Abstract
Background
Isolated systolic hypertension (ISH) in middle-aged and elderly is associated with high cardiovascular risk, but no randomised controlled trial has assessed the effect of antihypertensive treatment in ISH using today’s definition, i.e. systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP) <90 mmHg.
Methods
A systematic review and meta-analysis of randomised controlled trials was performed. Studies with ≥1000 patient-years of follow-up, comparing more intensive versus less intensive BP targets, or active drug versus placebo, were included if the mean baseline SBP was ≥140 mmHg and the mean baseline DBP was <90 mmHg. The primary outcome was major adverse cardiovascular events (MACE). Relative risks from each trial were pooled in random-effects meta-analyses, stratified by baseline and attained SBP level.
Results
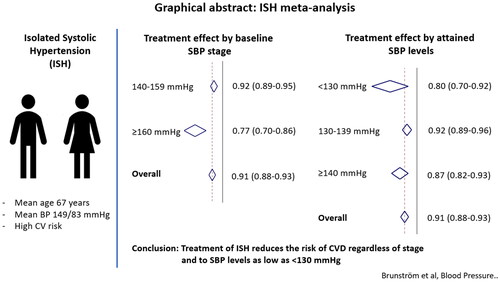
Twenty-four trials, including 113,105 participants (mean age 67 years; mean blood pressure 149/83 mmHg) were included in the analysis. Overall, treatment reduced the risk of MACE by 9% (relative risk 0.91, 95% confidence interval 0.88–0.93). Treatment was more effective if baseline SBP was ≥160 mmHg (RR 0.77, 95% CIs 0.70–0.86) compared to 140–159 mmHg (RR 0.92, 95% CIs 0.89–0.95; p = 0.002 for interaction), but provided equal additional benefit across all attained SBP levels (RR 0.80, 95% CIs 0.70–0.92 for <130 mmHg, RR 0.92, 95% CIs 0.89–0.96 for 130–139 mmHg, and RR 0.87, 95% CIs 0.82–0.93 for ≥140 mmHg; p = 0.070 for interaction).
Conclusions
These findings support antihypertensive treatment of isolated systolic hypertension, regardless of baseline SBP, to target SBP <140 mmHg and even <130 mmHg if well tolerated.
Introduction
Isolated systolic hypertension (ISH) in the most common form of hypertension in the elderly and it is an advanced form of hypertension, characterised by increased pulse pressure due to stiff arteries with reduced compliance [Citation1–3]. The aetiology of ISH includes vascular remodelling and endothelial dysfunction and it commonly occurs in people with comorbidities affecting vascular structure and function, such as diabetes mellitus and chronic kidney disease (CKD) [Citation1]. Although generally at an increased risk of cardiovascular events, treatment strategies for patients with ISH have been debated due to the fear of decreased coronary perfusion and myocardial oxygenation with lower diastolic pressure [Citation3–5].
Few trials have examined the effect of antihypertensive therapy in ISH patients specifically. In the Systolic Hypertension in the Elderly Program (SHEP), 4736 participants with baseline systolic blood pressure (SBP) higher than 160 mmHg and diastolic blood pressure (DBP) lower than 90 mmHg were randomly allocated to chlorthalidone-based antihypertensive treatment or placebo [Citation6]. This resulted in a 36% relative risk reduction for stroke and a 27% relative risk reduction for coronary events. The benefits of drug treatment in ISH were confirmed in the Systolic Hypertension in Europe (Syst-Eur) trial, in which 4695 participants with similar BP inclusion criteria were randomised to nitrendipine-based antihypertensive treatment or placebo, resulting in 42% lower risk of stroke and 33% lower risk of coronary events [Citation7].
No randomised clinical outcome trial has assessed the effect of antihypertensive treatment in patients recruited based on today’s definition of ISH, i.e. SBP ≥140 mmHg and DBP <90 mmHg [Citation8,Citation9]. However, many trials, which were not designed to address treatment in ISH specifically, have included large portions of participants with ISH. Thus, the available evidence for the effect of antihypertensive treatment in people with ISH goes beyond that generated from trials designed to investigate outcomes by treating ISH specifically. In the present study we aimed to assess the effect of antihypertensive drug treatment in trials with, on average, ISH at baseline, i.e. trials with a mean baseline SBP ≥140 mmHg and a mean baseline DBP <90 mmHg.
Methods
We performed a systematic review and meta-analysis, including trials with at least 1000 patient-years of follow-up, which randomly compared more intensive versus less intensive BP targets, or an antihypertensive agent or combination of antihypertensive agents against placebo, in other words trials aiming for a BP difference between treatment arms. Trials comparing one agent against another were not included because such trials generally aim to assess BP independent effects, and a small difference in BP can then untoward be associated with a major difference in cardiovascular outcomes between different antihypertensive drugs [Citation10,Citation11]. Furthermore, trials in patients with overt heart failure, left ventricular dysfunction or acute myocardial infarction at baseline were excluded because several antihypertensive drug classes have possible BP independent effects on clinical outcomes in these conditions [Citation12–14].
For the current analysis, we included trials with an average baseline SBP ≥140 mmHg and an average baseline DBP <90 mmHg, i.e. trials with on average ISH at baseline. Although such a selection will include participants with other BP phenotypes as well, our assumption was that a majority of participants in such trials would have ISH, and thus our findings may be applicable to this patient group.
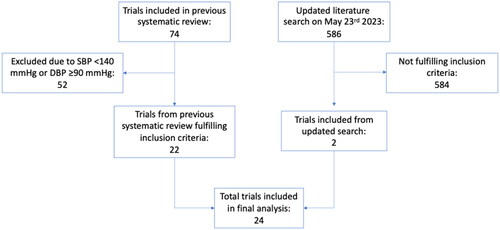
Trial selection was based on a previous systematic review [Citation15], in which PubMed, Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews (DARE), and Cochrane Central Register for Controlled Trials (CENTRAL) were searched in 2017, using the search terms (‘blood pressure lowering’ OR ‘blood-pressure lowering’ OR ‘blood pressure-lowering’ OR antihypertensive) AND (mortality OR myocardial OR stroke). We performed a complementary search in PubMed with the same search terms from the date of the previous search until 23 May 2023, resulting in 586 titles and abstracts screened, and two additional trials to be included () [Citation16,Citation17].
Risk of bias was assessed using Cochrane’s risk of bias assessment tool [Citation18]. Trials at high risk of selection bias, performance bias or detection bias were excluded from all analyses. This led to the exclusion of one potentially eligible trial, where treatment was assigned in a non-random fashion [Citation19]. Attrition bias was assessed on study level whereas selective reporting was assessed on outcome level. We systematically collected data on early termination, baseline imbalances and protocol changes as other potential sources of bias.
Data were extracted by two reviewers independently (MB and BC) with any discrepancies resolved by discussion and re-evaluation of original publications. Participant characteristics at baseline and design features were collected on study level, whereas follow-up BP levels and outcomes were collected on treatment arm-level. The primary outcome was major adverse cardiovascular events (MACE), defined as a composite of stroke, myocardial infarction, heart failure and cardiovascular death. For several trials, we had to accept slightly different definitions, most often either excluding heart failure or including revascularization, as defined by trial investigators. Secondary outcomes were stroke, myocardial infarction, heart failure, and all-cause and cardiovascular mortality.
We performed random-effects meta-analyses, pooling non-standardized relative risks (RR) from included trials to generate an average effect estimate with 95% confidence intervals (95% CIs) [Citation20]. Analyses were stratified according to baseline SBP level 140–159 mmHg versus ≥160 mmHg to explore potential differences in treatment effect between ISH stage 1 and stage 2. Additionally, analyses were stratified according to attained SBP level <130 mmHg, 130–139 mmHg and ≥140 mmHg to assess the potential effect of different guideline-defined SBP targets. Heterogeneity was assessed using the I-squared statistic and sources for heterogeneity explored in meta-regression analyses.
Sensitivity analyses were performed for the primary outcome MACE, using random-effects meta-regression analysis to assess the association between treatment effect and SBP measured as a continuous variable at baseline and during follow-up, respectively, and attained DBP to explore potentially detrimental effects of low DBP levels. Furthermore, we assessed the impact of type-2 diabetes and CKD on treatment effect using Cochrans Q to test for interaction, and by performing separate random-effects meta-analyses when trials in patients with type-2 diabetes and CKD were removed.
All analyses were performed using Stata/MP version 16.1 for Mac.
Results
Twenty-four trials [Citation6,Citation7,Citation16,Citation17,Citation21–40], including 113,105 participants, fulfilled our inclusion criteria. The mean age at baseline was 67 years and 45,140 participants (40%) were women. Mean baseline blood pressure was 149/83 mmHg. Eight trials, including 21,711 participants were restricted to people with type-2 diabetes, seven trials, including 9950 participants had chronic kidney disease (CKD) as an inclusion criterion, and five trials, including 31,440 participants, were performed post stroke ().
Table 1. Study characteristics.
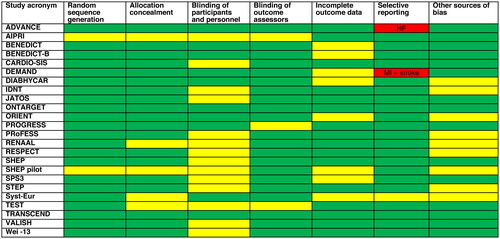
During an average of 3.6 years follow-up, 14,228 participants experienced a MACE and 9657 participants died, reflecting a mean 10-year risk for cardiovascular events of 35%, and a 10-year mortality of 23%. The risk of bias was judged as low or unclear for most included trials and outcomes (). However, the ADVANCE trial failed to report heart failure, and the DEMAND trial failed to report stroke and myocardial infarction, as intended, which may represent selective reporting [Citation21,Citation26].
Figure 2. Risk of bias assessment. Green marks low risk of bias, yellow marks unclear risk of bias, and red marks high risk of bias. The large portion of unclear risk of bias for the domain ‘blinding of participants and personnel’ comes from prospective randomised open-label blinded endpoint (PROBE) trials or target trials, which can never be blinded. Because we only assess objective cardiovascular outcomes, the impact of blinding in this meta-analysis is unclear. Under other sources of bias, trials are marked as unclear risk if they were stopped preterm as this may increase the risk of chance findings. HF: heart failure; MI: myocardial infarction.
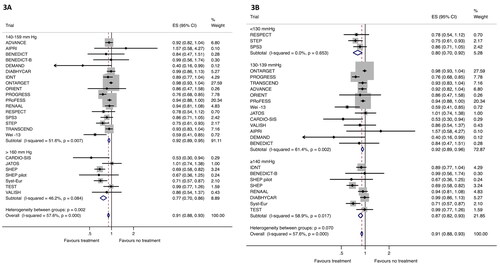
Overall, treatment reduced the risk of MACE by 9% (RR 0.91, 95% CIs 0.88–0.93) across all included trials (). When trials were stratified according to baseline SBP, the effect was significantly greater in trials with baseline SBP ≥160 mmHg (RR 0.77, 95% CIs 0.70–0.86) compared to the 140–159 mmHg range (RR 0.92, 95% CIs 0.89–0.95; p = 0.002 for interaction). However, when analyses were stratified based on attained SBP in the intervention arm, we found no difference in treatment effect between trials with an average in-treatment SBP <130 mmHg (RR 0.80, 95% CIs 0.70–0.92), 130–139 mmHg (RR 0.92, 95% CIs 0.89–0.96) and ≥140 mmHg (RR 0.87, 95% CIs 0.82–0.93; p = 0.070 for interaction).
Figure 3. Effect of antihypertensive treatment on MACE. (A) Analysis of MACE based on baseline BP 140–159 mmHg (upper part) and SBP ≥160 mmHg (lower part). (B) Analysis of MACE based on achieved SBP <140 mmHg (upper part) and achieved SBP ≥140 mmHg (lower part). MACE: major adverse cardiovascular event; ES: effect size reported as relative risk; CI: confidence interval.
Among secondary outcomes, treatment reduced the risk of stroke, myocardial infarction and heart failure, with a borderline trend towards reduced risk for cardiovascular mortality, but no significant effect on all-cause mortality, in the overall analyses (). As for the primary outcome, there was a significant interaction between baseline SBP and treatment effect, with more pronounced effect if baseline SBP was ≥160 mmHg for stroke, heart failure and cardiovascular mortality (p < 0.05), and borderline significant interactions for myocardial infarction (p = 0.062) and all-cause mortality (p = 0.063). Importantly, treatment reduced the risk of stroke, myocardial infarction and heart failure also in ISH stage 1.
Table 2. Effect of antihypertensive treatment on secondary outcomes.
In analyses stratified by attained SBP, treatment reduced the risk of stroke and heart failure across all SBP strata, with a 22% relative risk reduction for stroke if attained SBP was below 130 mmHg (RR 0.78, 95% CIs 0.66–0.93). For myocardial infarction, there was a non-significant trend towards benefit with attained SBP below 130 mmHg, virtually excluding potential harm (RR 0.77, 95% CIs 0.59-1.001). The effect on mortality outcomes was neutral across all attained SBP strata.
Overall, there was low to moderate statistical heterogeneity across primary and secondary outcome analyses ( and ). There was a significant interaction between baseline SBP as a continuous variable and treatment effect on MACE, with more pronounced effect in trials with higher baseline SBP (p = 0.021). Importantly, inclusion of baseline SBP as a covariate reduced statistical heterogeneity for all outcomes (from 58% to 41% for MACE), indicating that differences in treatment effect between trials could be partly explained by differences in baseline SBP level. Contrary to baseline levels, treatment was equally effective across attained SBP levels when assessed as a continuous variable (p = 0.996), indicating no threshold under which treatment was not beneficial within the attained SBP range studied (127–157 mmHg). Furthermore, there was no sign of harm in meta-regression analyses of treatment effect in relation to attained DBP (p = 0.16 for MACE; range 68 to 86 mmHg).
There was no interaction between diabetes status (p = 0.42) or CKD status (p = 0.39) and treatment effect, with a beneficial treatment effect in sensitivity analyses excluding trials in people with diabetes (RR 0.90, 95% CIs 0.87–0.93) as well as trials in people with CKD (RR 0.90, 95% CIs 0.87–0.93).
Discussion
With ISH being the most common form of hypertension in people older than 65 years, the effect of antihypertensive treatment on clinical cardiovascular outcomes in this patient group is of great interest to clinical practice. Whereas no RCT have addressed this question specifically using the current definition of ISH, this analysis summarises the findings from 24 trials, including more than 100,000 participants, with baseline ISH on average. Importantly, our findings confirm the protective effect of antihypertensive treatment on cardiovascular outcomes in this patient group, including those with ISH stage 1, i.e. baseline SBP 140–159 mmHg. Furthermore, analyses stratified by attained SBP found a significant benefit on MACE, as well as stroke and heart failure, in trials with average attained SBP down to below 130 mmHg, with no sign of harm at low BP levels in meta-regression analyses exploring the association between treatment effect and attained SBP and DBP, respectively .
These findings have important clinical implications as they provide evidence for a beneficial effect of antihypertensive treatment which goes beyond that of designated ISH RCTs. Firstly, all designated ISH trials have had average baseline SBP values >170 mmHg, thus representing ISH stage 2 [Citation6,Citation7,Citation35,Citation39]. Here, we include data from 17 trials with an average baseline SBP 140–159 mmHg (ISH stage 1), showing a beneficial effect on several clinically important cardiovascular outcomes, thereby supporting initiation of antihypertensive treatment in people with ISH stage 1. Secondly, the average attained SBP in the intensive treatment group in designated ISH RCTs have been in the 140–150 mmHg range, giving the impression that current evidence does not support antihypertensive treatment to below 140 mmHg in this group. Our findings clearly demonstrate that BP lowering treatment to below 140 mmHg, and possibly even to below 130 mmHg, reduces the risk of MACE, stroke and heart failure in trials with baseline ISH on average.
The effect of drug treatment in ISH stage 1 appears similar to that of stage 1 hypertension in general [Citation15]. Furthermore, the present analysis suggests that treatment targets recommended for the general hypertensive population [Citation8,Citation9] are also beneficial and safe in people with ISH. Thus, it simplifies hypertension guidelines in the sense that we may generalise and recommend therapeutic treatment target below 140/90 mmHg for all patients with Stage I hypertension if drug treatment is indicated, and even target SBP below 130 mmHg, if well-tolerated. Importantly, the studies that have been included in the present analysis included middle-aged and older patients [Citation41]. ISH is the dominant form of hypertension also in adolescence, with different risk factor profile compared to diastolic or combined hypertension [Citation42]. For young people with ISH, data on treatment effect are lacking and other considerations beyond the scope of the present meta-analysis may be relevant, like weighing lifelong treatment against low short-term risk. Likewise, several trials included here had an upper age limit of 80 years, making the applicability of our findings to very elderly ISH patients uncertain as well. In this patient group, the absolute risk of cardiovascular events is always very high, but at the same time arterial stiffens have progressed further, with increased risk of hypotension-related side effects, like dizziness, syncope and falls.
ISH represents an advanced form of hypertension with stiff arteries and high pulse pressure. Thus, ISH patients are already characterised by hypertensive mediated organ damage (HMOD) in the form of arterial stiffness. Stiff arteries in these patients are general and affect all large and to a certain degree medium sized arteries; the condition is characterised by deposition of collagen and general fibrotic tissue throughout the vessel walls, in contrast to atherosclerotic disease of the major and large arteries which are characterised by endothelial patchy plaques in certain areas of the vascular system. These pathophysiological aspects are important to understand the difference between stiff arteries, representing HMOD, in many ways similar to left ventricular hypertrophy of the heart and increased urinary albumin excretion from the kidneys, and atherosclerotic arterial disease for which the relationship to hypertension is different and far more complex, and other risk factors, like dyslipidaemia, play an important role.
Considering coexisting HMOD, patients with left ventricular hypertrophy (LVH) may be an exception from where target SBP <130 mmHg is beneficial. Patients with LVH have poor myocardial microcirculation [Citation43] and they may need a certain arterial-venous perfusion pressure to maintain tissue blood flow and avoid myocardial ischaemia, arrhythmias and sudden cardiac death [Citation44]. In the Losartan Intervention For Endpoint Reduction in Hypertension Study (LIFE), all 9193 patients had LVH diagnosed by ECG [Citation45]. The target SBP in LIFE was below 140 mmHg, but on average this target was not achieved and few patients attained a SBP below 130 mmHg. In line with common practice in hypertension outcome trials, patients attaining low BP levels were not back-titrated, however, and subsequent analyses have found increased all-cause mortality in these patients [Citation45]. These findings were confirmed in a pre-specified and similar analysis of patients in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial, who had qualified for participation in the study by having LVH on ECG, showing increased cardiac and all-cause mortality if averaged achieved SBP came below 130 mmHg [Citation46]. In the present analysis certainly some fractions of patients had LVH and their inclusion in the analysis may potentially have masked a larger benefit of SBP target below 130 mmHg in non-LVH patients with ISH.
This analysis has some limitations. Firstly, trials were included based on mean BP values at baseline; only four trials were strictly limited to ISH patients (stage 2), and none included participants based on the current definition of ISH (stage 1). Although this means that individuals with other blood pressure phenotypes contribute to the analyses, the majority of patients had ISH, and our findings are likely to be generalisable to both ISH stage 1 and 2. Secondly, the finding that treatment reduced the risk of MACE, stroke and heart failure when attained SBP was below 130 mmHg should be interpreted with some caution. This analysis included only three trials, all of which assessed the effect of intensive treatment targets post stroke. Whether our findings also apply to ISH patients without previous stroke is unknown, although we have no reason to suspect that the effect on MACE and heart failure should differ by cerebrovascular disease status.
In summary, this meta-analysis, including 24 randomised controlled outcome studies with more than 100,000 participants, of whom most had ISH at baseline, strongly suggests that patients with ISH stage 1 and 2 should be treated. Treatment seems effective and safe down to target SBP levels below 140 mmHg, and possibly even below 130 mmHg, supporting the general recommendation to get all hypertensive patients to a SBP <140 mmHg, and further below 130 mmHg if tolerated.
Disclosure statement
MB and BC report no conflict. SEK has in the past 3 years received lecture honoraria from Getz, Vector-Intas, Merck Healthcare KGaA, and Sanofi Aventis.
Additional information
Funding
References
- Bavishi C, Goel S, Messerli FH. Isolated systolic hypertension: an update after SPRINT. Am J Med. 2016;129(12):1–10. doi: 10.1016/j.amjmed.2016.08.032.
- Bejan-Angoulvant T, Saadatian-Elahi M, Wright JM, et al. Treatment of hypertension in patients 80 years and older: the lower the better? A meta-analysis of randomized controlled trials. J Hypertens. 2010;28(7):1366–1372. doi: 10.1097/HJH.0b013e328339f9c5.
- Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000;355(9207):865–872. doi: 10.1016/s0140-6736(99)07330-4.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively hypertensive patients with coronary artery lowering blood pressure in disease be dangerous? Ann Intern Med. 2006;144(12):884–893. doi: 10.7326/0003-4819-144-12-200606200-00005.
- Bangalore S, Messerli FH, Wun CC, et al. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial(dagger). Eur Heart J. 2010;31(23):2897–2908. doi: 10.1093/eurheartj/ehq328.
- Probstfield JL. Prevention of stroke by antihypertensive drug-treatment in older persons with isolated systolic hypertension - final results of the systolic hypertension in the elderly program (SHEP). JAMA. 1991;265(24):3255–3264.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350(9080):757–764. doi: 10.1016/s0140-6736(97)05381-6.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:1269–1324.
- Brunström M, Thomopoulos C, Carlberg B, et al. Methodological aspects of meta-analyses assessing the effect of blood pressure-lowering treatment on clinical outcomes. Hypertension. 2022;79(3):491–504. doi: 10.1161/HYPERTENSIONAHA.121.18413.
- Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3.
- Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501.
- Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362(9386):759–766. doi: 10.1016/s0140-6736(03)14282-1.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Brit Med J. 2009;338:34.
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease Across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(1):28–36. doi: 10.1001/jamainternmed.2017.6015.
- Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385(14):1268–1279. doi: 10.1056/NEJMoa2111437.
- Kitagawa K, Yamamoto Y, Arima H, et al. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke: a randomized clinical trial and meta-analysis. JAMA Neurol. 2019;76(11):1309–1318. doi: 10.1001/jamaneurol.2019.2167.
- Higgins JPT, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Brit Med J. 2011;343:d5928.
- Liu LS, Wang JG, Gong LS, et al. Systolic hypertension China C. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. J Hypertens. 1998;16(12 Pt 1):1823–1829. doi: 10.1097/00004872-199816120-00016.
- Brunström M, Carlberg B. Standardization according to blood pressure lowering in meta-analyses of antihypertensive trials: comparison of three methodological approaches. J Hypertens. 2018;36(1):4–15.doi:10.1097/HJH.0000000000001574.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8.
- Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334(15):939–945. doi: 10.1056/NEJM199604113341502.
- Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941–1951. doi: 10.1056/NEJMoa042167.
- Ruggenenti P, Fassi A, Ilieva AP, et al. Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens. 2011;29(2):207–216. doi: 10.1097/hjh.0b013e32834069bd.
- Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374(9689):525–533. doi: 10.1016/S0140-6736(09)61340-4.
- Ruggenenti P, Lauria G, Iliev IP, et al. Effects of manidipine and delapril in hypertensive patients With type 2 diabetes mellitus the Delapril and Manidipine for Nephroprotection in Diabetes (DEMAND) randomized clinical trial. Hypertension. 2011;58(5):776–783. doi: 10.1161/HYPERTENSIONAHA.111.174474.
- Marre M, Lievre M, Chatellier G, et al. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ. 2004;328(7438):495–499. doi: 10.1136/bmj.37970.629537.0D.
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303.
- JATOS Study Group . Principal results of the japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31(12):2115–2127.
- Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559.
- Imai E, Chan JCN, Ito S, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia. 2011;54(12):2978–2986. doi: 10.1007/s00125-011-2325-z.
- MacMahon S, Neal B, Tzourio C, et al. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033–1041.
- Yusuf S, Diener H, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225–1237. doi: 10.1056/NEJMoa0804593.
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161.
- Perry HM, Smith WM, McDonald RH, et al. Morbidity and mortality in the systolic hypertension in thE elderly program (shep) pilot-study. Stroke. 1989;20(1):4–13. doi: 10.1161/01.str.20.1.4.
- Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–515.
- Eriksson S, Olofsson BO, Wester PO. Atenolol in secondary prevention after stroke. Cerebrovasc Dis. 1995;5(1):21–25. doi: 10.1159/000107813.
- Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174–1183.
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56(2):196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035.
- Wei Y, Jin ZM, Shen GY, et al. Effects of intensive antihypertensive treatment on chinese hypertensive patients older than 70 years. J Clin Hypertens (Greenwich). 2013;15(6):420–427. doi: 10.1111/jch.12094.
- Mancia G, Kreutz R, Brunström M et al. 2023. ESH Guidelines for the management of arterialhypertension. J Hypertens.doi:10.1097/HJH.0000000000003480"
- Rietz H, Pennlert J, Nordström P, et al. Prevalence, time-trends and clinical characteristics of hypertension in young adults: nationwide cross-sectional study of 1.7 million Swedish 18-year-olds, 1969–2010. J Hypertens. 2022;40(6):1231–1238. doi: 10.1097/HJH.0000000000003141.
- Strauer BE. Ventricular function and coronary hemodynamics in hypertensive heart disease. Am J Cardiol. 1979;44(5):999–1006. doi: 10.1016/0002-9149(79)90235-2.
- Polese A, De Cesare N, Montorsi P, et al. Upward shift of the lower range of coronary flow autoregulation in hypertensive patients with hypertrophy of the left ventricle. Circulation. 1991;83(3):845–853. doi: 10.1161/01.cir.83.3.845.
- Okin PM, Hille DA, Kjeldsen SE, et al. Impact of lower achieved blood pressure on outcomes in hypertensive patients. J Hypertens. 2012;30(4):802–810; discussion 810. doi: 10.1097/HJH.0b013e3283516499.
- Heimark S, Mehlum MH, Mancia G, et al. Middle-aged and older patients with left ventricular hypertrophy: higher mortality with drug treated blood pressure below 130/80 mmHg. Hypertension. 2023; in press. doi: 10.1161/HYPERTENSIONAHA.123.21454.