal hemodynamic response to cardiovascular stress. Kidney Int. 2003;63(2):617–623.