Abstract
Purpose
We tested the sex-specific associations between primary aldosteronism (PA), left ventricular (LV) hypertrophy and LV systolic myocardial function.
Material and methods
Conventional and speckle tracking echocardiography was performed in 109 patients with PA and 89 controls with essential hypertension (EH). LV hypertrophy was identified if LV mass index exceeded 47.0 g/m2.7 in women and 50.0 g/m2.7 in men. LV systolic myocardial function was assessed by global longitudinal strain (GLS) and midwall shortening.
Results
PA patients had higher prevalence of LV hypertrophy (52 vs. 21%, p < 0.001) than EH patients in both sexes, while GLS did not differ by sex or hypertension aetiology. In multivariable analyses, presence of LV hypertrophy was associated with PA and obesity in both sexes, while lower systolic myocardial function, whether measured by GLS or midwall shortening, was not associated with PA, but primarily with higher body mass index and LV mass index, respectively, in both sexes (all p < 0.05).
Conclusion
Having PA was associated with higher prevalence of LV hypertrophy both in women and men, compared to EH. PA was not associated with LV systolic myocardial function in either sex.
Introduction
Uncontrolled hypertension induces preclinical cardiac disease like left ventricle (LV) hypertrophy or LV systolic dysfunction, which are precursors for clinical cardiovascular disease [Citation1,Citation2]. The severity of preclinical cardiac disease is influenced by blood pressure load and the presence of other cardiometabolic risk factors, in particular sex, obesity and diabetes mellitus [Citation3]. Primary aldosteronism (PA) is the most common cause of secondary hypertension and is often found in patients with uncontrolled hypertension [Citation2]. PA causes a variety of cardiovascular, renal, metabolic and bone complications relatively independent of blood pressure (BP) [Citation4]. PA is associated with metabolic syndrome [Citation5], and it has recently been suggested that obesity-related factors contribute to the pathogenesis of idiopathic hyperaldosteronism [Citation6]. It is well known that LV hypertrophy is more common in PA than in essential hypertension (EH) [Citation7,Citation8]. This has been related to aldosterone induced myocyte hypertrophy and cardiac interstitial collagen deposition and fibrosis [Citation8,Citation9]. Despite more prevalent LV hypertrophy, several studies have demonstrated comparable LV ejection fraction in PA and EH [Citation8,Citation10], but few studies in PA have assessed LV myocardial function by speckle tracking echocardiography.
Sex differences in LV hypertrophy and LV myocardial function are well described in EH, but scarcely reported in PA. Previous studies in EH have demonstrated that women are more prone to develop LV hypertrophy than men [Citation11,Citation12], and that regression of LV hypertrophy during antihypertensive treatment is attenuated in women, when obesity is co-present [Citation13]. Several studies have reported that women with LV hypertrophy retain higher systolic function than their male counterparts [Citation11,Citation14,Citation15]. Whether these sex-specific characteristics also apply for patients with PA remains to be explored. The present study aimed at exploring the sex-specific associations of PA with LV hypertrophy and LV myocardial function.
Materials and methods
Study population
We recruited consecutively 109 patients diagnosed with PA at Haukeland University hospital between 2013 and 2016. In patients with elevated aldosterone-to-renin ratio, PA was confirmed by recumbent saline infusion testing, with a positive test defined as post-infusion plasma aldosterone level > 140 pmol/L [Citation16]. Adrenal vein sampling, under continuous cosyntropin infusion, was performed for subtype differentiation at time of study inclusion. Unilateral disease was found in 47%, with no difference between sexes. Interfering medication was withdrawn for 2–4 weeks before diagnostic assessments. Controls were identified among patients with EH that participated in the FAT-associated CardiOvasculaR dysfunction (FATCOR study), another study cohort established at the European Society of Hypertension Excellence Centre in Bergen [Citation17]. EH and PA patients were matched for sex, presence of obesity and age within a five-year range. The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethics Committee. All participants signed written informed consent before inclusion.
Cardiovascular risk assessment
Attended clinic BP was measured in triplets in the seated position after at least 5 min rest, using a calibrated aneroid sphygmomanometer, in accordance with current guidelines [Citation2]. Clinic BP was taken as the average of the two last measurements. Obesity was identified as body mass index (BMI) ≥30.0 kg/m2. Diabetes mellitus was considered present if history of diabetes mellitus or haemoglobin (Hb) A1c >6.5% was found. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation [Citation18]. Hypokalaemia was considered present in the individual patients if serum potassium <3.5 mmol/L, or the patient was taking potassium supplementation.
Echocardiography
All patients underwent conventional and two-dimensional speckle tracking echocardiography following a standardised imaging protocol using a GE Vivid E9 scanner (GE Vingmed Ultrasound, Horten, Norway). In the PA group, echocardiography was performed as soon as a conclusive diagnosis was reached. Images were analysed in the Echocardiography Core Laboratory at the University of Bergen, Bergen, Norway, on workstations equipped with Image Arena Software version 4.2 (TomTec Imaging systems GmbH, Unterschleissheim, Germany) for conventional measurements. All images were read by a junior investigator and proofread by a single expert reader following current guidelines for chamber quantification [Citation19]. LV hypertrophy was identified by validated prognostic sex-specific cut-off values for LV mass index (>47.0 g/m2.7 in women and >50.0 g/m2.7 in men), as recommended in the current guidelines on management of arterial hypertension [Citation2]. Relative wall thickness was calculated from posterior wall thickness/LV internal radius ratio and considered increased if >0.42 (Citation19]. LV geometry was defined from relative wall thickness and LV mass index in combination. Concentric remodelling was defined as increased relative wall thickness with normal LV mass index, while concentric LV hypertrophy was defined as increased relative wall thickness and increased LV mass index. Eccentric LV hypertrophy was defined as increased LV mass index with normal relative wall thickness [Citation19]. LV ejection fraction was calculated using biplane method and discs summation (modified Simpson’s rule) [Citation19]. Midwall shortening was calculated by validated equations [Citation20]. LV filling pressure was estimated from the ratio of early transmitral filling velocity(E) to the average of septal and lateral early diastolic mitral annular plane velocity (e’) ratio (E/e’ ratio) [Citation21].
Workstations equipped with EchoPac software version BT202 (GE Vingmed Ultrasound, Horten, Norway) were used for two-dimensional speckle tracking echocardiography. The average value of peak systolic global longitudinal strain (GLS) was calculated from 17 individual LV segments based on three apical imaging planes using automatic function imaging. The endocardial border was tracked automatically and adjusted manually when considered suboptimal by visual assessment. Segments with inadequate tracking were excluded. End-systole was defined by aortic valve closure using pulse wave Doppler. In 13 patients average GLS could not be calculated, because two or more segments were not adequately tracked in the same view.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 26 software (IBM, Armonk, NY). Data is reported as mean and standard deviation for continuous variables and as percentages for categorical variables. The study compared PA and EH groups and was further divided into four subgroups: PA women, PA men, EH women and EH men. Groups are compared with un-paired t-tests and chi square tests, and with one-way analysis of variance (ANOVA) with Scheffe’s post-hoc test for continuous variables and general linear model with Sidak’s post hoc test for categorical variables as appropriate. Uni- and multivariable logistic regression analyses were used to assess the association of PA with presence of LV hypertrophy in the total study population and separately in women and men. All variables with a significant univariable association were included in the multivariable models. Results from logistic regression analyses are reported as odds ratio with corresponding 95% confidence intervals and p values. Uni- and multivariable linear regression analyses were used to assess the association of PA with LV systolic myocardial function measured by GLS and midwall shortening in the total study population and separately in women and men. In the primary multivariable models for GLS and midwall shortening, we adjusted for clinical variables with significant univariable associations. In secondary models, echocardiographic variables with significant univariable associations were added as covariables. Collinearity tools were used to document sound statistics. Results from linear regression analyses are reported as standardised β-coefficients and p values. Statistical significance was defined as p < 0.05 in all analysis. The study had statistical power to identify differences in prevalence of LV hypertrophy in sex-specific analysis (β = 80% and α < 0.05).
Results
Clinical characteristics
In the total study population PA patients were older, had higher systolic BP, lower BMI and lower renal function compared to EH patients (). PA men were older and had lower renal function than EH men, while PA women and EH women had similar baseline characteristics (). Seventy-four percent of PA patients had hypokalaemia, with no difference between sexes (p = 0.680).
Table 1. Characteristics of women and men with PA compared to EH.
Echocardiographic characteristics
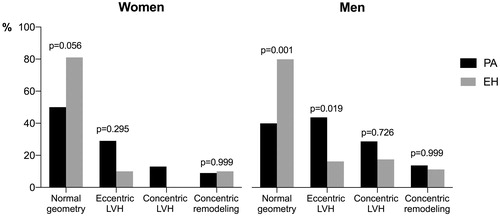
PA patients had higher prevalence of abnormal LV geometry than EH patients (). LV hypertrophy was more common in PA than EH patients both in women (41 vs. 10%) and men (57 vs. 27%, both p < 0.05). In the total population women had lower prevalence of LV hypertrophy than men (26 vs 44%, p = 0.012). In PA patients with LV hypertrophy, eccentric LV hypertrophy was the domination type in both sexes (71% in women and 60% in men). LV systolic myocardial function assessed by GLS was similar in PA and EH patients in the total study population, but GLS was lower in men than in women in both groups (). Midwall shortening was significantly lower in PA men than EH men (14.0 vs. 15.4%, p = 0.023), but did not differ in women ().
Table 2. Echocardiographic findings in women and men with PA compared to EH.
Factors associated with LV hypertrophy in women and men
In univariable analysis, LV hypertrophy was associated with PA, male sex, age, obesity, higher systolic BP and lower eGFR in the total study population, while no association with HbA1c was found (). With exception of male sex and eGFR, these factors remained associated with presence of LV hypertrophy in multivariable analysis (). In sex-specific analyses, PA was associated with LV hypertrophy in both sexes independent of a significant association with obesity ().
Table 3. The association of PA with LVH in the total study population, and in women and men separately.
Factors associated with LV myocardial function in women and men
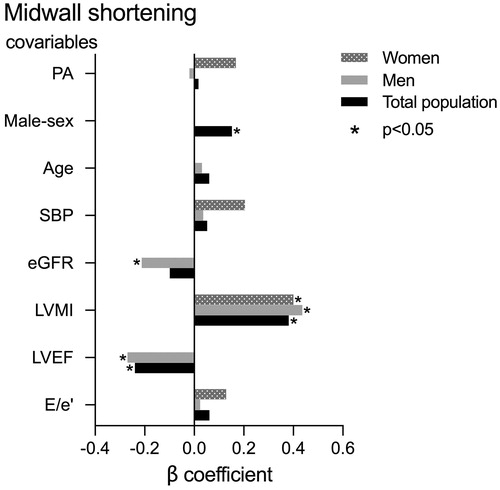
In univariable analysis, lower midwall shortening was associated with PA, male sex, higher age, systolic BP, E/e’ ratio and LV mass index and with lower eGFR and LV ejection fraction in the total study population (all p < 0.05). In the primary multivariable model, including clinical covariables, lower midwall shortening remained associated with male sex (β = 0.29) and higher systolic BP (β = 0.15, both p < 0.05), while the association with PA became statistically not significant. In sex-specific analysis, including clinical covariables, lower midwall shortening was associated with higher systolic BP in women (β = 0.26, p < 0.05), whereas no covariables in the model were significantly associated with midwall shortening in men. In a second multivariable model we adjusted also for LV mass index, E/e’ ratio and LV ejection fraction. In this second model, no significant association of PA with midwall shortening was found. However, lower midwall shortening was associated with male sex, higher LV mass index and lower LV ejection fraction in the total study population, with higher LV mass index and lower LV ejection fraction and eGFR in men, and with higher LV mass index in women. (all p < 0.05; ).
Figure 2. The association of PA with lower midwall shortening in the total study population, and in women and men separately.
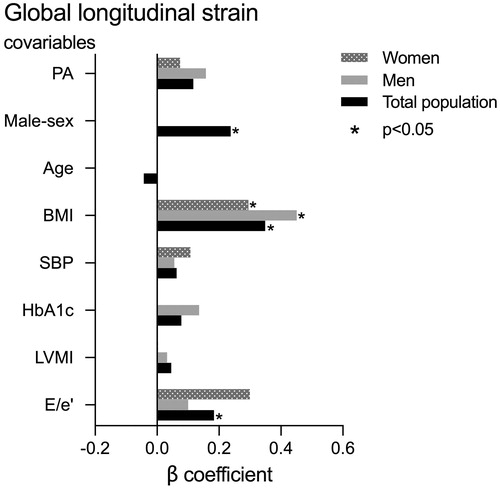
PA was not associated with GLS neither in univariable nor in multivariable analysis. In univariable analysis, lower GLS was associated with male sex, higher age, BMI, systolic BP, HbA1c, LV mass index and E/e’ ratio in the total study population (all p < 0.05). In the primary model adjusting for clinical variables, only male sex (β = 0.23) and higher BMI (β = 0.38, both p < 0.05) remained associated with GLS. In sex-specific analysis adjusting for clinical variables, higher BMI (β = 0.47, p < 0.001) was the only significant covariable for GLS in men, while no covariables were significantly associated with lower midwall shortening in women. In a second multivariable model additionally adjusting for LV mass index and E/e’ ratio, lower GLS was associated with male sex, higher BMI and E/e’ ratio in the total study population, while higher BMI was the main covariable of lower GLS in sex-specific analyses (all p < 0.05; ).
Discussion
This study adds to previous knowledge by demonstrating that PA, as compared to EH, is associated with higher prevalence of LV hypertrophy both in women and men. However, PA was not associated with lower LV systolic myocardial function whether measured by GLS or midwall shortening in either sex.
In line with previous reports, prevalence of LV hypertrophy was higher in PA than EH [Citation7,Citation22,Citation23]. However, the predominate type of LV hypertrophy varied in previous studies comparing LV geometry in PA and EH [Citation10]. Salvetti et al. recently reported higher prevalence of concentric LV hypertrophy in PA [Citation22], whereas Yang et al. reporting sex-specific results, found a higher prevalence of eccentric LV hypertrophy in PA women, but not in men [Citation24]. In our cohort, eccentric LV hypertrophy was the dominating type in PA in both sexes. The independent association of PA with presence of LV hypertrophy in sex-specific multivariable analysis in our study, supports that aldosterone excess contributed to LV hypertrophy both in women and men [Citation25]. However, independent of hypertension aetiology, obesity was strongly associated with LV hypertrophy in both sexes, in line with previous reports in EH [Citation26,Citation27]. Previous reports in larger EH studies have demonstrated that LV hypertrophy is more common in women [Citation11,Citation12]. This was not found in the present smaller cohort, possibly related to women being younger and less obese than women in previous studies. Furthermore, there is evidence that oestrogen inhibits synthesis of aldosterone in women [Citation28]. Oestrogen has been found to attenuate cardiac hypertrophy and fibrosis in studies both in humans and mice [Citation3]. Thus, we can speculate that a protective effect of oestrogen may contribute to the observed lower prevalence of LV hypertrophy in women in our cohort. However, serum oestrogen was not measured in the present study.
Previous reports comparing LV systolic myocardial function by midwall shortening in PA and EH have yielded diverging results, some reporting lower midwall shortening in PA compared to EH [Citation7,Citation29], whereas others found no difference [Citation8,Citation30]. However, none of these studies reported sex-specific results. Thus, the present study adds to previous knowledge by demonstrating that although midwall shortening was lower in PA men than in EH men, midwall shortening did not differ between PA and EH women, and PA was not associated with lower midwall shortening in the multivariable models. PA women had lower LV mass index than PA men, which may explain why midwall shortening was less deteriorated in PA women [Citation20]. However, independent of hypertension aetiology, higher systolic BP was associated with lower midwall shortening, in line with previous publications [Citation14,Citation27].
GLS is a measure of LV longitudinal myocardial function which has been associated with presence and extent of myocardial fibrosis assessed by gadolinium enhanced cardiac magnetic resonance imaging [Citation31]. Few PA studies have reported LV systolic myocardial function by GLS. Two smaller studies demonstrated lower GLS in PA than EH [Citation23,Citation32]. In contrast, we found comparable GLS in PA and EH, both in women and men, in the present study. The association between obesity and lower GLS is well demonstrated, particularly when hypertension co-exists [Citation27,Citation33], as also demonstrated in the present study for both sexes. However, EH controls had higher BMI than PA, which may have reduced GLS particularly in the EH group. Our finding of comparable GLS in PA and EH groups may also be explained by a low prevalence of myocardial fibrosis in the PA patients in our cohort, as demonstrated in a sub study of 32 PA patients who underwent cardiac magnetic resonance imaging with late gadolinium enhancement or T1 mapping, and who did not have increased myocardial fibrosis compared to healthy subjects [Citation34]. In line with previous reports in EH, midwall shortening and GLS were both higher in women than in men with PA, and male sex was independently associated with lower midwall shortening and GLS in multivariable analysis in the total population [Citation14,Citation15].
Study limitations
There are several limitations to this study. First, the EH and PA patients were not matched for BP values, and duration of hypertension was not known. Furthermore, the clinic BP in the PA group may have been influenced by the routine change of BP medications during the diagnostic work up, and therefore not reflect the individual BP burden preceding the diagnostic work up. The underrepresentation of women in our study cohort is another important limitation, and type 2 error may be present, since power calculation was solely based upon prevalence of LV hypertrophy.
Disclosure statement
The authors declare no conflict of interest.
References
- Devereux RB, Alderman MH. Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation. 1993;88:1444–1455.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314–340.
- Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666.
- Mulatero P, Sechi LA, Williams TA, et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38(10):1929–1936.
- Fallo F, Pilon C, Urbanet R. Primary aldosteronism and metabolic syndrome. Horm Met Res. 2012;44(03):208–214.
- Ohno Y, Sone M, Inagaki N, et al. Obesity as a key factor underlying idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 2018;103(12):4456–4464.
- Muiesan ML, Salvetti M, Paini A, et al. Inappropriate left ventricular mass in patients with primary aldosteronism. Hypertension. 2008;52(3):529–534.
- Rossi GP, Di Bello V, Ganzaroli C, et al. Excess ldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension. 2002;40(1):23–27.
- Freel EM, Mark PB, Weir RA, et al. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: a cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5(6):740–747.
- Milan A, Magnino C, Fabbri A, et al. Left heart morphology and function in primary aldosteronism. High Blood Press Cardiovasc Prev. 2012;19(1):11–17.
- Gerdts E, Okin PM, De Simone G, et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51(4):1109–1114.
- Gerdts E, Izzo R, Mancusi C, et al. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania Salute Network). Int J Cardiol. 2018;258:257–261.
- Lønnebakken MT, Izzo R, Mancusi C, et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania Salute Network). J Am Heart Assoc. 2017;6(3):e004152.
- Gerdts E, Zabalgoitia M, Björnstad H, et al. Gender differences in systolic left ventricular function in hypertensive patients with electrocardiographic left ventricular hypertrophy (the LIFE study). Am J Cardiol. 2001;87(8):980–983.
- Tadic M, Cuspidi C, Grassi G. The influence of sex on left ventricular remodeling in arterial hypertension. Heart Fail Rev. 2019;24(6):905–914.
- Funder J, Carey R, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266–3281.
- Halland H, Lønnebakken M, Pristaj N, et al. Sex differences in subclinical cardiac disease in overweight and obesity (the FATCOR study). Nutr Metab Cardiovasc. 2018;28(10):1054–1060.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–271.
- de Simone G, Devereux RB, Roman MJ, et al. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23(6):1444–1451.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur J Echocardiogr. 2016;17(12):1321–1360.
- Salvetti M, Paini A, Bertacchini F, et al. Myocardial mechano-energetic efficiency in primary aldosteronism. J Hypertens. 2021;139(2):318–324.
- Chen Z-W, Huang K-C, Lee J-K, et al. Aldosterone induces left ventricular subclinical systolic dysfunction: a strain imaging study. J Hypertens. 2018;36(2):353–360.
- Yang Y, Zhu L-m, Xu J-z, et.al. Comparison of left ventricular structure and function in primary aldosteronism and essential hypertension by echocardiography. Hypertens Res. 2017;40(3):243–250.
- Rossi GP, Sacchetto A, Visentin P, et al. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension. 1996;27(5):1039–1045.
- Mancusi C, Gerdts E, Losi MA, et al. Differential effect of obesity on prevalence of cardiac and carotid target organ damage in hypertension (the Campania Salute Network). Int J Cardiol. 2017;244:260–264.
- Herfindal B, Gerdts E, Kringeland EA, et al. Concomitant hypertension is associated with abnormal left ventricular geometry and lower systolic myocardial function in overweight participants: the FAT associated Cardiovascular dysfunction study. J Hypertens. 2020;38(6):1158–1164.
- Rossi GP, Caroccia B, Seccia TM. Role of estrogen receptors in modulating aldosterone biosynthesis and blood pressure. Steroids. 2019;152:108486.
- Kozàkovà M, Buralli S, Palombo C, et al. Myocardial ultrasonic backscatter in hypertension: relation to aldosterone and endothelin. Hypertension. 2003;41(2):230–236.
- Catena C, Colussi G, Lapenna R, et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50(5):911–918.
- Spartera M, Damascelli A, Mozes F, et al. Three-dimensional speckle tracking longitudinal strain is related to myocardial fibrosis determined by late-gadolinium enhancement. Int J Cardiol. 2017;33(9):1351–1360.
- Wang D, Xu J-Z, Chen X, et al. Speckle-tracking echocardiographic layer-specific strain analysis on subclinical left ventricular dysfunction in patients with primary aldosteronism. Am J Hypertens. 2019;32(2):155–162.
- Orhan AL, Uslu N, Dayi SU, et al. Effects of isolated obesity on left and right ventricular function: a tissue Doppler and strain rate imaging study. Echocardiography. 2010;27(3):236–243.
- Grytaas MA, Sellevåg K, Thordarson HB, et al. Cardiac magnetic resonance imaging of myocardial mass and fibrosis in primary aldosteronism. Endocr Connect. 2018;7(3):413–424.