?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background/Aims
The reactive hyperaemia index (RHI) assesses endothelial function, with a proposed cut-off of <1.67 for prevalent endothelial dysfunction (ED). However, uncertainties remain about whether this cut-off is age-dependent and applicable in healthy individuals. We aimed to explore ED in relation to age within a large population-based cohort of young to middle-aged, healthy individuals.
Methods
Within the Malmö Offspring Study, a total of 1812 subjects (50.9% women, mean age 48 ± 11 years) were included. Post-occlusion/pre-occlusion ratio of the pulsatile signal amplitudes in the non-dominant upper arm was used to calculate RHI by EndoPat®. ED was defined as RHI < 1.67. Multivariable regression models were used to explore associations between ED and age.
Results
Prevalent ED was found in 534 (29.5%) participants. In subjects aged ≤30 years, ED was present in 47.4% compared to 27.6% in subjects ≥30 years (p < 0.001). In multivariable logistic regression analyses, ED was associated with younger age (p < 0.001), higher BMI (p < 0.001) and current smoking (p < 0.001). No sex differences were observed.
Conclusion
In a large healthy population, RHI < 1.67, an early marker of endothelial dysfunction, was more prevalent in younger individuals, implying that RHI might not be a suitable measure of endothelial function in individuals under 30 years of age. Our findings suggest that low RHI in young, healthy individuals may not necessarily indicate true ED but rather an artefact of the limited ability of young and healthy arteries to dilate post-occlusion. Therefore, the term "pseudo-ED" may be applicable to young individuals with low RHI values.
PLAIN LANGUAGE SUMMARY
What is the context?
The endothelium is a thin layer of cells that lines the inside of blood vessels, and its proper function is crucial for the maintenance of vascular health. Endothelial dysfunction (ED) is an early marker of cardiovascular disease and is characterised by impaired dilation of blood vessels, which can lead to reduced blood flow and increased risk of heart attacks and strokes. The reactive hyperaemia index (RHI) is a widely used non-invasive test that measures endothelial function by evaluating the dilation of blood vessels in response to temporary occlusion.
What is new?
This study aimed to investigate the relationship between age and ED in a large population-based cohort of young to middle-aged healthy individuals. The results showed that prevalent ED was more common in younger individuals, with 47.4% of participants aged ≤30 years having ED, compared to 27.6% in those ≥30 years. The study also found that ED was associated with higher BMI and current smoking, but no sex differences were observed.
What is the impact?
The findings suggest that the proposed RHI cut-off of <1.67 for prevalent ED may not be applicable to individuals under the age of 30, as young and healthy arteries may have limited ability to dilate post-occlusion, resulting in low RHI values that do not necessarily indicate true ED. Therefore, the term "pseudo-ED" may be more appropriate for young individuals with low RHI values.
Introduction
Under normal conditions, the endothelium maintains the balance of vascular tone and blood fluidity. Vascular endothelial dysfunction (ED) is a term that refers to reduced production and/or availability of vasoactive nitric oxide, and/or an imbalance in relaxing and contracting factors derived by the endothelium. This might result in the incapacity of the endothelium to respond to shear stress because of changes in blood flow, which in turn affects the arterial tone [Citation1]. Thus, endothelial function can be assessed by measuring the arterial dilation in response to ischaemia (reactive hyperaemia index (RHI)) assed by peripheral arterial tonometry (PAT) [Citation2].
ED is an early contributor to the development of clinical cardiovascular disease (CVD), a major cause of mortality and morbidity globally [Citation3,Citation4], which is reflected by decreased RHI in patients with coronary artery disease, hyperlipidaemia, hypertension, glucose intolerance and diabetes [Citation1]. Apart from the Framingham Heart Study [Citation5], in which associations between RHI and cardiovascular risk factors were examined in 1957 subjects, most studies up to date consist of rather small study populations (n ≤ 100 subjects) [Citation6]. A proposed RHI cut-off indicating endothelial dysfunction is <1.67 [Citation7–9], which has been shown to independently predict peak troponin I concentrations in ST-elevation myocardial infarction (STEMI) patients treated with primary angioplasty [Citation10]. However, concerns have been raised that the proposed cut-off is: a) not applicable to healthy individuals, and b) is age-dependent, as illustrated by earlier preliminary findings in a sub-population of the Malmö Offspring Study (MOS), where 47% of young healthy subjects had RHI below the proposed cut-off value of 1.67 [Citation11]. Therefore, we aimed to present values of RHI in subjects free from diabetes and CVD across different sex and age groups, with a focus on values in the young (age 30 years).
Methods
Study population
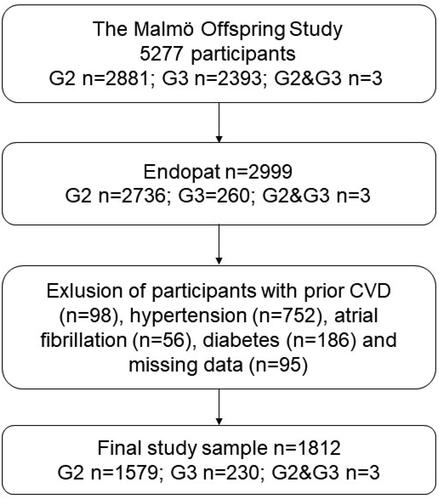
The Malmö Offspring Study (MOS) [Citation12] is a population-based study including 5277 adult participants, children (generation 2) and grandchildren (generation 3) of individuals previously examined within the framework of the Malmö Diet and Cancer–Cardiovascular Cohort (MDC-CC, generation 1; n = 6100) [Citation13]. The primary aim of MOS is to describe hereditary patterns (family clusters) behind non-communicable diseases such as diabetes, cardiovascular disease, cancer, and dementia. Endothelial function was assessed in 2736 participants from Generation 2 (children), 260 participants in Generation 3, and 3 participants that belonged to both Generation 2 and 3 (ntotal=2999). From this population, subjects with prior CVD (n = 98), hypertension (n = 752), atrial fibrillation (n = 56), or diabetes (n = 186) were excluded, along with subjects with missing data on any co-variate, rendering a final study sample of 1812 subjects with complete data on all covariates. All participants provided written informed consent and the study obtained ethical approval from the Regional Ethics Review Board in Lund, Sweden (DNR. 2012/594).
Assessment of endothelial function
Endothelial function was measured using the EndoPat® device (Itamar, Israel) to estimate the endothelium-dependent vasodilation following post-ischemic hyperaemia. A cuff was placed on the non-dominant upper arm, and the index or middle fingers were placed in pneumo-electric tubes. Arterial pulsatile volume changes were recorded continuously. After a 5 min period of rest, the cuff was inflated to 60 mmHg above baseline systolic blood pressure and no less than 200 mmHg, with the possibility to increase the pressure to a maximum of 300 mmHg if necessary. After occlusion of 5 min, the pressure of the cuff was released, and the subsequent arterial dilatation assessed as an increase in the signal amplitude was recorded for another 5 min. The reactive hyperaemia index (RHI) was calculated as a post-occlusion to pre-occlusion ratio of the signal amplitudes [Citation12].
Clinical assessment
As described in previous publications [Citation12], data on medical history (prevalent myocardial infarction, prevalent heart failure or heart valve surgery, prior atrial fibrillation, prior stroke, smoking status, blood pressure and diabetes medication) and lifestyle were acquired through a self-administered questionnaire. Weight (kg) and height (m) were measured in light indoor clothing, and BMI (kg/m2) was subsequently calculated. Resting blood pressure (mmHg) and heart rate (beats/min) were measured as a mean of two readings in the supine position after 5 min rest by use of an automatic device (Omron®). Hypertension was defined as either systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg, or the use of anti-hypertensive drug treatment. Prevalent diabetes was defined as either a self-reported diagnosis of type 2 diabetes, use of antidiabetic medication, or fasting plasma glucose >7 mmol/L on two separate occasions. Prevalent cardiovascular disease was defined as having either prior myocardial infarction, prior heart failure or heart valve surgery, or prior stroke. Blood samples were acquired after an overnight fast and stored at −80 °C.
Laboratory analyses
Fasting blood samples were analysed for total cholesterol, low-density lipoprotein cholesterol (LDL-c) and fasting plasma glucose (FPG) at the Department of Clinical Chemistry, Skåne University Hospital in Malmö, participating in a national standardisation and quality control system [Citation12].
Statistics
Group differences in continuous variables between study participants ≤30 years vs. >30 years of age were compared using Students t-tests for normally distributed variables, or Mann–Whitney U-test for skewed variables. Categorical variables were compared using the χ2 test. Variables are presented as means (standard deviation; SD) and medians (25–75 interquartile range).
In 1812 participants with a complete dataset on all co-variates, unadjusted logistic regressions were carried out exploring associations between 1) age as a continuous variable and ED, and 2) age categorised by ≤30 vs >30 years of age and ED. In Model 1, an adjustment was done for sex. Thereafter, further adjustment according to Model 2 (current smoking, BMI, SBP, total cholesterol, LDL-C and FPG) was carried out. Linear regressions were carried out to analyse associations between 1) age as a continuous variable and RHI, and 2) age categorised by ≤30 vs >30 years of age and RHI adjusted in the same models as described above. Due to skewed distribution, RHI was ln-transformed. All analyses were performed using SPSS version 27.0. A p-value of <0.05 was considered statistically significant.
Results
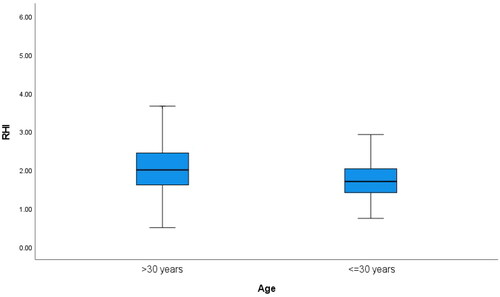
Characteristics of the study population are presented in . Overall, in the total study population, the mean age was 48.8 ± 10.9 years, with 50.9% women (Figure 1). Subjects aged ≤30 years differed in all aspects from subjects aged >30 years, except for FPG levels. Younger adults (aged ≤30 years) presented with lower RHI values, and a higher prevalence of ED defined as RHI <1.67 (, ). In logistic regression models, increasing age was associated with a lower risk of prevalence of ED (OR 0.97; CI95% 0.96–0.98, p < 0.001) in the adjusted Model 2, while being aged ≤30 years was associated with a higher risk of prevalent ED (OR 2.40; CI95% 1.71–3.38, p < 0.001) ().
Table 1. Characteristics of study participants by age groups and by endothelial dysfunction.
Table 2. Logistic regression models displaying associations between age RHI <1.67.
In linear regression models, increasing age was associated with higher RHI (β 0.005; p < 0.001), and being aged ≤30 years was associated with lower RHI (β −0.167; p = 0.024) in the adjusted Model 2 (). No association was observed between ED and sex.
Table 3. Linear regression models displaying associations between age and RHI.
Discussion
In this cross-sectional, observational study, we demonstrated that endothelial dysfunction defined as a reactive hyperaemia index (RHI) <1.67 is cross-sectionally associated with lower age. Additionally, younger individuals below the age of 30 years demonstrated an inverse association with endothelial dysfunction. In this paper, we describe the relationship between RHI and ED, specifically focusing on the potential for “pseudo-ED” in young individuals, which we will discuss further below.
Endothelial dysfunction has previously been shown to be an early marker of cardiovascular (CV) risk. Growing evidence has shown that disrupted endothelial tone is profoundly implicated in the pathogenesis of endothelial dysfunction, thereby promoting vasoconstriction, inflammation, and thrombosis [Citation14]. Thus, several methods have been developed in order to assess vascular tone including laser Doppler flowmetry and pulse amplitude tonometry (PAT) [Citation15]. The use of PAT has been widely studied and is considered a useful method to assess endothelial dysfunction, at least in middle-aged or elderly subjects, or in patients with established disease [Citation16]. Additionally, studies on treatment targeting vascular dysfunction have shown that concomitant improvement of PAT response leads to improved cardiovascular health [Citation17]. Also, a diminished PAT response has been shown to be associated with a higher incidence of adverse cardiovascular events, as well as to predict CV symptoms such as chest pain leading to recurrent hospitalisations for suspected acute coronary syndrome [Citation18]. However, the assessment of PAT has mainly been applied in patients with prevalent CVD, whereas its accuracy in healthy individuals remains unclear. In the Framingham Heart Study including healthy individuals (40 ± 9 years), a lower PAT ratio was shown to be associated with male sex, younger age, BMI, total/HDL cholesterol ratio, diabetes, smoking and lipid-lowering treatment [Citation5]. Additionally, like the Framingham Heart Study, our study showed an unexpected positive association between increasing age and a higher PAT ratio. This has previously been suggested to be an effect of the narrow age range of the study sample. However, the positive relationship between increasing age and higher PAT ratio in the current study was seen within a broad age range (18–75 years) which emphasises that there may be differential age-related changes in vasomotor hyperaemic response in the fingertip micro-vessels as compared to other vascular beds. Also, the paradoxical relationship between age and PAT ratio (RHI) might be due to the method itself, and not a consequence of different vasomotor hyperaemic responses. Therefore, the proposed cut-off value for endothelial dysfunction of RHI <1.67 seems not to be applicable in young individuals (“pseudo-ED”). One potential hypothesis for the lower reactive hyperaemia index (RHI) observed in young individuals is that their vasculature may have greater baseline vasodilation, leading to a relatively smaller increase in post-occlusion dilation compared to older individuals. This could be due to age-related changes in vascular tone regulation, as well as other physiological factors such as differences in endothelial function or sympathetic nervous system activity. A study conducted on young and older males examining the relationship between PAT ratio and brachial artery flow-mediated dilation (FMD) revealed that FMD decreased with age, while PAT ratio increased. This suggests that the microvascular vasodilator regulation evaluated by EndoPat may not be entirely consistent with that of the larger upstream arteries [Citation19]. Also, the use of a fixed time frame during hyperaemia for RHI determination by the automated EndoPat analysis may not be suitable for ageing individuals. However, in older and sick individuals, low RHI values may indeed indicate true ED. Therefore, we propose that the term “pseudo-ED” may be applicable to young healthy individuals with low RHI values. This term suggests that there may be a different underlying cause for low RHI in younger individuals that is not related to true ED. Further research is needed to explore the underlying mechanisms of this proposed “pseudo-ED”.
To maintain vascular homeostasis, endothelial cells release several molecules with the ability to alter the vascular tone. The presence of vascular risk factors such as hypertension, diabetes mellitus and smoking impairs endothelial cellular function and thereby also lowers NO synthesis which has previously been reported to decrease during ageing [Citation20]. Previous findings show that infusion of NO synthase inhibitor dampened the PAT hyperaemic response suggesting that the increased pulse amplitude following hyperaemic flow has to be partly dependent on NO bioavailability [Citation21].
PAT is used as an office-based non-invasive clinical method to assess ED, but it might also serve as an indication of therapy choice in the presence of cardiovascular disease. Still, the contradictory associations observed between RHI and age in the current study suggest that its usefulness for risk stratification in healthy, younger persons may still be limited. Further research would be needed to explore potential mechanisms underlying age-related differences in RHI.
Strengths and limitations
Assessment of PAT response in the current study is limited to small arteries in the fingertip vascular bed. However, arterial physiology and mechanisms of vessel distensibility may vary considerably between different vascular beds through the phenomena of impedance mismatch and resultant pulse pressure amplification.
Impedance mismatch refers to the difference in characteristic impedance between large arteries and smaller arterioles and capillaries. This leads to pulse pressure amplification, where the pulse pressure increases as blood moves from the larger arteries to the smaller vessels due to the increased resistance encountered by the pressure wave in the smaller vessels. Pulse pressure amplification affects peripheral measurements of blood pressure and may not reflect the true central arterial physiology.
The strength of the study is that it is population-based and recruited a high number of healthy young to middle-aged participants, not often screened with PAT in previous studies that mostly recruited patients. A shortcoming might be that the technical device itself and its results may not reflect true ED in the young, as a precursor of atherosclerosis, but rather influenced by other age-related factors such as the diameter of small arteries, finger volume, hyperreactivity to examination stress and occlusion pain, or other unknown factors, i.e. “pseudo-ED”. The paradoxical relationship between age and PAT ratio might also be due to the method itself rather than different vasomotor hyperaemic responses. Overall, more research is needed to determine the accuracy of RHI in young, healthy individuals, as well as to investigate the potential limitations of this method and its applicability across different age groups.
Conclusion
In a population-based, observational study, younger individuals below the age of 30 years unexpectedly demonstrated a higher prevalence of endothelial dysfunction than older subjects, which emphasises that future research is necessary to identify the age cut-off at which this technique may be valid among healthy, young to middle-aged individuals. In addition, other age-related factors that may disturb the interpretation of results have to be investigated to reduce the influence of spurious results or even artefacts in the young. Our findings suggest that low RHI in young individuals may not necessarily indicate true ED but rather an artefact of the limited ability of young and healthy arteries to dilate post-occlusion. Therefore, the term “pseudo-ED” may be applicable to young individuals with low RHI values.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hadi HAR, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):1–7.
- Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the working group on endothelin and endothelial factors of the European society of hypertension. J Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659.
- Widlansky ME, Gokce N, Keaney JF, Jr., et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160. doi: 10.1016/s0735-1097(03)00994-x.
- Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham heart study. Circulation. 2008;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574.
- McCrea CE, Skulas-Ray AC, Chow M, et al. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17(1):29–36. doi: 10.1177/1358863X11433188.
- Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062.
- Heffernan KS, Patvardhan EA, Kapur NK, et al. Peripheral augmentation index as a biomarker of vascular aging: an invasive hemodynamics approach. Eur J Appl Physiol. 2012;112(8):2871–2879. doi: 10.1007/s00421-011-2255-y.
- Tanaka A, Tomiyama H, Maruhashi T, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72(5):1060–1071. doi: 10.1161/HYPERTENSIONAHA.118.11554.
- Baptista SB, Faustino M, Simões J, et al. Endothelial dysfunction evaluated by peripheral arterial tonometry is related with peak TnI values in patients with ST elevation myocardial infarction treated with primary angioplasty. Microvasc Res. 2016;105:34–39. doi: 10.1016/j.mvr.2015.12.010.
- Nilsson PM, Östling G, Kennbäck C, et al. [PP.12.11] endothelial function IN young healthy subjects. Journal of Hypertension. 2016;34(Supplement 2):e187. doi: 10.1097/01.hjh.0000491862.58954.4e.
- Brunkwall L, Jönsson D, Ericson U, et al. The Malmö offspring study (MOS): design, methods and first results. Eur J Epidemiol. 2021;36(1):103–116. doi: 10.1007/s10654-020-00695-4.
- Manjer J, Carlsson S, Elmstahl S, et al. The Malmo diet and cancer study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10(6):489–499. doi: 10.1097/00008469-200112000-00003.
- Sun HJ, Wu ZY, Nie XW, et al. Role of endothelial dysfunction in cardiovascular diseases: the link Between inflammation and hydrogen sulfide. Front Pharmacol. 2019;10:1568. doi: 10.3389/fphar.2019.01568.
- Sandoo A, van Zanten JJ, Metsios GS, et al. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–312. doi: 10.2174/1874192401004010302.
- Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2.
- Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41(10):1761–1768. doi: 10.1016/s0735-1097(03)00329-2.
- Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010.
- Babcock MC, DuBose LE, Witten TL, et al. Assessment of macrovascular and microvascular function in aging males. J Appl Physiol (1985). 2021;130(1):96–103. doi: 10.1152/japplphysiol.00616.2020.
- Sverdlov AL, Ngo DT, Chan WP, et al. Aging of the nitric oxide system: are we as old as our NO? J Am Heart Assoc. 2014;3(4):e000973. doi: 10.1161/JAHA.114.000973.
- Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19(1):6–11. doi: 10.1016/j.tcm.2009.03.001.