Abstract
Purpose
Although 24-hour ambulatory blood pressure measurement (24-h ABPM) is the most important method to establish true hypertension, in clinical practice often repeated automated office blood pressure (AOBP) measurements are used because of convenience and lower costs. We aimed to assess the agreement rate between a 30 and 60 min AOBP and 24-h ABPM.
Materials and methods
Patients with known hypertension (cohort 1) and patients visiting the neurology outpatient clinic after minor stroke or transient ischaemic attack (cohort 2) were selected. We performed AOBP for 30–60 min at 5-min intervals followed by 24-h ABPM and calculated average values of both measurements. Agreement between the two methods was studied with McNemar and Bland–Altman plots with a clinically relevant limit of agreement of ≤10 mm Hg difference in systolic BP.
Results
Our final cohort consisted of 135 patients from cohort 1 and 72 patients from cohort 2. We found relatively low agreement based on the clinical relevant cut-off value; 64.7% of the measurements were within the limits of agreement for 24-h systolic and 50.2% for 24-h diastolic. This was 61.4% for daytime systolic and 56.6% for daytime diastolic. In 73.5% of the patients, both methods led to the same diagnosis of either being hypertensive or non-hypertensive. This resulted in a significant difference between the methods to determine the diagnosis of hypertension (p < 0.0001).
Conclusion
We conclude that 30–60 min AOBP measurements cannot replace a 24-h ABPM and propose to perform 24-h ABPM at least on a yearly basis to confirm AOBP measurements.
Introduction
There are various ways to measure blood pressure (BP). Unfortunately, the most reliable method to establish true hypertension, the 24-hour (24-h) ambulatory blood pressure measurement (ABPM), is also the least patient-friendly and most time-consuming method [Citation1]. Therefore, many previous studies have tried to determine if less costly and time-consuming methods like home blood pressure monitoring (HBPM) or repeated, automated, unattended office blood pressure measurements (AOBP) are as reliable as the 24-h ABPM to detect hypertension. Reliable measurements are pivotal for the diagnosis of hypertension and several factors influence this diagnosis.
The first factor is the definition of hypertension with corresponding BP values that are continuously subject to change due to emerging evidence of the benefits of low BP on morbidity and mortality endpoints [Citation2,Citation3]. Furthermore, BP is influenced by day-to-day changes, for instance by the so-called white coat effect and masked hypertension [Citation4–6]. The way BP is measured, is, therefore, crucial to diminish these influences to assess the true blood pressure.
However, each method to measure BP has its own pros and cons. HBPMs, on the one hand, are very convenient for the patient and seem reliable, and especially during the COVID pandemic contributing to limiting the number of hospital visits. On the other hand, new challenges arise like the quality of the BP monitor, costs for the patient to purchase the monitor, and instruction of the patient to use the monitor correctly [Citation7,Citation8].
Considering these different factors, AOBP measurements may be an important alternative in clinical practice. In the most recently published hallmark study SPRINT on systolic blood pressure (SBP) targets, three unobserved or partially unobserved AOBP measurements with an interval of 1 min were used to measure BP [Citation9,Citation10]. This led to some discussion about the feasibility and reliability of this method in clinical practice. The discussion point on feasibility can easily be refuted as AOBP measurements of 30 min to 1 h with intervals of 5 min are routinely used in secondary and tertiary hospitals in the Netherlands. However, only a few studies investigated the agreement between this type of AOBP measurements with daytime or 24-h ABPM [Citation11,Citation12].
In most comparisons between AOBP and APBM, usually using the SPRINT regime with 1-min intervals for AOBP, a Bland-Altman plot was used to determine the agreement between both BP monitoring methods [Citation13]. Furthermore, several studies that compared AOBP with ABPM used a 2-SD cut-off in the Bland–Altman plot to determine agreement [Citation12,Citation14–16]. However, this cut-off value results in a wide range of measurements that are within the limits of agreement without taking clinical relevance into account. Therefore, we suggest defining a clear clinically relevant cut-off value beforehand, as earlier suggested by Bland and Altman [Citation16–18]. We think this leads to a better comparison between the two methods and will lead to a conclusion that in turn may inform clinical practice. Also, it is important to identify specific patient characteristics that are more prone to deviating blood pressure measurements when AOBP is used compared to ABPM.
Therefore, in the present study, we compared an unattended 30–60 min AOBP with a 24-ABPM including daytime and night-time values and applied predefined limits of agreement. As an exploratory analysis, we aimed to examine associations between clinically relevant factors explaining disagreement between these measurements. For this purpose, we analysed data from two cohorts of patients that recently had a minor stroke or TIA or a diagnosis of hypertension.
Methods
Patients
Patients were recruited from the hypertension outpatient clinic and neurology outpatient clinic of the Erasmus University Medical Centre (UMC), Rotterdam, The Netherlands. Based on the department, patients were divided into two cohorts. Cohort 1 consisted of patients from the hypertension outpatient clinic and was recruited from May 2009 up to and till December 2011. Although this study did not match the criteria of the Medical Research Involving Human Subjects Act, written informed consent was obtained from each patient.
The second cohort consisted of patients visiting the neurology outpatient clinic after they experienced a minor stroke with a modified Rankin Scale score of ≤3 or a transient ischaemic attack (TIA) [Citation19]. Patients were recruited when they participated in another prospective study concerning dipping patterns in patients after a minor stroke or TIA (unpublished). This study was approved by the Medical Ethics Committee of the Erasmus MC and written informed consent was obtained. For the study of dipping patterns, they already underwent a 24-h ABPM and because of this only the AOBP was added to the measurements. The diagnosis of pre-existent hypertension in cohort 2 was based on the use of antihypertensive drugs prior to the event instead of the BP itself. We thereby excluded patients that accidentally had high BP due to the acute phase reaction during a stroke event.
Study design
A prospective cohort study was performed to collect all the blood pressure data. At the time of inclusion, all patients from the hypertension patient clinic underwent both a 60-min AOBP and 24-h ABPM as part of the usual care. Patients from cohort 2 received the measurements as part of a study. Only measurements from patients who underwent a 30–60 min AOBP on the day before or after a 24-h ABPM were used for further analyses. The application of the BP measurement devices was performed by trained nurses. If the BP measurements were incorrect or incomplete, patients were excluded from the analysis.
Demographic data, vascular history, and risk factors, use of antihypertensive drugs, renal function, and data on event characteristics were collected.
Blood pressure measurements and target values
Using oscillometric SpaceLabs 90207 monitor (SpaceLabs Healthcare, Issaquah, Washington, United States of America), 24-h ABPM was recorded [Citation20] with the device attached to the non-dominant arm. Patients were instructed to relax their arms during the measurement and to pursue their normal daily activities. They were asked to write down these activities in a diary, as well as sleep quality and their true sleeping times. BP was measured at 20-min intervals between 6:00 AM and 10:00 PM and at 30-min intervals between 10.00 PM and 6.00 AM. Measurements were included if >70% of the 24-h measurements were successful including 20 valid awake measurements and 7 valid asleep measurements [Citation8, Citation21]. Unattended AOBP was measured for 30 min up and till 1 h at 5 min intervals using an oscillometric device (Accutor Plus, Datacope, Paramus, USA) with the patient awake, in a seated position, and the cuff attached to the non-dominant arm [Citation22]. Measurements were included if at least 6 measurements were available.
Based on the current guidelines the following target values were used [Citation8]: average 24-h ABPM <130/80 mm Hg; daytime ABPM <135 mm Hg/85 mm Hg and nighttime ABPM <120/70 mm Hg. For AOBP no clear cut-off values are defined, but it was suggested to be 5–15 mm Hg lower compared to office blood pressure [Citation8, Citation23]. We, therefore, used a cut-off for normal AOBP values of <135/85 mm Hg and <130/80 mm Hg.
To gain more inside into the difference between a 30 min BP measurement and other AOBP methods like the one described in SPRINT, we did additional measurements in approximately 50 patients that came for an AOBP at the cardiovascular outpatient clinic at the Erasmus Medical Centre. These patients were similar to the hypertension cohort as described previously. As part of the clinical BP measurements, patients first underwent a 30-min AOBP with 5-min intervals, which was followed by a rest period of 5 min and 3 measurements at 1-min intervals. Data were collected anonymously.
Statistical analysis
We analysed the data in several steps. BP measurements were checked for outliers by means of a boxplot. Values beyond the mean ± 2 standard deviations (SD) were considered outliers and were double-checked. Outliers were only omitted from the dataset if BP measurements were not compatible with life. This did not occur in our dataset. General characteristics from cohort 1 and cohort 2 were determined with a Shapiro–Wilk test and verified visually for normal distribution by means of a histogram. Subsequently, characteristics were compared between both cohorts using t-test for continuous variables when data were normally distributed and chi-square test for categorical variables. In the case of a skewed distribution, a Mann–Whitney test was used for continuous variables.
Second, the relations between 24-h ABPM and AOBP were determined by a Pearson’s correlation. Subsequently, we used a Deming regression to determine proportional or constant bias between the two BP measurement methods. To quantify the agreement between 24-h ABPM, night-time ABPM, and daytime ABPM and AOBP, Bland–Altman analysis was used before and after correction of the bias found with Deming regression [Citation17]. We used a similar methodology as suggested by papers explaining the Bland-Altman analysis [Citation17,Citation24]. To compare our 30-min AOBP method with the more frequently used 1-min interval AOBP as used in the SPRINT trial [Citation9], we performed a paired sample t-test and Bland-Altman analysis in the additional group of approximately 50 patients with hypertension as described previously.
We decided to use a difference of 10 mm Hg SBP and 5 mm Hg diastolic blood pressure (DBP) between both BP measuring methods as clinically relevant and therefore as the limit of agreement in the Bland–Altman analysis. This was based on the fact that a difference of >10 mm Hg systolic and >5 mm Hg diastolic is associated with an increase in cardiac events, but this difference can also lead to misclassification of hypertension [Citation8,Citation9]. We also included a limit of agreement of 20 mm Hg SBP, 10 mm Hg DBP and 2 × SD to compare our results with previously published comparisons.
BP measurements were used to determine if patients were misdiagnosed with hypertension when using AOBP. For the diagnosis of hypertension, we used a target value of 135/85 mm Hg for AOBP and 130/80 mm Hg for daytime ABPM. If either the average SBP or average DBP was above this target value, the measurement was marked as hypertension. Subsequently, a McNemar test was used to assess whether the conclusions on reaching the target values did agree between AOBP and ABPM.
Third, an exploratory analysis was carried out to identify factors influencing agreement between the two methods. A logistic regression analysis was performed with the correspondence between AOBP and ABPM found with the Bland–Altman as the outcome variable. Agreement between the two methods was defined as the difference in SBP ≤ 10 mm Hg or DBP ≤ 5 mm Hg and a broader difference of SBP ≤ 20 mm Hg or DBP ≤ 10 mm Hg. Age, sex, type of cohort (TIA/minor stroke vs hypertension), BMI, and a number of used antihypertensive drugs were considered as explanatory factors. The binary logistic regression was carried out as described by Field [Citation25]. We used the SPSS version 24.0 for Windows (IBM Corp, Armonk, NY), and GraphPad Prism 9 software (GraphPad Software, La Jolla, CA) for analysis.
Results
General characteristics
We evaluated the BP measurements of 168 patients from cohort 1 and 279 of cohort 2. Patients were mainly excluded because of an AOBP not performed on the same date as the 24-h ABPM, which led to the exclusion of 112 patients. Furthermore, patients were excluded due to an incomplete 24-h ABPM (n = 6), fewer than 70% correct ABPM measurements or <6 AOBP measurements (n = 30), and missing data (n = 73). Our final cohort consisted of 135 patients from cohort 1 and 72 patients from cohort 2. Patients had on average 12 BP (range 6–16) measurements with the AOBP. All continuous variables were normally distributed and no outliers were found in the BP data. As expected, since cohort 1 existed of patients with hypertension only, BP values in cohort 2 were significantly lower for all measurements as compared to cohort 1 (). No clinically relevant differences were found between Cohorts 1 and 2 with regard to patient characteristics () except for the expected difference prevalence of a TIA, age, smoking, and use of antihypertensive medication. As smoking was earlier determined as a factor to lower AOBP, but not ABPM [Citation26], we performed a separate Bland-Altman analysis with only non-smoking patients. However, the agreement between both methods stayed the same in comparison to the analysis where smokers were included. Since all other factors were unlikely to influence the correlation between 24 h ABPM and AOBP, further analyses were done with all patients from cohorts 1 and 2 combined.
Table 1. Automated office and ambulatory systolic and diastolic blood pressure values.
Table 2. General characteristics.
(Dis)agreement between AOBP and ABPM measurements
A statistically significant correlation was found between AOBP and 24-h ABPM for SBP (r = 0.631, p < 0.0001) as well as DBP (r = 0.719, p < 0.0001). Comparable correlations were found for AOBP and daytime ABPM (SBP: r = 0.659, p < 0.0001, DBP: r = 0.721, p < 0001).
We found no proportional or constant bias between the AOBP and day or mean ABPM for SBP or DBP in the Deming regression analysis. Therefore, the Bland-Altman analysis was performed without correcting the data for bias.
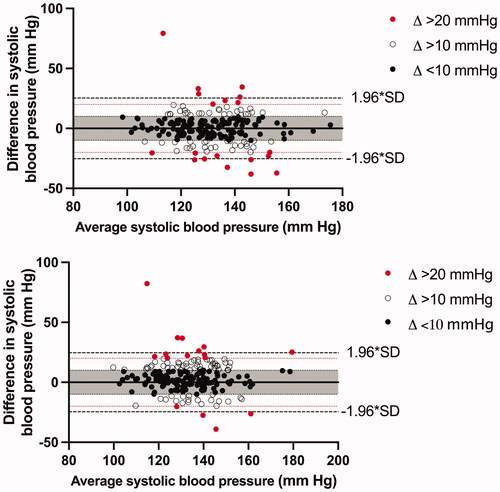
We found that 24-h SBP and DBP measured with ABPM agreed most with the AOBP measurements with 61.4% within the predetermined limits of agreement for SBP and 54.6% for DBP ( and ). When comparing daytime BP from the ABPM with average AOBP, 64.7% was within the predetermined limits for the SBP and 50.2% for the DBP ( and ). In comparison, when using a limit of agreement of 2-SD, the agreement increased to 95.7% for daytime SBP (2 × SD = 24.6 mm Hg) and 96.1% DBP (2 × SD = 15.9 mm Hg), 94.7% for 24-h SBP (2 × SD = 25.3 mm Hg) and 97.6% for 24-h DBP (2 × SD = 15.4 mm Hg). Results on the agreement were the same when 30-min measurements (6–8 measurements) or 60-min measurements (13–14 measurements) were used.
Figure 1. Bland–Altman plot for systolic blood pressure for (a) 1-h AOBP vs 24-h ABPM and (b) AOBP vs daytime ABPM. The average blood pressure values on the x-axis are the combined blood pressure averages of AOBP and ABPM.
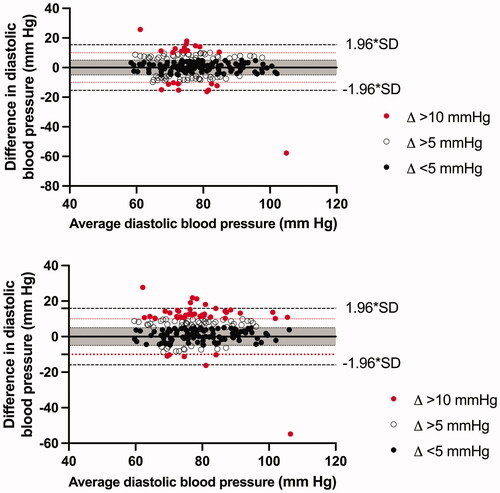
Figure 2. Bland–Altman plot for diastolic blood pressure for (a) 1-h AOBP vs 24-h ABPM and (b) AOBP vs daytime ABPM. The average blood pressure values on the x-axis are the combined blood pressure averages of AOBP and ABPM.
For the comparison of our 30-min AOBP with the 1-min interval AOBP, 77 patients were measured. For the 30-min AOBP average, BP was 134.8/81.5 mm Hg and 133.1/81.4 mm Hg without the first measurement. There was a significant difference between SBP with and without this first measurement (p < 0.001), but not for DBP (p = 0.441). The average BP for the 1-min interval AOBP was 131.6/80.4 mm Hg. The results from the 30-min AOBP were statistically different from the 1-min AOBP (SBP p < 0.001, DBP p = 0.028). In the Bland-Altman analysis with the predetermined limits of agreement of 10 mm Hg for SBP and 5 mm Hg for DBP, we found the best agreement when leaving out the first measurement of the 30-min AOBP. Agreement of SBP improved from 83.1% to 89.6% without the first measurement and from 79.2% to 80.5% for DBP.
When comparing 24-h ABPM with AOBP in patients on BP target, defined as <130/80 mm Hg measured with ABPM or <135/85 mm Hg measured with AOBP, or not on target, we found 142 (68.5.%) patients that were on target and 31 (15.0%) patients that were off target with both methods. So in total 83.6% of the patients, both methods led to the same diagnoses of either being hypertensive or non-hypertensive. Five (2.4%) patients were off target with AOBP, but on target with ABPM and 29 (14.0%) patients were off target with ABPM, but on target with AOBP (masked hypertension). These results led to a statistically significant difference between the outcome of hypertension diagnosis measured with both methods (p < 0.0001).
When using cut-off values of 130/80 mm Hg for AOBP more patients were off target with both methods (n = 40, 20.2%), but around the same percentage of patients were diagnosed the same after measurement with both methods (n = 167, 80.7%). The number of patients off-target AOBP and on target with ABPM or vice versa, on target with AOBP and off-target with ABPM, were the same with 20 (9.7%) patients in each group. No statistically significant difference was found in the outcome of hypertension diagnosis between ABPM and AOBP when using a cut-off of 130/80 mm Hg for AOBP (p = 1.000).
Binary logistic regression analysis
describes the exploratory analysis to identify factors influencing agreement between the two methods. We found that TIA patients as compared to hypertension patients have a lower likelihood of agreement between AOBP and daytime ABPM with a cut-off value of 10 mm Hg for SBP (OR 0.26, 95% CI 0.12–0.56), and 5 mm Hg for DBP (OR 0.48, 95% CI 0.24–0.99), whereas the TIA patients as compared to hypertension patients showed no significant differences with respect to AOBP and 24-h ABPM. As for age, we found that patients with a higher BMI have a lower likelihood of agreement when comparing AOBP and daytime SBP ABPM (OR 0.93 per 1 unit difference, 95% CI 0.88–0.99), whereas BMI is not associated with a lower agreement in the other three regressions. Furthermore, when using a broader cut-off of 20 mm Hg SBP and 10 mm Hg DBP, many associations disappeared. These results are presented in Supplementary Table 1.
Table 3. Binary logistic regression analysis with (dis)agreement as outcome.
Discussion
This is the first study to investigate the appropriateness of a 30–60 min AOBP with a 5 min interval to substitute or complement 24-h ABPM in patients diagnosed with hypertension or stroke and included clinically relevant cut-off values to assess agreement. We found that, despite high correlations, the agreement between the average BP measured with AOBP and ABPM was mediated with pre-specified cut-off values of SBP <10 mm Hg and DBP <5 mm Hg. However, when the most commonly used limit of agreement of 2-SD was used, the agreement between both methods was near to 100%. This high agreement with 2 × SD can be expected when taking into account the properties of a normal distribution.
Our findings are in line with previous studies on the substitution of 24-h ABPM by AOBP with 5–6 measurements and an interval of 1–2 min, and the agreement between both methods [Citation27]. These studies also found a large proportion of masked hypertension and a small proportion of hypertension during AOBP but not ABPM (‘white coat’, although apparently the repeated measurements already correct for the ‘white coat’ effect) [Citation28]. This small proportion is also the reason that, when used correctly, AOBP is preferred over office BP measurements and recommended by the guidelines to use in clinical practice [Citation8,Citation29]. The high prevalence of masked hypertension could implicate the presence of bias as it implicates a constant deviation from BP measured with ABPM. However, no systematic bias was present after Deming-regression and AOBP gave higher as well as lower BP values compared to 24-h ABPM. Also, the high prevalence of masked hypertension was expected as certain patient characteristics such as obesity, diabetes, and high-normal office BP, which were common features in our population, are associated with a higher prevalence of masked hypertension [Citation8]. The prevalence of masked hypertension advocates the use of 24-h ABPM to prevent missing diagnosis of hypertension.
It should be mentioned that the diagnosis of hypertension with AOBP and thereby the existence of white coat or masked hypertension, was based on the assumption of a cut-off value of 135/85 mm Hg. Although previous research suggested a cut-off of 130/85 mm Hg, no clear cut-off values are established yet [Citation30]. We also found that more patients were wrongly diagnosed with hypertension by the use of either one of the methods (AOBP or ABPM) when a cut-off of 130/80 mm Hg as compared to a cut-off of 135/85 mm Hg. Therefore, we suggest using <135/85 mm Hg as normal values for 30–60 min AOBP for now until more is known about the actual cut-off values.
In both our cohorts we found higher BP averages with AOBP as compared with 24-ABPM overall averages, but lower BP averages as compared with daytime ABPM. These results were compared with a meta-analysis from Pappaccogli et al. [Citation13], which showed that the overall differences between methods on average were small, but when looking at individual studies large differences between AOBP and daytime ABPM were observed. The large differences between individual studies are probably due to the selection of the population and suggest that the optimal method of BP measurement may be different for specific populations [Citation27].
Until now, no conclusive evidence was found to justify the substitution of ABPM with AOBP [Citation8]. Unfortunately, even a 30–60 min AOBP with on average 12 measurements was not in agreement with daytime ABPM or 24-h ABPM. Moreover, the highest percentage of measurements within the limits of agreement was only 65% and found when comparing average 24-h ABPM with AOBP. It was expected to find more agreement when daytime ABPM was used. This could be a result of the fact that most AOBPs were performed before noon. As most antihypertensive drugs are used in the morning, we presume most patients were measured at peak drug levels which could have led to slightly lower blood pressures. The exact influence of antihypertensive drugs on the drop in blood pressure remains unknown, while the time interval between intake and BP measurement was unknown. Furthermore, as night-time ABPM is often lower, it will lower the total average of the ABPM. In this case, both explanations could have resulted in a closer agreement with the AOBP.
Furthermore, we demonstrated that there was a strong correlation between AOBP and ABPM for SBP and DBP, but only a limited degree of agreement. This apparent discrepancy was explained in a meta-analysis of Jegatheswaran et al. [Citation27] where they indicated that the use of a correlation coefficient for examining agreement between AOBP and ABPM could result in a bias. This bias suggests when comparing two measures a correlation will always be found, but will not necessarily lead to more agreement. To overcome bias, Deming regression and subsequently Bland-Altman analysis are important [Citation18,Citation31]. Although, most previous studies performed a Bland-Altman analysis, we are the first to check for bias with a Deming regression and use clinically relevant cut-off values in this analysis. When applying these limits of agreement to previous studies, it may have resulted in a lower agreement between both methods. Furthermore, no effect was seen in the Bland-Altman analysis of the actual blood pressure on the difference between AOBP and ABPM as was found in previous studies [Citation32]. Therefore, we think that if there is any influence of the actual blood pressure on this difference, the effect will be small or may be isolated to specific populations that are not included in our current study. Also, we found no difference in the Bland-Altman analysis between 30-min and 60-min AOBP measurements in comparison to ABPM. As both 30 and 60 min BP measurements will lead to the same BP values, we recommend performing at least a 30-min AOBP to save time. Furthermore, it seems reasonable to leave out the first measurement. Furthermore, a shorter method with unobserved measurements and 1-min intervals including a rest period of 5-min before the start of the measurements might be an even more convenient alternative for a 30-min AOBP. We found that this shorter method was not different from a 30-min AOBP and even resulted in lower BP values as compared to the 30-min AOBP. However, these measurements were performed directly after the AOBP, giving the patients time to get comfortable and more at rest. We postulate that the shorter method with 1-min intervals would have higher BP values as compared to the 30-min AOBP if we started with these measurements. Because simultaneous measurements of two different BP methods are impossible, this issue will remain and should be taken into account when interpreting results. For future studies addressing these shorter measurements, the order of measurements ideally would be randomised.
As for our binary logistic regression analysis, we attempted to identify factors associated with agreement between these measurements. However, the regressions showed mixed results, and those associations that were significant were not easily explainable and inconsistent when compared to others. This is possibly due to the variability in BP during the day, which is influenced by various factors independently of behaviour [Citation33]. Although, no unambiguous factors could be identified in our analysis, it does not imply that no factors are associated with more agreement between AOBP and ABPM while this was only investigated in exploratory research.
Finally, with our results, we were able to make a suggestion on the cut-off values for AOBP measurements to diagnose hypertension.
This study has two important limitations. Firstly, no office BP was taken. This makes comparison with earlier studies comparing office and 24-h ABPM and defining the percentage of ‘white coat’ hypertension identified by AOBP difficult. However, the limited value of single office measurements has unequivocally been established previously [Citation34]. Finally, the population of our study was formed by combining two different cohorts. A mixture of cohorts can lead to a biased representation of the actual population. However, since the assessment of patients and BP measurement was executed in the same way, combining the two cohorts has led to a larger cohort with participants with both high and normal BP values. We have no indication that the origin of the cohort influences the results since we focussed on the methodology of BP measurement.
Conclusion
We conclude that 30–60 min AOBP measurements cannot replace a 24-h ABPM and propose to perform 24-h ABPM at least on a yearly basis to confirm AOBP measurements in all patients suspected of hypertension or who suffered a minor stroke or TIA.
Authors’ contributions
All listed authors have contributed to the manuscript substantially and have agreed to the final submitted version.
Supplemental Material
Download MS Word (14 KB)Disclosure statement
L.E.J. Peeters received lecture fees from Astellas Pharma. The other authors declare that they have no conflict of interest.
References
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of hypertension (ESH) and of the european society of cardiology (ESC). J Hypertens. 2013;31(7):1281–1357.
- The SPRINT Research Group. A randomized trial of intensive versus standard Blood-Pressure control. New Eng J Med. 2015;373(22):2103–2116.
- Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in Middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287(8):1003–1010.
- Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29(1):102–111.
- Koroboki E, Manios E, Psaltopoulou T, et al. Circadian variation of blood pressure and heart rate in normotensives, white-coat, masked, treated and untreated hypertensives. Hellenic J Cardiol. 2012;53(6):432–438.
- Huang Y, Huang W, Mai W, et al. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens. 2017;35(4):677–688.
- Parati G, Stergiou GS, Asmar R, et al. European society of hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24(12):779–785.
- Williams B, Mancia G, Spiering W, et al. [2018 ESC/ESH Guidelines for the management of arterial hypertension]. Kardiol Pol. 2019;77(2):71–159.
- Group SR, Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116.
- Johnson KC, Whelton PK, Cushman WC, et al. Blood pressure measurement in SPRINT (systolic blood pressure intervention trial). Hypertension. 2018;71(5):848–857.
- van der Wel MC, Buunk IE, van Weel C, et al. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9(2):128–135.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit. 2006;11(1):37–42.
- Pappaccogli M, Di Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out-off-Office measurement techniques. Hypertension. 2019;73(2):481–490.
- Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure - being alone and not location is what matters most. Blood Press Monit. 2015;20(4):204–208.
- Lamarre-Cliche M, Cheong NN, Larochelle P. Comparative assessment of four blood pressure measurement methods in hypertensives. Can J Cardiol. 2011;27(4):455–460.
- Edwards C, Hiremath S, Gupta A, et al. BpTRUth: do automated blood pressure monitors outperform mercury? J Am Soc Hypertens. 2013;7(6):448–453.
- Giavarina D. Understanding bland altman analysis. Biochem Med. 2015;25(2):141–151.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310.
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607.
- O’Brien E, Mee F, Atkins N, et al. Accuracy of the SpaceLabs 90207 determined by the british hypertension society protocol. J Hypertens. 1991;9(6):573–574.
- O’Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62(6):988–994.
- Khawaja RA, Qureshi R, Mansure AH, et al. Validation of datascope accutorr plus™ using british hypertension society (BHS) and association for the advancement of medical instrumentation (AAMI) protocol guidelines. J Saudi Heart Assoc. 2010;22(1):1–5.
- Filipovský J, Seidlerová J, Kratochvíl Z, et al. Automated compared to manual office blood pressure and to home blood pressure in hypertensive patients. Blood Press. 2016;25(4):228–234.
- Andreadis EA, Geladari CV, Angelopoulos ET, et al. Attended and unattended automated office blood pressure measurements have better agreement with ambulatory monitoring than conventional office readings. J Am Heart Assoc. 2018;7(8):e008994.
- Field A. Discovering statistics using SPSS. 2nd ed. London (UK): SAGE Publications Ltd.; 2005.
- Mann SJ, James GD, Wang RS, et al. Elevation of ambulatory systolic blood pressure in hypertensive smokers. A case-control study. JAMA. 1991;265(17):2226–2228.
- Jegatheswaran J, Ruzicka M, Hiremath S, et al. Are automated blood pressure monitors comparable to ambulatory blood pressure monitors? A systematic review and meta-analysis. Can J Cardiol. 2017;33(5):644–652.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280–286.
- Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(3):351–362.
- Wohlfahrt P, Cífková R, Krajčoviechová A, et al. Comparison of three office blood pressure measurement techniques and their effect on hypertension prevalence in the general population. J Hypertens. 2020;38(4):656–662.
- Paul M, Robert MC, Kenneth J, et al. Rationale for ambulatory and home blood pressure monitoring thresholds in the 2017 American College of Cardiology/American Heart Association guideline. Hypertension. 2019;73(1):33–38.
- Parati G, Ochoa JE, Bilo G, et al. SPRINT blood pressure: sprinting back to smirk’s basal blood pressure? Hypertension. 2017;69(1):15–19.
- Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension. 2012;60(2):512–517.
- Ohkubo T, Hozawa A, Nagai K, et al. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama Study. J Hypertens. 2000;18(7):847–854.
