Abstract
Purpose
This study examines the effect of antihypertensive drugs on ACE2 and Angiotensin II levels in hypertensive COVID-19 patients.
Introduction
Hypertension is a common comorbidity among severe COVID-19 patients. ACE2 expression can be modulated by antihypertensive drugs such as ACEis and ARBs, which may affect COVID-19's prognosis. BB and CCB reduce mortality, according to some evidence. Their effect on circulating levels of ACE2 and angiotensin II, as well as the severity of COVID-19, is less well studied.
Materials and methods
The clinical data were collected from 200 patients in four different antihypertensive medication classes (ACEi, ARB, BB, and CCB). Angiotensin II and ACE2 levels were determined using standard ELISA kits. ACE2, angiotensin II, and other clinical indices were evaluated by linear regression models.
Results
Patients on ACEi (n = 57), ARB (n = 68), BB (n = 15), or CCB (n = 30) in this study had mild (n = 76), moderate (n = 76), or severe (n = 52) COVID-19. ACE2 levels were higher in COVID-19 patients with severe disease (p = 0.04) than mild (p = 0.07) and moderate (p = 0.007). The length of hospital stay is correlated with ACE2 levels (r = 0.3, p = 0.003). Angiotensin II levels decreased with severity (p = 0.04). Higher ACE2 levels are associated with higher CRP and D-dimer levels. Elevated Angiotensin II was associated with low levels of CRP, D-dimer, and troponin. ACE2 levels increase with disease severity in patients taking an ARB (p = 0.01), patients taking ACEi, the degree of disease severity was associated with a decrease in angiotensin II. BB patients had the lowest disease severity.
Conclusion
We found different levels of soluble ACE2, and angiotensin II are observed among COVID-19 patients taking different antihypertensive medications and exhibiting varying levels of disease severity. COVID-19 severity increases with elevated ACE2 levels and lower angiotensin II levels indicating that BB treatment reduces severity regardless of levels of ACE2 and angiotensin II.
Introduction
Coronavirus disease 2019 (COVID-19) pandemic was a major threat engendering the lives of millions around the globe, yet effective treatment is still lacking, especially in highly vulnerable populations [Citation1]. The presence of cardiovascular disease (CVD) is a primary risk factor for coronavirus infection with the severe acute respiratory syndrome (SARS-CoV-2) because 2.5–15% of COVID-19 patients present with coronary heart disease and 15–30% suffer from hypertension [Citation2]. During SARS-CoV-2 infection, the virus utilises the angiotensin-converting enzyme 2 (ACE2) receptor on the cell membrane to infect the host cells [Citation3]. The involvement of ACE2 in CVD has observed increased attention because ACE2 plays an influential role in the renin-angiotensin system (RAS), which is responsible for converting angiotensin II into angiotensin-1-7. ACE2 is highly expressed in cardiac fibroblasts, cardiomyocytes, and endothelium. The loss of membrane-bound cardiac ACE2 is associated with the progression of heart failure, whereas overexpression of ACE2 reverses the heart failure phenotype. A downregulation of ACE2 produces increased angiotensin II, which leads to cardiovascular complications, noting that angiotensin II is proinflammatory and vasoconstrictor, whereas angiotensin 1-7 is anti-inflammatory and vasorelaxant [Citation4].
The ACE2 enzyme may exist in two forms: tissue-specific (membrane-bound) and soluble (circulating). Besides the cardiac tissues, ACE2 is expressed on various other organs; however, its function in these organs is not well understood. Pulmonary ACE2 expression is lower than the cardiac expression and is mainly concentrated to alveolar type 2 (AT2) cells which also possess transmembrane protease, serine 2 (TMPRSS2). This protease is critical for priming the ACE2–SARS-CoV-2 complex, a step needed for host cell entry [Citation5,Citation6]. The induction of acute respiratory distress syndrome (ARDS) downregulates pulmonary ACE2 expression in vivo, similarly to the SARS-CoV-induced ARDS, suggesting that viral infection deactivates pulmonary ACE2 and deteriorates lung function [Citation7]. In contrast, higher expression of ACE2 on lung cells was suggested to increase the risk of SARS-CoV-2 infection. Nonetheless, it has not been confirmed [Citation8]. Although the function of pulmonary and cardiac expression of ACE2 during SARS-CoV-2 infection and cardiac disease is better understood, the possible influence of soluble ACE2 on the RAS pathway remains controversial.
Several studies have shown that viral infection, as well as lung and heart disease, increase sera-circulating ACE2, partly due to the shedding of membrane-bound ACE2 [Citation9,Citation10]. In patients with heart failure, shedding of the membrane-bound ACE2 may be responsible for the increased circulating activity of ACE2, which is associated with a poor prognosis [Citation11]. An increase in soluble ACE2 levels in CVD patients was attributed to dysfunctional RAS [Citation10,Citation12]. Considering this phenomenon, it may be possible to explain the high prevalence of cardiovascular disease among COVID-19 patients. Alternatively, it is argued that high levels of circulating ACE2 could protect against hypertension in hypertensive patients since soluble ACE2 is capable of cleaving angiotensin II and can bind and neutralise SARS-CoV-2 [Citation13–15]. Consequently, it is not clear how changes in the levels of especially soluble ACE2 will affect hypertensive patients under COVID-19 conditions.
A variety of antihypertensive drugs are used in the current clinical practice to control blood pressure in hypertensive patients. The RAS-blocking drugs, including angiotensin-converting-enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB), are primarily used to restore cardiac function and decrease angiotensin II levels. In addition to their role in blocking ACE1 and angiotensin II, these drugs can increase ACE2 expression as well [Citation16–18]. Accordingly, ACEi and ARB were thought to predispose CVD patients to infection [Citation19]. A large multi-centre retrospective study concluded that the use of RAS inhibitors did not increase the risk of death in the hospital [Citation20]. Contrary to this, other classes of antihypertension drugs, such as beta-blockers and calcium channel blockers, may exhibit a protective effect in COVID-19 hypertensive patients [Citation20]. Nevertheless, their impact on circulating ACE2, angiotensin II, and the severity of the disease remains unclear. The purpose of this multicenter cross-sectional study is to compare the severity of the disease, the level of circulating ACE2, and the amount of circulating angiotensin II in COVID-19 hypertensive patients who are treated with ACEi, ARB, BB, and CCB. Also, to determine how these drugs affect the levels of circulating ACE2 and angiotensin II.
Methods
Study design
The study consists of 200 hypertensive COVID-19 patients (71% males, 29% females) recruited from Hamad Medical Corporation (HMC) hospitals, the primary facility in Qatar. The project protocols were approved by the Institutional Review Boards (IRBs) of the Harvard Medical Centre (MRC-02-20-382) and Qatar University (IRB-QU-2020-006). All methods were conducted according to the relevant guidelines and regulations. The inclusion criteria for patients require that they be hypertensive and under regular and specific antihypertensive medication for at least 30 days before admission to the hospital. All participants provided informed consent as directed in the protocol. Four groups of patients were categorised based on their use of antihypertension medications (ACEi, N = 57, ARB, N = 68, BB, N = 15, and CCB, N = 30). Thirty patients did not have available data regarding the type of antihypertensive medication administered. Among the RAS blockers were ramipril, perindopril, lisinopril, captopril, candesartan, irbesartan, and losartan. A further grouping of patients was made according to their severity of disease (mild, n = 76, moderate, n = 72, and severe, n = 52). According to World Health Organisation (WHO) guidelines, the severity of disease was defined as follows: mild disease included symptomatic patients without evidence of viral pneumonia or hypoxia; moderate disease defined as the presence of clinical signs of pneumonia without signs of severe pneumonia; severe and critical disease are defined as the presence of severe pneumonia, ARDS, sepsis, ICU admission or in-hospital death [Citation21]. Demographic and anthropometric data, as well as medical history information, were collected. This included age, ethnicity, vital signs, BMI, comorbidities, complete blood count (CBC), C-reactive protein (CRP), D-dimer, troponin, kidney, and liver functions.
Measurement of circulating ACE2 and angiotensin II
Three days after the patient's admission to the hospital, blood samples were collected. We measured the levels of ACE2 and angiotensin II in the plasma of all participants using enzyme-linked immunosorbent assays (ELISA) with a quenched-fluorescent substrate, as described previously [Citation22–24]. In a solid-phase sandwich ELISA, ACE2, and angiotensin II proteins were detected and quantified. Angiotensin II ELISA kit (Catalog# NBP2-62135) and Novusbio ACE2 Chemiluminescence ELISA kit (Catalog# NBP2-66387) were used. In separate assays, highly specific antibodies are used that target different epitopes on one protein to quantify all protein targets simultaneously using a Luminex instrument, which only requires 50 µL of plasma or serum, and takes four hours for analysis to be completed. ACE2 ELISA kit measures the relative light unit (RLU) value associated with human ACE2 concentrations. The ACE2 concentration is calculated from the measured RLU using a standard curve. According to the manufacturer, the chemiluminescence ELISA kit has a sensitivity of 46.88 pg/mL as well as a detection range of 78.13–5000 pg/mL. Additionally, no significant cross-reactivity or interference was observed between human ACE2 and analogs, according to the manufacturer. Concerning the Angiotensin II ELISA kit, the manufacturer determined cross-reactivity between Ang2 and many related compounds including Ang(1-7) by diluting cross-reactants to concentrations ranging from 0.1 pM to 500 nM and then monitoring the results by assay. It was found that the cross-reactivity with Ang(1-7) was only 0.053. Moreover, the sensitivity of the Ang2 assay was assessed using six independent standard curves and reported to be 4.6 pg/ml. Under biosafety level 2 (BSL2) standards, all protocols were conducted per the manufacturer's instructions.
Statistical analysis
The clinical traits were analysed with IBM SPSS version 25 and GraphPad Prism version 9.0.1. To ensure normal distributions, variables with skewed distributions (ACE2 and angiotensin II) were log-transformed. There were several comparisons performed, including t-tests, Wilcoxon–Mann–Whitney tests, one-way ANOVA, Chi-squared tests, and linear regression as appropriate. The significance level was defined as p ≤ 0.05.
Results
General characteristics of participants
Two hundred hypertensive COVID-19 patients (56.2 years ±11.2) were recruited amongst patients admitted to HMC hospitals. A total of 57 (34%) of the recruited patients were taking ACE inhibitors, 68 (40%) were taking ARBs, 15 (9%) were taking BBs, and 30 (18%) were taking CCBs. In , it is shown that the average stay in the hospital was 14 days (SD = 8.7). Patients in the CCB group were a bit younger (51.4 years ±10.1) and had higher CRP levels (66.2 mg/L ± 79.0). In the BB treated group, the duration of hospital stay was shortest (10.2 days ±5.3). Angiotensin II levels were lowest in the CCB treated group (2266.8 ng/ml, ±1061.2), whereas ACE2 levels were highest in this group (630.2 ng/ml, ±947.3).’s count was highest in ACEi treated group (1.8 × 10^3/µL ± 1.1) compared to ARB treated group which had the lowest count (1.3 × 10^3/µL ± 0.69). It was not found that there were significant differences among the four treated groups in procalcitonin levels, systolic blood pressure, or diastolic blood pressure. The most common comorbidity among all patients was diabetes mellitus (64.5%), followed by coronary artery disease (12.0%), congestive heart failure, and chronic kidney disease (5.5%). As shown in , other comorbidities such as solid tumours, myocardial infarction, asthma, end-stage renal disease, hypothyroidism, and others are also reported, however at a less frequent rate. In addition, 86.5% of the patients were given co-medications treatments specifically to treat COVID-19, including Azithromycin, Lopinavir, Dexamethasone, and Hydroxychloroquine
Table 1. Age, body mass index (BMI), length of stay at the hospital, levels of angiotensin-converting-enzyme-2 (ACE2) angiotensin II, C-reactive protein (CRP), D-dimer, and troponin in hypertensive COVID-19 patients treated with ACE inhibitors (ACEi), angiotensin receptor blockers (ARB), beta-blockers (BB) and calcium channel blockers (CCB).
Circulating levels of ACE2 and angiotensin II in COVID-19 severity groups
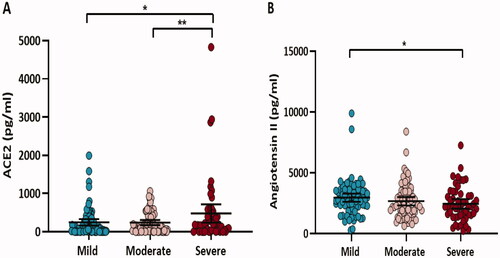
illustrates that circulating ACE2 levels were 1.9-fold higher in patients with severe COVID-19 symptoms compared to mild (p = 0.04) and moderate (p = 0.007) counterparts and they were positively correlated with the amount of time spent in the hospital (r = 0.3, p = 0.003) (data not shown). In contrast, levels of angiotensin II decreased as disease severity increased, whereas patients with mild symptoms had 1.2 times the levels of their severe counterparts (p = 0.04).
Figure 1. Circulating ACE2 (A) and angiotensin II (B) levels in COVID-19 hypertensive patients grouped according to disease severity (mild, moderate, and severe). Data are presented as dot plots with the mean (95% CI). Differences in log-transformed ACE2 and angiotensin II were assessed by ANOVA, *p < 0.05, **p < 0.01.
Circulating levels of ACE2 and angiotensin II in different medication groups
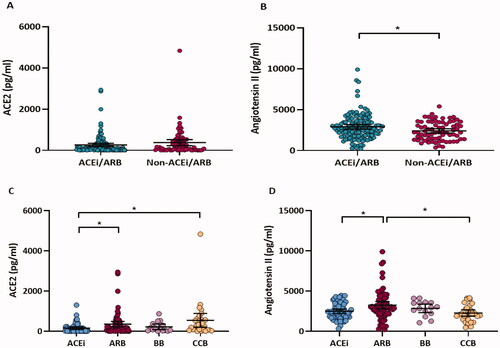
An analysis of pairwise correlations between circulating ACE2 levels and angiotensin II levels in all participants revealed a negative correlation (r = −0.17, p = 0.02) (Figure S1). The levels of circulating ACE2 and angiotensin II were compared between patients who had received an ACEi/ARB and those who had not (). ACE2 levels did not differ significantly between the ACEi/ARB group and the non-ACEi/ARB group (p = 0.1, ), but angiotensin II levels did differ significantly between the two groups (0.8-fold change, p = 0.02, ). A comparison was also conducted between four different antihypertension medication groups (ACEi, ARB, BB, and CCB). As demonstrated by , there was a significant difference in the levels of ACE2 (p = 0.008, ) and angiotensin II (p = 0.005, ) among the four medication groups. The ARB group displayed higher levels of ACE2 (1.5-fold change, p = 0.03, ) and angiotensin II (1.2-fold change, p = 0.01, ) than the ACEi group. Furthermore, CCBs treated patients showed higher levels of ACE2 compared to patients treated with ACEi (2.7 folds change, p = 0.01, ), as well as lower levels of angiotensin II compared to patients treated with ARBs (0.7-fold change, p = 0.02, ).
Figure 2. Circulating ACE2 and angiotensin II levels in COVID-19 hypertensive patients grouped according to two (ACEi/ARB, non-ACEi/ARB) (A, B) or four (ACEi, ARB, BB, and CCB) (C, D) medication groups. Differences in log-transformed ACE2 and angiotensin II were assessed by ANOVA, followed by Tukey's post-hoc analysis. *p < 0.05.
Circulating levels of ACE2 and angiotensin II in different medication groups by severity
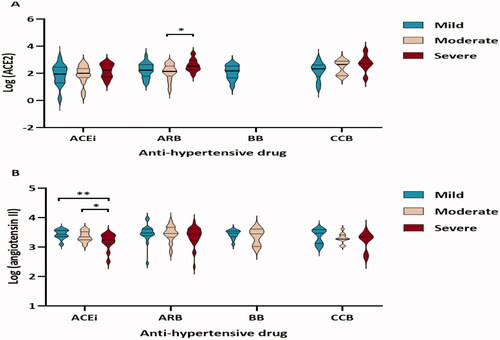
ACE2 and angiotensin II levels in patients with mild, moderate, or severe COVID-19 treated with ACEi, ARB, BB, or CCB were also compared. The patients on ARB who had a severe disease exhibited higher levels of ACE2 than those who had a moderate disease severity (2.5-fold change, p = 0.01, ). In contrast, patients on ACEi with mild disease severity had higher levels of angiotensin II than those who had high disease severity (1.7-fold change, p = 0.003, ). Patients with moderate disease severity who were treated with ACEIs had higher levels of angiotensin II than patients with severe disease (1.2-fold difference, p = 0.04, ).
Figure 3. Circulating ACE2 (A) and angiotensin II levels (B) in hypertensive patients with different COVID-19 severity (mild, moderate, or severe) under each medication group (ACEi, ARB, BB, and CCB). Differences in ACE2 and angiotensin II levels were assessed by a linear regression model. *p < 0.05, **p < 0.01.
Correlation of circulating ACE2, angiotensin II, and disease severity with clinical indices
The correlation between COVID-19 severity and CRP severity for the patients with CRP showed a significant difference between patients with mild disease and patients with severe disease (2.0-fold change, p = 0.002, Figure S2A), and between patients with moderate and severe disease (1.5-fold change, p = 0.01, Figure S2A). There was also a significant difference in D-dimer levels between patients with mild and severe COVID-19 (2.7-fold change, p = 0.04, Figure S2B). The troponin levels did not differ significantly between severity groups (Figure S2C). ACE2 levels correlated positively with CRP (r = 0.26, p = 0.0007, Figure S3A) and D-dimer levels (r = 0.17, p = 0.03, Figure S4A). A negative correlation was observed between angiotensin II levels and CRP (r = −0.18, p = 0.008, Figure S3B), D-dimer (r = −0.15, p = 0.03, Figure S4B), and troponin (r = −0.24, p = 0.019, Figure S5B).
Differences in the prevalence of patients on different antihypertension drug treatment in different disease severity groups
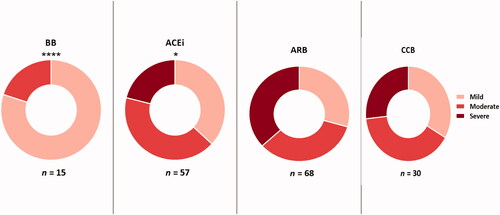
For the analysis of the relationship between antihypertension drug treatment and disease severity, the percentage of patients belonging to the four different medication groups (ACEi, ARB, BB, and CCB) in mild, moderate, and severe disease groups was compared. There is a statistically significant difference in the prevalence of patients in severity groups among the four treatment groups (p = 0.007) (). According to these comparisons, patients on ARB were associated with the highest prevalence of the severe phenotype, whereas no patients were diagnosed with the phenotype in BB patients.
Discussion
Previously it has been established that RAS plays a role in SARS-CoV-2 infection-associated acute lung failure [Citation25]. In previous studies, it was found that SARS-CoV-2 infection reduced pulmonary ACE2 expression and increased angiotensin II signalling via angiotensin II type 1a receptor (AT1a), which led to inflammatory responses, contributing to the progression of the disease, pulmonary edoema, and respiratory function impairment [Citation7,Citation26]. Several studies have also shown that inhibiting the RAS pathway and lowering levels of angiotensin II may reduce lung injury [Citation7,Citation26,Citation27] suggesting that the use of ACEi and ARB can ameliorate pneumonia [Citation28]. Studies of the ACEi/ARB have reported conflicting results with regards to the COVID-19 outcomes [Citation29–33]. It has been observed that ACEi/ARB drugs enhance ACE2 expression or activity in experimental animals, but the evidence is not consistent and varies among RAS blockers and different organs [Citation26,Citation34,Citation35]. As SARS-CoV-2 exploits the ACE2 receptor for cellular entry [Citation36], it remains unclear whether ACEi/ARBs are safely administered to COVID-19 patients with hypertension. As well, further research is needed to determine the clinical significance of circulating ACE2 and its potential influence on the functioning of the RAS pathway in these patients. Hence, the purpose of this study is to examine the levels of soluble ACE2 and angiotensin II in hypertensive COVID-19 patients receiving various RAS inhibitors as well as other medications. Additionally, to determine whether the level of circulating ACE2 and angiotensin II correlates with disease outcome and other clinical variables in these patients [Citation37]. This study involved 200 hypertensive patients with COVID-19 who were being treated with one of four classes of antihypertensive drugs. The present study suggests that elevated levels of soluble ACE2 and reduced levels of angiotensin II were associated with disease severity and clinical indices independent of the treatment received. Additionally, in our study, we found that patients receiving different antihypertensive medications experienced significant differences in disease severity.
In this study, circulating ACE2 levels were elevated in patients with severe disease compared to patients with mild or moderate disease severity. Additionally, ACE2 levels correlated positively with the length of hospital stay, CRP, and D-dimer levels, regardless of the antihypertensive medication used. According to recent studies, increased ACE2 expression in the lungs increases the risk of being infected with SARS-CoV-2 [Citation3], and increased ACE2 shedding is associated with elevated levels of circulating ACE2 and poor prognosis in patients with heart failure [Citation11]. The significance of soluble ACE2 shedding is not completely understood, but it could be related to membrane expression, with a relatively constant rate of conversion between soluble and membrane-bound forms. It is also possible that increased circulating ACE2 is a result of the upregulation or downregulation in the proteases that are responsible for cleaving ACE2 from the membrane [Citation38]. Several studies have suggested that inflammation may be responsible for the cleavage of ACE2 [Citation9–11], which may result in the increased release of soluble ACE2. Moreover, some studies suggest that ADAM17 is upregulated because of the internalisation of the virus, with ADAM17 primarily responsible for shedding membrane-bound and catalysing its conversion into soluble ACE2.
Elevated levels of serum ACE2 in critically ill COVID-19 patients were reported in a case report, which indicates that circulating ACE2’s dramatic rise may act as a non-specific protective mechanism against SARS-CoV-2 infection that preceded recovery [Citation39]. Additionally, Yeung et al. demonstrated that the interaction between SARS-CoV-2 spike protein and soluble ACE2 mediates receptor-mediated endocytosis of the virus [Citation40] possibly contributing to the pathogenesis of COVID-19. A further investigation is warranted into whether an elevated circulating level of ACE2 negatively influences blood pressure in critically ill hypertensive patients. In contrast, angiotensin II levels decreased with increased disease severity, with patients with mild symptoms exhibiting higher levels than their severe counterparts. The lower levels of angiotensin II may be due to an increase in circulating ACE2 with increasing severity of the disease [Citation41–43]. However, it is difficult to understand the weak negative correlation between Ang II and sACE2, perhaps simply due to low ACE levels in patients with severe COVID-19 caused by endothelial damage resulting in lower angiotensin II levels [Citation41–45]. The decrease in levels of circulating angiotensin II was also associated with increased levels of inflammatory markers, including CRP, D-dimer, and troponin, which were all thought to contribute to the progression of COVID-19 [Citation46,Citation47].
Notably, in this study, we observed high levels of angiotensin II (in the order of 1 ng/ml) in hypertensive COVID-19 patients which could possibly indicate a significant finding. Such elevated angiotensin II levels in hypertensive COVID-19 patients were also reported in a previous study comparing angiotensin II levels in three groups of COVID-19 patients (non-hypertensive, hypertensive with no co-morbidities and hypertensive with one or more other chronic diseases) showing levels within the range of 143.56–1466.03, 150.55–1551.62, and 101.30–2997.91 pg/ml respectively [Citation48]. However, similar to our study, they used an ELISA-based method to quantify angiotensin II which has been recently criticised by Chappell et al. [Citation49] due to its poor specificity in measuring Ang II and Ang-(1–7) in human plasma showing higher levels compared to other assays [Citation24]. Therefore, the use of ELISA to quantify RAS peptides is not considered the optimal method and needs further confirmation by direct measurement using a mass spectrometry-based approach following the inactivation of plasma proteases and potential SARS-CoV-2 in the collected blood samples [Citation50].
The results of the study showed that patients on different antihypertensive medications have varying levels of serum ACE2 and angiotensin II. A significant reduction in levels of angiotensin II was observed as expected, but an interesting trend was detected for increased levels of ACE2 in patients treated with ACEi/ARB. When comparing the ACEi treated group with the ARB treated group, the ARB treated patients had significantly higher levels of ACE2 and angiotensin II. In line with other studies, this study's results suggest that higher levels of angiotensin II are expected. However, more investigation is needed to determine the functional significance of the higher levels of ACE2. The expression of ACE2 both at the mRNA and protein level is altered by ARBs in several studies [Citation17–19]. In addition, ARB dosage was reported to increase circulating levels of angiotensin II [Citation20,Citation21]. Moreover, AT1R blockade in ARB administration may lead to an increase in AngII concentration due to non-binding with AT1R. Inactive sACE2 cleaved by TMPRSS2 could account for the higher sACE2 concentration [Citation51]. Furthermore, other differences were observed between the ACE inhibitor/ARB group and the non-ACE inhibitor/ARB group. It was expected that the ARB-treated group would have higher angiotensin II levels than its CCB counterpart, but the higher ACE2 levels in the CCB-treated group as compared to the ACEi-treated group were surprising (). This is because, in contrast to the mechanism of action of ACEi, that of CCB does not directly involve modulation of ACE2. Given this, further investigation of the functional relevance of these associations is warranted. One explanation might be that when CCB binds to the AT1R and activates ADAM17, it increases inflammatory markers expressed in sACE2, which might lead to an increase in shedding caused by an increase in ADAM17 [Citation52]. Additionally, Ca2+ also plays a role in inflammatory cellular responses by disrupting mitochondrial function. In the case of SARS-COV-2, CCBs can inhibit the virus' replication [Citation53,Citation54]. ACE2 and angiotensin II levels also differed in treatment groups based on disease severity. The patients treated with ARB in the severe disease group had higher levels of ACE2 compared to their counterparts in the moderate severity group. It is possible that enhanced ACE2 expression might be related to severity and treatment. Animal studies have demonstrated increased ACE2 expression with ACEi and ARB, although the mechanism of the link between SARS-CoV-2 infection and ARB treatment has not yet been determined [Citation16,Citation55]. In contrast, patients taking ACEIs with mild disease severity had higher levels of angiotensin II than did their counterparts with moderate and high severity diseases, thus confirming the interactions between disease severity and medication regarding soluble ACE2 levels.
Lastly, to determine the treatment associated with the least disease severity, the prevalence of patients in each medication group was compared among the three disease severity groups (). Patients on ARB were more likely to have severe COVID-19 symptoms, whereas patients on BB exhibited mild disease symptoms. However, the number of patients in this group was too small to be able to interpret the results without further validation by a larger cohort. Interestingly, patients on BB also experienced the shortest duration of hospital stay compared to other groups, which was not related to variations in circulating levels of ACE2 or angiotensin II suggesting that this outcome is independent of the RAS pathway. BB was previously reported to play a protective role, with patients taking BB having a lower risk of contracting SARS-CoV-2 [Citation29]. Beta-2-adrenergic receptors expressed on lung and airway cells as well as various immune cells are blocked by BB. Along with their role in regulating heart rate, BB also exhibits anti-inflammatory properties as a result of reducing the Th-17 immune response, which plays an important role in driving the cytokine storm associated with COVID-19 [Citation56]. Previous studies have described multiple potential mechanisms underlying BB's anti-inflammatory properties, including blockade of viral entry, reduction of pulmonary edoema, hypercoagulation, and improvement of oxygenation in addition to their putative antioxidant effects [Citation57]. Additionally, non-selective BB has been reported to reduce the excessive inflammatory burst of severe COVID-19 [Citation56] and other studies also suggested that pre-treatment with BB might be protective against SARS-CoV-2 infection, complications, and death [Citation29,Citation33,Citation56–58]. According to a multicenter retrospective study comprising more than 2190 patients diagnosed with COVID-19, BB is the most effective medication for COVID-19 [Citation33].
Conclusion
According to our findings, COVID-19 severity is associated with elevated levels of circulating ACE2 and lower levels of angiotensin II. There are no solid data to indicate that ACEIs or ARBs are associated with a higher risk associated with COVID-19 infection and there is no reason to discontinue these medications because of concerns related to COVID-19. Further studies are required to evaluate the possible effects of different antihypertensive medications on COVID-19 infection, and as the ESC-COVID-19 guidance clearly states, there is no solid evidence that ACEIs or ARBs are associated with an increased risk associated with COVID-19.
Limitations
We encountered several limitations in this study, including its cross-sectional nature and the relatively small number of patients in each of the medication groups, especially those on BB. Therefore, further prospective studies are recommended to confirm these findings. Further, data on some proinflammatory cytokines, such as IL-6, which could be used for interpreting COVID-19 severity groups, were not available for the patients. Additional confounding factors may also have influenced our results, which are unidentified. In addition, the presence of control groups would have contributed significantly to our study.
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Hamad Medical Corporation (protocol code MRC-02-20-382and date of approval 06/06/2020.
Supplemental Material
Download MS Word (254.3 KB)Acknowledgments
Open Access funding is provided by the Qatar National Library. This report was made possible by an RRC award [RRC-2-076] from the Qatar National Research Fund (a member of The Qatar Foundation). The statements made herein are solely the responsibility of the authors. We would like to acknowledge Qatar BioBank for helping with the logistics of the collected samples, Prof. Nahla Afifi, Dr. Marwa A. El Deeb, and Ms. Sidra Abdulshakoor. The publication of this paper is covered by Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Attaway AH, Scheraga RG, Bhimraj A, et al. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436.
- Guo J, Huang Z, Lin L, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of Angiotensin-Converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7):e016219.
- Tikellis C, Thomas MC. Angiotensin-Converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012; (2012), :80.
- South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H1090.
- Zhang X, Li S, Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad Med J. 2020;96(1137):403–407.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease Inhibitor. Cell. 2020;181(2):271–280.e8.
- Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116.
- Medina-Enríquez MM, Lopez-León S, Carlos-Escalante JA, et al. ACE2: the molecular doorway to SARS-CoV-2. Cell Biosci. 2020;10(1):148.
- Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–1205.
- Epelman S, Tang WHW, Chen SY, et al. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-renin ratio and high soluble ACE2 system. J. Am. Coll. Cardiol. 2008;52(9):750–754.
- Oudit GY, Pfeffer MA. Plasma angiotensin-converting enzyme 2: novel biomarker in heart failure with implications for COVID-19. Eur Heart J. 2020;41(19):1818–1820.
- Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100.
- Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206–206.
- Patel SK, Juno JA, Lee WS, et al. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 2021;57.
- van Lier D, Kox M, Santos K, et al. Increased blood angiotensin converting enzyme 2 activity in critically ill COVID-19 patients. ERJ Open Res. 2021;7(1):00848–2020.
- Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610.
- Karram T, Abbasi A, Keidar S, et al. Effects of spironolactone and eprosartan on cardiac remodeling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am J Physiol Heart Circ Physiol. 2005;289(4):H1351–8.
- Ishiyama Y, Gallagher PE, Averill DB, et al. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976.
- Razeghian-Jahromi I, Zibaeenezhad MJ, Lu Z, et al. Angiotensin-converting enzyme 2: a double-edged sword in COVID-19 patients with an increased risk of heart failure. Heart Fail Rev. 2021;26(2):371–380.
- Chouchana L, Beeker N, Garcelon N, et al. Association of antihypertensive agents with the risk of in-hospital death in patients with covid-19. Cardiovasc Drugs Ther. 2021.
- World Health Organization, Living guidance for clinical management of COVID-19. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2.
- Lew RA, Warner FJ, Hanchapola I, et al. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp Physiol. 2008;93(5):685–693.
- Ramchand J, Patel SK, Srivastava PM, et al. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLOS One. 2018;13(6):e0198144.
- Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879.
- Huang F, Guo J, Zou Z, et al. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun. 2014;5:3595.
- Mortensen EM, Nakashima B, Cornell J, et al. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55(11):1466–1473.
- Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-renin ratio and high soluble ACE2 system inhibitors and risk of covid-19. N Engl J Med. 2020;382(25):2441–2448.
- Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760.
- Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-renin ratio and high soluble ACE2 inhibitors. Eur Heart J. 2020;41(19):1810–1817.
- Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-renin ratio and high soluble ACE2 system blockers and the risk of covid-19. N Engl J Med. 2020;382(25):2431–2440.
- Yan F, Huang F, Xu J, et al. Antihypertensive drugs are associated with reduced fatal outcomes and improved clinical characteristics in elderly COVID-19 patients. Cell Discov. 2020;6(1):77.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422.
- Danser AHJ, Epstein M, Batlle D. Renin-Angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75(6):1382–1385.
- Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473.
- Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625.
- Grobe JL, Mecca AP, Lingis M, et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7). Am J Physiol Heart Circ Physiol. 2007;292(2):H736–42.
- Nagy B, Jr,Fejes Z, Szentkereszty Z, et al. A dramatic rise in serum ACE2 activity in a critically ill COVID-19 patient. Int J Infect Dis. 2021;103:412–414.
- Yeung ML, Teng JLL, Jia L, et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell. 2021;184(8):2212–2228.e12.
- Gerard L, Lecocq M, Bouzin C, et al. Increased angiotensin-converting enzyme 2 and loss of alveolar type II cells in COVID-19-related acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204(9):1024–1034.
- Chen Y, Huang D, Yuan W, et al. Lower serum angiotensin-converting enzyme level in relation to hyperinflammation and impaired antiviral immune response contributes to progression of COVID-19 infection. Infect Dis Ther. 2021;10(4):2431–2446.
- Zhu Z, Cai T, Fan L, et al. The potential role of serum angiotensin-converting enzyme in coronavirus disease 2019. BMC Infect Dis. 2020;20(1):883.
- Yalcin HC, Sukumaran V, Al-Ruweidi MKAA, et al. Do changes in ACE-2 expression affect SARS-CoV-2 virulence and related complications: a closer look into membrane-bound and soluble forms. IJMS. 2021;22(13):6703–6703.
- Akin S, Schriek P, van Nieuwkoop C, et al. A low renin ratio and high soluble ACE2/renin ratio and high soluble ACE2 associate with COVID-19 severity. J. Hypertens. 2021;395(10229):1033–1034.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034.
- Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402.
- Biberoğlu S, İpekci A, İkizceli İ, et al. Role of plasma angiotensin II and angiotensin-converting enzyme 2 levels on prognosis and mortality in hypertensive patients with COVID-19. Biomark Med. 2021;15(17):1581–1588.
- Chappell MC, Pirro NT, South AM, et al. Concerns on the specificity of commercial ELISAs for the measurement of angiotensin (1-7) and Angiotensin II in human plasma. Hypertension. 2021;77(3):e29–e31.
- Martins ALV, da Silva FA, Bolais-Ramos L, et al. Increased circulating levels of angiotensin-(1–7) in severely ill COVID-19 patients. ERJ Open Res. 2021;7(3):00114–2021.
- Heurich A, Hofmann-Winkler H, Gierer S, et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307.
- de Queiroz TM, Lakkappa N, Lazartigues E. ADAM17-Mediated shedding of inflammatory cytokines in hypertension. Front Pharmacol. 2020;11:1154.
- Straus MR, Bidon M, Tang T, et al. FDA approved calcium channel blockers inhibit SARS-CoV-2 infectivity in epithelial lung cells, bioRxiv 2020.
- Tao X, Zhang L, Du L, et al. Allosteric inhibition of SARS-CoV-2 3CL protease by colloidal bismuth subcitrate. Chem Sci. 2021;12(42):14098–14102.
- Wan Y, Shang J, Graham R, et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–20.
- Barbieri A, Robinson N, Palma G, et al. Can beta-2-Adrenergic pathway be a new target to combat SARS-CoV-2 hyperinflammatory syndrome?-lessons learned from cancer. Front Immunol. 2020;11:588724.
- Kjeldsen SE, Narkiewicz K, Burnier M, et al. Potential protective effects of antihypertensive treatments during the covid-19 pandemic: from inhibitors of the renin-angiotensin system to beta-adrenergic receptor blockers. Blood Press. 2021;30(1):1–3.
- Pinto-Sietsma S-J, Flossdorf M, Buchholz VR, et al. Antihypertensive drugs in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother. 2020;6(6):415–416.