Abstract
Background
Successful antihypertensive management can limit left ventricular hypertrophy (LVH) and improve the clinical prognosis. However, it remains unclear whether intensive blood pressure (BP) lowering has a greater effect on the occurrence and regression of LVH compared to standard BP lowering.
Methods
We searched the electronic databases of PubMed, EMBASE and Web of Science from inception to 2 June 2023. Relevant and eligible studies were included. A random-effects model was used to estimate the pooled odds ratio (OR) and 95% confidence intervals (CI).
Result
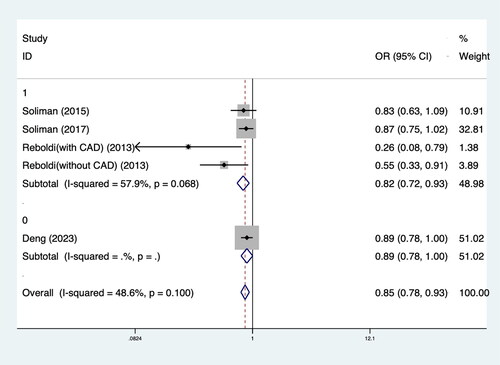
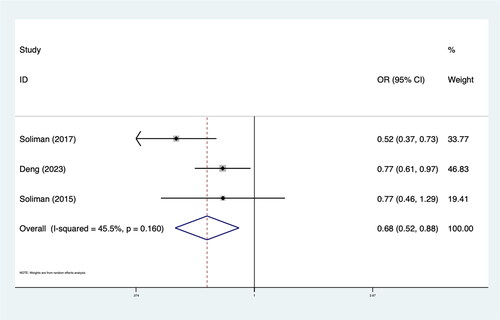
Four RCTs including 20,747 patients met our inclusion criteria. The results demonstrated that intensive BP lowering was associated with a significantly lower rate of LVH (OR 0.85; 95%CI: 0.78–0.93; I2 48.6%) in patients with hypertension compared to standard BP lowering. Subgroup analysis revealed that the effect of intensive BP lowering on LVH was more pronounced in patients with high cardiovascular disease (CVD) risk factors (OR 0.82; 95%CI: 0.72–0.93; I2 57.9%). In addition, intensive BP lowering led to significant regression of LVH (OR 0.68; 95%CI: 0.52–0.88; I2 45.5%).
Conclusions
Our study suggests that intensive BP lowering should be instigated as soon as possible for optimal control of BP and to prevent regression of LVH, especially in patients with high risk of CVD. However, caution is warranted when treating hypertensive patients with LVH to systolic blood pressure (SBP) targets below 130 mm Hg.
PLAIN LANGUAGE SUMMARY
We conducted a meta-analysis of four randomised controlled trials involving 20,747 patients with hypertension. The result suggested that intensive BP lowering should be instigated as soon as possible for optimal control of BP and to prevent regression of LVH, especially in patients with high risk of CVD. However, caution is warranted when treating hypertensive patients with LVH to SBP targets below 130 mm Hg.
Introduction
Hypertension remains an important public health problem associated with considerable morbidity and mortality [Citation1]. It is the leading risk factor for cardiovascular disease (CVD) worldwide. Hypertension has long been associated with asymptomatic cardiac geometric and functional alterations [Citation2]. The long-term increase in afterload results in the enlargement and hypertrophy of the myocardium and cardiac remodelling. Initially, this response may be beneficial, but over time, it can lead to decreased cardiac function, which is a key characteristic in the natural history of hypertension. The left ventricle is particularly vulnerable to end-organ damage caused by elevated blood pressure [Citation3]. An increase in left ventricular wall stress is the principal mechanical factor in the development of left ventricular hypertrophy (LVH). LVH is a well-established pivotal biomarker of cardiac injury and an indicator of incident cardiovascular events and mortality [Citation4]. In observational studies, LVH has been identified as the strongest independent predictor of CVD events after age, with an average risk ratio of 2.5 for overall CVD events including myocardial infarction, heart failure, stroke and death [Citation5–8].
Successful antihypertensive management can limit LVH and improve the clinical prognosis [Citation9,Citation10]. The regression of LVH during antihypertensive therapy is a significant predictor of CVD events [Citation11,Citation12]. However, LVH often develops in patients receiving standard blood pressure (BP) control [Citation13]. Emerging evidence suggests an early change in the structure and geometry of the left ventricle in patients with high normal BP, defined as a systolic BP (SBP) from 120 mmHg to 139 mmHg or a diastolic BP from 80 mmH to 89 mmHg. It was indicated that intensified antihypertensive strategies might be more effective in preventing cardiac hypertrophy [Citation14,Citation15]. Intensive BP lowering in patients with LVH can lead to the regression of myocardial hypertrophy and subsequently exert beneficial effects on CVD outcomes [Citation16–18]. However, relatively few randomized control trials (RCTs) have examined the effect of BP lowering on LVH and a comprehensive systematic review of this evidence is lacking [Citation19–22].
Therefore, we conducted this meta-analysis with the following objectives: (1) to compare the effects of intensive antihypertensive therapy and standard antihypertensive therapy on the occurrence of LVH in hypertensive patients and (2) to compare the regression of LVH between intensive antihypertensive therapy and standard antihypertensive therapy.
Methods
The systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2015 [Citation23]. We prospectively registered the analysis at the PROSPERO international prospective register of systematic reviews (CRD42023432009).
Data sources and search strategy
We searched the electronic databases of PubMed, EMBASE and Web of Science from inception to 2 June 2023. No language restrictions were applied. Our search strings included (‘blood pressure’ OR ‘hypertens*’ OR ‘antihypertens*’ OR ‘anti-hypertens*’) and (‘Hypertrophy, Left Ventricular’ OR ‘Left Ventricular Hypertroph*’). The scope of the literature was restricted to RCTs. We screened the reference lists of included studies and related publications. The results were then hand-searched for eligible trials. This search strategy was performed iteratively until no new potential citations could be found on review of the reference lists of retrieved articles.
We specifically included only RCTs that compared intensive BP lowering with standard BP lowering. The terminology used to define intensive BP lowering and standard BP lowering was based on the definitions provided in the respective published studies. We included studies focusing on the occurrence and regression of LVH as the outcomes.
Study eligibility
We excluded any duplicate published or same sample origin studies and only included those with the longest follow-up period. Publications that had no relevant data (e.g. reviews, case reports, editorials and comments) were excluded.
Outcome
The primary outcome assessed was the occurrence of LVH at the longest available follow-up period. The secondary outcome was the regression of LVH.
Data extraction
Two authors searched the studies and extracted the data independently and in duplicate. Data were extracted using standardized protocol and reporting forms. Disagreements were resolved by discussion. For each included study, we mainly collected the following data: the first author, publication year, country, characteristics of the included population (e.g. inclusion criteria, age, number of males, criteria definition of intensive BP lowering and standard BP lowering), number of events and sample size.
Quality assessment
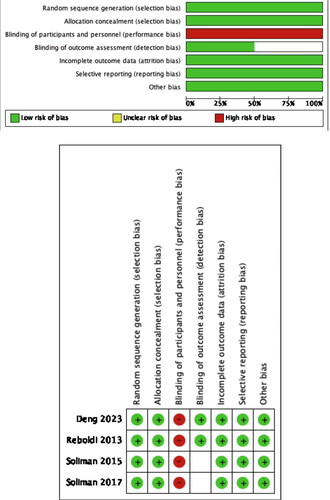
Quality assessment of all included studies was done by using the six domains of the Cochrane tool for assessing risk for bias. Two authors independently assessed the risk of bias of the included RCTs with the recommended version 2 of the Cochrane risk of bias tool for randomized trials (RoB2) [Citation24]. The risk of bias in each domain (randomization process, intervention assignment, missing outcome data, measurement of outcome, selection of the reported result, other bias) and the overall bias were judged as low, with some concerns, or high.
Statistical analysis
The meta-analysis was performed using STATA 15.0 and Revman 4.2. The statistical analysis was performed using the Cochran–Mantel Haenszel method under the random-effects model to calculate the odds ratio (OR) and 95% confidence interval (CI) for the primary and secondary outcomes. We used a p < .05 as statistically significant in our analyses. The I2 statistic test was used for the assessment of between-study heterogeneity, with values >50% was corresponding to have heterogeneity. To find the possible sources of heterogeneity, subgroup analysis was used. Subgroup analysis was performed based on the presence or the absence of CVD risk factors within the study population. In addition, we performed a sensitivity analysis by removing each study from the meta-analysis. Due to the limited number of included literature, we did not conduct a publication bias analysis. p Value < .05 was considered statistically significant.
Results
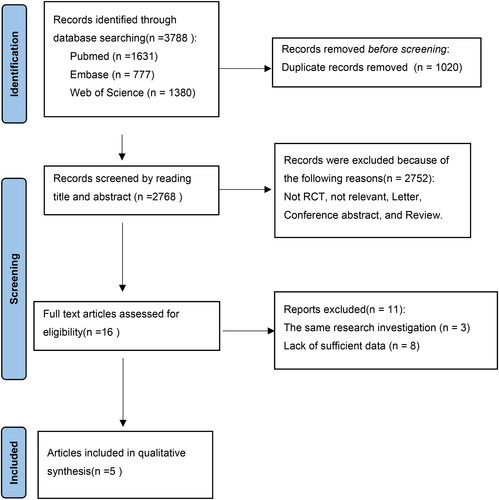
Finally, 3788 studies were retrieved by the search strategy, including 1631 from PubMed, 777 from Embase and 1380 from Web of science. Subsequently, 1020 duplicates were excluded. 2768 studies were selected for initial screening by abstract and title, and 2752 studies were further excluded. Ultimately, after reading the full text of 16 studies, 4 studies were included in this meta-analysis. The flow diagram of study selection is shown in .
Clinical trials and baseline patient characteristics are shown in online and . All included studies were multicentre RCTs. One study specifically focused on individuals with diabetes, while another study stratified participants based on the presence or the absence of CVD. All studies were followed up for more than 2 years. LVH was diagnosed by electrocardiogram. Three studies provided data on the occurrence of LVH in individuals without baseline LVH and the regression of LVH in those with baseline LVH. All included studies were of high quality, and the quality evaluation is detailed in .
Table 1. Baseline characteristics of the included studies.
Table 2. Occurrence/regressive of LVH in the included studies.
Primary endpoint
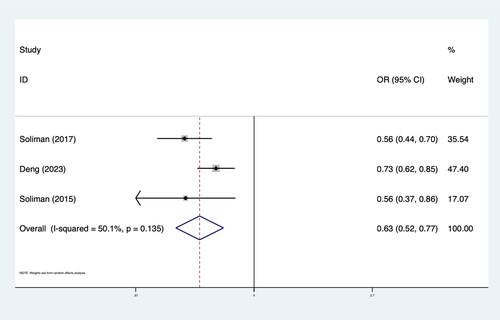
The results showed that intensive BP lowering in comparison with the control group was associated with significantly lower LVH rates (OR 0.85; 95%CI: 0.78–0.93; I2 48.6%) in hypertension patients. Subgroup analysis showed that the effect of intensive antihypertensive treatment on LVH was more obvious in patients with high CVD risk factors (OR 0.82; 95%CI: 0.72–0.93; I2 57.9%). In patients without LVH at baseline, the effect of intensive BP control on LVH is more significant (OR 0.63; 95%CI: 0.52–0.77; I2 50.1%). The results of the meta-analysis and subgroup analysis are shown in and .
Discussion
In this meta-analysis of four RCTs including 20,747 patients with hypertension suggested that intensive BP lowering results in a statistically significant 15% reduction in the risk of LVH. Specifically, individuals with CVD appear to benefit the most, experiencing a 39% reduction in the risk of LVH. Furthermore, intensive BP lowering led to a higher rate of regression of LVH in those who already had LVH at baseline, with a reduction of 32%. However, it should be noted that the evidence base remains relatively limited, with only a small number of RCTs available for inclusion in this analysis. Further studies with larger sample sizes and longer follow-up periods would provide more robust evidence and enhance the generalizability of the findings.
Hypertension has long been associated with asymptomatic geometric and functional changes in the heart (such as LVH). It is well established that LVH increases the risk of CVD events [Citation25–27] and that this risk could be reduced by regression of LVH [Citation27]. The long-term benefits of antihypertensive therapy in hypertensive patients are well established [Citation28–30]. The prognostic benefit of LVH regression on CVD has been demonstrated in two meta-analyses, which revealed a significant reduction (approximately 50%) in CVD risk associated with the resolution of LVH in hypertensive individuals [Citation31,Citation32]. Intensive BP lowering in patients with LVH can lead to the regression of myocardial hypertrophy and subsequently exert beneficial effects on CVD outcomes. LVH is a maladaptive response of cardiomyocytes to chronic pressure overload. Intensive BP control could halt or reverse this process and subsequently exert beneficial effects on CVD outcomes [Citation21]. Accordingly, current hypertension guidelines recommend a strict BP goal, in most cases, below 130/80 mm Hg [Citation28,Citation29]. Nevertheless, excessive BP lowering may impede adequate myocardial perfusion and increase the risk of CVD events due to increased myocardial compressive pressure on coronary arteries and impaired left ventricular filling in LVH [Citation16,Citation33]. Consequently, the 2018 European Society of Cardiology/European Society of Hypertension guidelines incorporated a lower limit for BP control of 120/70 mm Hg [Citation29]. This reflects the need to balance the benefits of BP reduction with the potential risks associated with excessive lowering.
Several studies indicated that excessive BP lowering can also increase CVD events in high-risk patients or those with established CVD risk factors [Citation34–36]. Therefore, determining the appropriate target BP level for hypertension patients remains a critical issue. Post hoc analyses of the The Losartan Intervention For Endpoint reduction (LIFE) study have shed light on this topic. They showed that compared to in-treatment SBP levels of at least 142 mmHg, patients with in-treatment SBP levels between 131 and 141 mmHg had significantly lower risk of all events [Citation37]. On the other hand, patients with SBP of 130 mmHg or less had less reduction in myocardial infarction, stroke and composite endpoint, and no significant decrease in CVD or all-cause mortality. Even after adjusting for LVH and other factors using multivariate regression analysis, patients who achieved an SBP of 130 mmHg or less did not show a significant reduction in the risk of MI, stroke or composite endpoint. Instead, they exhibited a trend towards increased CVD mortality and a statistically significant 37% increased risk of death from all causes. Likewise, post hoc analysis of The Valsartan Antihypertensive Long-term Use Evaluation (VALUE) study found similar results in the LVH population [Citation38]. The analysis revealed that CVD and all-cause mortality were increased in LVH patients with an average SBP below 130 mmHg. In contrast, in patients without LVH, average achieved BP below 130/80 mmHg was associated with reduced stroke, heart failure, cardiac morbidity and cardiovascular mortality. It is important to note that both the VALUE [Citation39] and LIFE [Citation40] studies were not specifically designed to compare outcomes between BP lowering and standard targets. The conclusions drawn from these studies are derived from post hoc analyses, which may introduce potential confounding due to differences between the SBP groups at baseline and during the trial.
Indeed, the underlying mechanisms linking hypertension treatment, LVH regression and reduction in CVD are not yet fully understood. The reasons for the increased mortality associated with intensive BP lowering may include the following considerations: Firstly, excessively low SBP can result in reduced perfusion pressure in the microcirculation, potentially leading to fatal ischaemic complications, particularly in patients with LVH who may have compromised microvascular function [Citation33]. Secondly, early deaths at low SBP levels may reflect undetected underlying diseases or frailty conditions, introducing the possibility of reverse causation bias. Patients with lower baseline and in-treatment SBP levels may have a higher burden of undetected underlying disease or frailty, which can contribute to increased early mortality [Citation41,Citation42]; Finally, it is possible that lower in-treatment achieved SBP could be a marker for subclinical left-ventricular dysfunction or heart failure. Therefore, prevention of LVH or its regression with treatment should continue to be considered important target to achieve. However, caution should be exercised in the selection of populations. In our research, we discovered that implementing intensive antihypertensive treatment leads to a significant reduction in the incidence of LVH. Furthermore, aggressive BP management can effectively diminish pre-existing LVH. It’s worth noting that none of the four RCTs included in the study specified a minimum SBP threshold [Citation19–21,Citation43]. The participants in these studies were elderly, thus highlighting the effectiveness and safety of intense blood pressure control in addressing LVH. However, caution is warranted when treating hypertensive patients with LVH to SBP targets below 130 mm Hg.
The optimal antihypertensive regimen to target LVH regression has been a topic of longstanding interest. Historically, ACE inhibitors (ACEi) have been favoured due to the perceived benefit of neurohormonal blockade in promoting LV mass regression compared to other classes of BP-lowering medications. Schmieder et al. [Citation44] conducted the first meta-analysis of randomised, double-blinded, placebo-controlled trials, demonstrating the clear benefits of antihypertensive therapies and associated BP reduction in the regression of echocardiographic LVM. This effect was consistent across several drug classes, including ACE inhibitors (ACEi), calcium channel blockers (CCBs), and beta-blockers. Subsequent meta-analyses by Klingbeil et al. [Citation45] and Fagard et al. [Citation9] supported these earlier findings, even when considering studies evaluating newer classes of antihypertensive medications. These above-mentioned studies collectively indicate that the type of antihypertensive medication has a minimal impact on LVH. In the studies included, there was no exclusive emphasis on anti-hypertension drugs [Citation19–21,Citation43], it could potentially have an impact on the study results.
There are several limitations to our study. First, due to the nature of meta-analysis, we were unable to access patient-level data, which limited our ability to adjust for potential confounding variables. This could have influenced the accuracy and precision of our findings. Second, our investigation included four articles comprising five studies, with three of them involving patients with LVH at baseline. Although there was no significant difference in baseline LVH between the two groups in each study, it could have influenced the research outcomes. Therefore, we conducted an additional meta-analysis on studies that included data from the population without LVH at baseline and obtained similar conclusions [Citation19–21]. Detailed results are presented in . Finally, there were variations in the blood pressure targets among the included studies. This heterogeneity in treatment protocols may have contributed to the variability in the outcomes observed in our analysis.
Conclusions
Our study adds to the growing body of evidence supporting the benefits of intensive BP lowering in the management of hypertension and prevention/regression of LVH. However, the limitations of our study should be taken into consideration, and further research is needed to provide more robust and conclusive evidence in this field.
Authors’ note
Conceptualisation: Jingjing Hu.
Data curation: Jingjing Hu, Yidan Zhou.
Formal analysis: Jingjing Hu, Yidan Zhou.
Methodology: Jingjing Hu.
Writing – original draft: Jingjing Hu.
Writing – review and editing: Yidan Zhou.
Acknowledgements
All the authors of the manuscript are immensely grateful to their respective universities and institutes for their technical assistance and valuable support in the completion of this research project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):1–10. doi: 10.1016/S0140-6736(21)01330-1.
- Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1992;19(1):130–134. doi: 10.1016/0735-1097(92)90063-s.
- Yan Y, Li S, Guo Y, et al. Life-course cumulative burden of body mass index and blood pressure on progression of left ventricular mass and geometry in midlife: the Bogalusa Heart Study. Circ Res. 2020;126(5):633–643. doi: 10.1161/CIRCRESAHA.119.316045.
- Manyari DE. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;323(24):1706–1707. doi: 10.1056/NEJM199012133232413.
- Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study . N Engl J Med . 1990;322(22):1561–1566. doi: 10.1056/NEJM199005313222203.
- Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141(3):334–341. doi: 10.1067/mhj.2001.113218.
- Gardin JM, McClelland R, Kitzman D, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001;87(9):1051–1057. doi: 10.1016/s0002-9149(01)01460-6.
- Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52(25):2148–2155. doi: 10.1016/j.jacc.2008.09.014.
- Fagard RH, Celis H, Thijs L, et al. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension. 2009;54(5):1084–1091. doi: 10.1161/HYPERTENSIONAHA.109.136655.
- Mathew J, Sleight P, Lonn E, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104(14):1615–1621. doi: 10.1161/hc3901.096700.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3.
- Okin PM, Devereux RB, Harris KE, et al. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147(5):311–319. doi: 10.7326/0003-4819-147-5-200709040-00006.
- Izzo R, Losi MA, Stabile E, et al. Development of left ventricular hypertrophy in treated hypertensive outpatients: the Campania Salute Network. Hypertension. 2017;69(1):136–142. doi: 10.1161/HYPERTENSIONAHA.116.08158.
- Simpson HJ, Gandy SJ, Houston JG, et al. Left ventricular hypertrophy: reduction of blood pressure already in the normal range further regresses left ventricular mass. Heart. 2010;96(2):148–152. doi: 10.1136/hrt.2009.177238.
- Cuspidi C, Sala C, Tadic M, et al. High-normal blood pressure and abnormal left ventricular geometric patterns: a meta-analysis. J Hypertens. 2019;37(7):1312–1319. doi: 10.1097/HJH.0000000000002063.
- Westerhof N, Boer C, Lamberts RR, et al. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev. 2006;86(4):1263–1308. doi: 10.1152/physrev.00029.2005.
- Ascher SB, de Lemos JA, Lee M, et al. Intensive blood pressure lowering in patients with malignant left ventricular hypertrophy. J Am Coll Cardiol. 2022;80(16):1516–1525. doi: 10.1016/j.jacc.2022.08.735.
- Lee HH, Lee H, Cho SMJ, et al. On-treatment blood pressure and cardiovascular outcomes in adults with hypertension and left ventricular hypertrophy. J Am Coll Cardiol. 2021;78(15):1485–1495. doi: 10.1016/j.jacc.2021.08.015.
- Deng Y, Liu W, Yang X, et al. Intensive blood pressure lowering improves left ventricular hypertrophy in older patients with hypertension: the STEP trial. Hypertension. 2023;80. doi: 10.1161/HYPERTENSIONAHA.122.20732.
- Soliman EZ, Byington RP, Bigger JT, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with diabetes mellitus: action to control cardiovascular risk in diabetes blood pressure trial. Hypertension. 2015;66(6):1123–1129. doi: 10.1161/HYPERTENSIONAHA.115.06236.
- Soliman EZ, Ambrosius WT, Cushman WC, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (systolic blood pressure intervention trial). Circulation. 2017;136(5):440–450. doi: 10.1161/CIRCULATIONAHA.117.028441.
- Randall O, Kwagyan J, Retta T, et al. Effect of intensive blood pressure control on cardiovascular remodeling in hypertensive patients with nephrosclerosis. Int J Nephrol. 2013;2013:120167. doi: 10.1155/2013/120167.
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Bang CN, Soliman EZ, Simpson LM, et al. Electrocardiographic left ventricular hypertrophy predicts cardiovascular morbidity and mortality in hypertensive patients: the ALLHAT study. Am J Hypertens. 2017;30(9):914–922. doi: 10.1093/ajh/hpx067.
- O’Neal WT, Almahmoud MF, Qureshi WT, et al. Electrocardiographic and echocardiographic left ventricular hypertrophy in the prediction of stroke in the elderly. J Stroke Cerebrovasc Dis. 2015;24(9):1991–1997. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.044.
- Leigh JA, O’Neal WT, Soliman EZ. Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged ≥65 years. Am J Cardiol. 2016;117(11):1831–1835. doi: 10.1016/j.amjcard.2016.03.020.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2017;138(17):e426–e483.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339.
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
- Verdecchia P, Angeli F, Borgioni C, et al. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens. 2003;16(11 Pt 1):895–899. doi: 10.1016/s0895-7061(03)01018-5.
- Pierdomenico SD, Cuccurullo F. Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta-analysis. Am J Hypertens. 2010;23(8):876–881. doi: 10.1038/ajh.2010.80.
- Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens. 2011;2011:495349. doi: 10.4061/2011/495349.
- Böhm M, Schumacher H, Teo KK, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from Ontarget and Transcend trials. Lancet. 2017;389(10085):2226–2237. doi: 10.1016/S0140-6736(17)30754-7.
- Böhm M, Schumacher H, Teo KK, et al. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120–140 mmHg) and cardiovascular outcomes in high-risk patients: results from Ontarget and Transcend trials. Eur Heart J. 2018;39(33):3105–3114. doi: 10.1093/eurheartj/ehy287.
- Douros A, Tölle M, Ebert N, et al. Control of blood pressure and risk of mortality in a cohort of older adults: the Berlin Initiative Study. Eur Heart J. 2019;40(25):2021–2028. doi: 10.1093/eurheartj/ehz071.
- Okin PM, Hille DA, Kjeldsen SE, et al. Impact of lower achieved blood pressure on outcomes in hypertensive patients. J Hypertens. 2012;30(4):802–810. doi: 10.1097/HJH.0b013e3283516499.
- Heimark S, Mehlum MH, Mancia G, et al. Middle-aged and older patients with left ventricular hypertrophy: higher mortality with drug treated systolic blood pressure below 130 mm Hg. Hypertension. 2023;80(8):1739–1748. doi: 10.1161/HYPERTENSIONAHA.123.21454.
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9.
- Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X.
- Pastor-Barriuso R, Banegas JR, Damián J, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med. 2003;139(9):731–739. doi: 10.7326/0003-4819-139-9-200311040-00007.
- Boutitie F, Gueyffier F, Pocock S, et al. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med. 2002;136(6):438–448. doi: 10.7326/0003-4819-136-6-200203190-00007.
- Reboldi G, Angeli F, de Simone G, et al. Tight versus standard blood pressure control in patients with hypertension with and without cardiovascular disease. Hypertension. 2014;63(3):475–482. doi: 10.1161/HYPERTENSIONAHA.113.02089.
- Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studies. JAMA. 1996;275(19):1507–1513. doi: 10.1001/jama.1996.03530430051039.
- Klingbeil AU, Schneider M, Martus P, et al. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115(1):41–46. doi: 10.1016/s0002-9343(03)00158-x.