Abstract
Purpose
Ambulatory, cuff-less blood pressure (BP) measurement devices are a promising trend to alleviate the strains of conventional, cuff-based BP determination. Cuff-less devices circumvent discomfort and nocturnal arousal reactions which can be triggered by cuff inflation from conventional, cuff-based ambulatory blood pressure measurement devices. Mitigating these discomforts is especially desirable when performing measurement in children. In this study we want to assess the clinical validity of a cuff-less BP measurement device for 24-h measurements in children and adolescents.
Materials and methods
We compared the simultaneously retrieved BP data of the cuff-less SOMNOtouch NIBP and the cuff-based Mobil-O-Graph in 24-h use in 90 children in the range from 5 to 17 years old.
Results
A total of 1218 valid measurement pairs showed a mean deviation of 0.99 mmHg (limits of agreement: 21.44/-19.46) for systolic and 3.03 mmHg (limits of agreement: 24.37/−18.31) for diastolic BP values. Patient-specific difference of means was within 15 mmHg in 97.7% (systolic BP) and 93.2% (diastolic BP) patients. 25.6% of nocturnal cuff inflations led to determinable, BP-relevant arousal reactions.
Conclusions
The SOMNOtouch NIBP demonstrated little measurement deviation of mean BP compared to the cuff-based technique over a broad spectrum of 24-h, ambulatory BP measurements in children and adolescents. Cuff-less blood pressure measurement relieves the issue of nocturnal arousal reactions which are shown to be frequently induced by cuff-based measurements. Driven by these promising results, we encourage ongoing efforts to create enough evidence on cuff-less BP measurement to promote it into broad clinical application.
PLAIN LANGUAGE SUMMARY
What is known about the topic?
Hypertension is of increasing relevance in children and adolescents
Blood pressure measurement is difficult, especially in younger individuals
What this study adds?
The study investigated the accuracy of a cuff-less blood pressure (BP) measurement device, SOMNOtouch™ NIBP, in children and adolescents for 24-h measurements, in comparison to a traditional cuff-based device.
The experiment involved 90 participants aged between 5 and 17 years old, with both devices worn simultaneously for 24 h.
The results indicated that the cuff-less device showed a minor average deviation in BP measurements. The mean deviation for systolic and diastolic BP values was 0.99 mmHg and 3.03 mmHg, respectively.
About 25.6% of night-time cuff inflations in traditional devices led to significant arousal reactions.
The study concluded that the cuff-less device had a slight measurement deviation and could alleviate issues like nocturnal arousal reactions that are common with cuff-based devices.
These findings suggest the potential for broad clinical application of cuff-less BP measurement devices, especially in children and adolescents, to reduce discomfort and improve patient adherence. However, more research is needed to solidify these findings.
Introduction
Blood pressure (BP) management is one of the most abundant and important tasks clinicians face in everyday practice. Even young children and adolescents are subject to hypertension (2–4%) in relevant numbers [Citation1,Citation2]. Childhood BP is highly predictive of BP levels in adulthood. There is a special, medical imperative to mitigate cardiovascular risk-factors in the young, as risks accumulate and multiply over time. Therefore, proper BP determination in children is of utmost importance [Citation3,Citation4].
Unfortunately, assessing BP in children poses a conflict of goals: Naturally, clinicians want the best data available to diagnose and treat juvenile hypertension correctly. For this reason, 24-h ambulatory BP measurement is recommended for diagnosis confirmation, classification, and treatment response monitoring [Citation4–6]. On the other side, there is the will to reduce the burden of diagnostic procedures as much as possible, especially for children. Ambulatory BP measurements are prone to create patient discontent, mainly due to the repeated discomforting cuff inflations [Citation7–9]. Subsequently, doctors are facing the choice of either running to little diagnostics or exposing their young patients to unnecessary discomfort. As a result, hypertension is frequently undiagnosed in children and adolescents, which can lead to severe secondary complications such as left ventricular hypertrophy [Citation10–12].
Cuff-less ambulatory BP measurement devices could be the solution to this dilemma: Firstly, they have the capability to provide continuous and non-disturbing 24-h BP measurements. Moreover, cuff-less BP monitoring devices ensure the recording of undisturbed nocturnal BP values, which have the highest predictive value for cardiovascular events [Citation13–16]. Before all considerations of a comforting measurement character, BP measurement device first and foremost must be accurate. There are multiple reports of promising measurement accuracy of cuff-less BP measurement devices, but predominantly in a laboratory setting examined in adults. However, the European Society of Hypertension (ESH) has released a recent position paper stating the ‘considerable potential’ but still lacking evidence for the applicability of cuff-less BP measurement devices [Citation17].
In this study, we investigated the measurement deviation and clinical applicability of cuff-less 24-h ambulatory BP measurements in 100 children and adolescents. We compared the SOMNOtouch NIBP cuff-less BP measurement device (SOMNOmedics GmbH) to a validated and widely used cuff-based ambulatory BP measurement device (Mobil-O-Graph, IEM GmbH). The SOMNOtouch™ NIBP was chosen as it is commercially available and had undergone validation while showing promising results in adults. The device is based on relating the pulse wave velocity to arterial BP [Citation18–20].
Ultimately, we want to contribute to provide the evidence demanded by the ESH: To what degree can a cuff-less BP measurement device prove sufficient measurement accuracy in a real-world, clinical setting?
Methods
Devices
We compared the SOMNOTouch™ NIBP (SOMNOmedics GmbH) cuff-less device for non-invasive continuous, beat-to-beat ambulatory BP measurement to the cuff-based Mobil-O-Graph (IEM GmbH). The Mobil-O-Graph is featured by the manufacturer for BP measurement in children and appropriate cuff sizes were used. It was set to run measurements every 30 min during the day and every hour during night-time (21:00–07:00). At the time the study commenced, there was no clear consensus recommendation for the measurement interval, especially for the BP measurement in children.
The cuff-less system consisted of a wrist-worn data recorder, a three-lead ECG (with integrated body-position sensor and accelerometer) and an oxygen-saturation sensor to be placed on the patient’s finger. Additionally, the SOMNOtouch NIBP recorded the Mobil-O-Graph’s pressure curves via an attached Y-manometer. This information was recorded for ensuring time synchronicity of both devices and identifying flawed cuff-based BP recordings. Throughout the 24 h measurement period both devices were worn simultaneously on contralateral arms. The cuff-based device was placed on the patients’ right arm and the cuff-less device on the patient’s left arm, respectively.
The SOMNOtouch™ NIBP determined the BP by analysing the beat-to-beat changes of the pulse-wave velocity. The device derives the BP by measuring the pulse arrival time. Latter is the time from the ECG R-wave to arrival of the peripheral pulse wave detected via an oxygen saturation sensor. The apparatus translates this signal into BP data using a proprietary transfer function. The device relies on a carefully performed calibration measurement at total rest (simultaneous measurement of cuff-based device and pulse arrival time) to adapt the patient specific transfer function. We performed this calibration using the Mobil-O-Graph®. Subsequently, the cuff-based BP reading had to be typed into the device via its touchscreen. From there it performed independently for the measurement duration.
Recruitment and ethics approval
A total of 100 patients aged 5–17 years with an indication for a 24 h BP measurements were recruited in a cardiologic-paediatric office. All subjects underwent regular and medically warranted 24-h ambulatory BP measurements, in reaction to elevated BP levels in an in-office spot measurement. All patients were checked for and confirmed to be free of secondary hypertension prior to the measurement. The study has been conducted with approval of the local ethics committee (ethics committee of the university hospital Göttingen, Germany; application number: 9/9/15). Exclusion criteria comprised: (i) any medication except ACE inhibitors, calcium antagonists, AT1 antagonists, methylphenidate, (ii) arrhythmia and (iii) coarctation of the aorta. We carried out the experiments in accordance with the declaration of Helsinki and under the protection of European and national laws and guidelines, as well as accepted by the stated local ethics committee. An informed consent was obtained from all subjects and their legal guardian(s).
Procedure
All subjects needed an ambulatory 24-h BP measurement for diagnostic reasons. After prior consent of the patients, as well as their parents or legal guardian the patients were included in the study. The interarm BP difference was not determined to spare the children of additional stress. As every patient underwent echocardiography and aortic coarctation was an exclusion criterion, a relevant interarm difference was highly unlikely. Ensuing, we proceeded with attaching the Mobil-O-Graph cuff-based device on the patient’s right arm with an appropriate cuff-size. Subsequently, we placed the SOMNOtouch NIPB cuff-less device dependent on the age of the patient either at the thorax via belt (5–12 years) or on the left wrist (13–17 years). We connected the ECG, manometer and oxygen-saturation sensor (placed on a finger of the left arm). Then we requested the patient to sit down and stay at total rest for at least five minutes before starting the initialisation process. After initialisation, the patient was allowed to leave the office and returned on the next day with the devices. The measurement ended regularly after 24-h. All patients were at liberty to end the measurement beforehand for any reason.
Data processing and artefact criteria
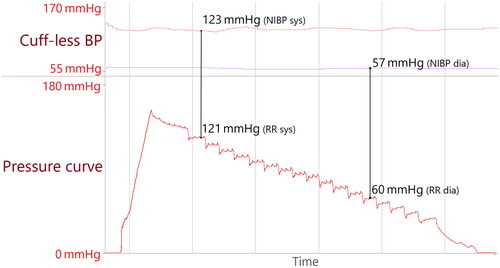
After the recording, we matched the cuff-based BP values with the results from the cuff-less device. To do so, we identified the exact time at which the cuff pressure reached its reported systolic/diastolic BP and read out the corresponding cuff-less BP at this time. Measurements were only considered if the SOMNOtouch NIBP’s signal had been stable for at least 20 s before the start of the cuff inflation. This was done because the SOMNOtouch NIBP calculates the BP as a moving average over multiple beat-to-beat measurements (systolic: 5 beats and diastolic: 20 beats). In this way, data pairs for systolic and diastolic cuff-less and cuff-based BP were obtained ().
Figure 1. Original data; example of measurement pair generation. The upper panel displays the cuff-less, beat-to-beat BP with systolic BP in red and diastolic values in violet. The lower panel displays the inflation/deflation curve. The cuff-based measurement proved a measurement of 121/60 mmHg. Cuff-less BP values (123/57 mmHg) were retrieved at the exact time the pressure curve matched the cuff-based BP values, indicated by the black lines.
In a first step, cuff-based measurements which the Mobil-O-Graph® recognised as flawed were excluded from the analysis. To further ensure representational data, we defined three artefact criteria in the presence of which a BP measurement must be scored as invalid and excluded from the final analysis. This procedure is in line with the recommendations from a recently published study on the importance of correcting for measurement artefacts [Citation21]
High motor activity: Children are and should be active, but excessive movement during cuff-based measurements does affect measurement precision. We therefore excluded measurements under strong motor activity (acceleration greater than 0.8 of earth’s gravity) and measurements with impeded inflation/deflation curves due to activity ().
Cuff error: Cuff inflation/deflation curves can reveal issues in the BP measuring process the device itself did not recognise. We therefore visually scored the inflation/deflation curves and excluded heavily impeded measurements ().
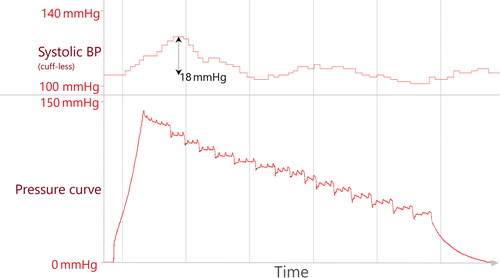
Arousal reactions: Nocturnal cuff-based measurements can lead to arousal reactions, leading to BP spikes themselves. We identified arousal reactions if cuff-less systolic BP increased by more than 12 mmHg (automatically detected by the SOMNOtouch™ NIBP software) in direct association to the inflation process. To unequivocally determine cuff-related arousals, only arousals were scored as ‘cuff-dependent’ and thus excluded if no other arousal had been present at least one minute prior to inflation ().
Figure 2. Original data; example of cuff-based measurement matching criteria for artefact 1 (activity) and 2 (cuff error). The red line in the Top panel represents 800 mg (0.8 times the earth’s gravitational force) and is our cut-off for excessive motor activity. The lower panel displays the heavily impeded inflation/deflation curve.
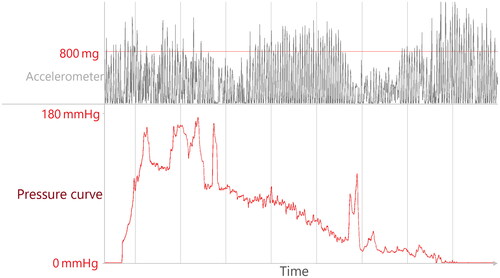
Figure 3. Original data; example of cuff-based measurement matching criteria for artefact 3 (nocturnal arousal). The increase in cuff-less BP of more than 12 mmHg is automatically detected by the software.
Single measurements did fulfill multiple artefact criteria, regularly the case for artefact 1 and 2 ().
After excluding measurements flagged by the Mobil-O-Graph and invalid measurements following our artefact criteria, only valid measurement pairs (simultaneous measurement of both the cuff-based and cuff-less device) were included in the further analysis. The sleep-period was determined via body-position (non-upright) and end of noteworthy motoric activity.
Data analysis
We analysed the data on an interindividual and individual level to provide insight into the general measurement deviation between the oscillometric and of the cuff-less device as well as into the clinical grading of individual patients.
To assess overall measurement deviation between the devices, we conducted related Student’s t-tests (mean difference) and Levene-tests (difference in variance) to compare the oscillometric and cuff-less device. For improved visual interpretability, we provided Bland-Altman-plots for overall, daytime, and nocturnal measurements.
Additionally, we calculated the B-Score to assess the BP measurement uncertainty of the cuff-less device more robustly. The B-Score is a metric used to evaluate the accuracy of BP measurement devices. It measures the absolute error (cuff-less BP – cuff derived BP) of the proposed device and sets it into contrast with the dataset interindividual and intraindividual BP variability, as well as to the overall predictability of BP values in the dataset using a standardised Deep Learning network. via this process, the B-Score provides a single, reliable score to compare “true” measurement performance across different studies relying on different datasets. Higher scores indicating desirable performance while scores below zero indicate lower measurement accuracy [Citation22].
Subsequently, to gain insight into the clinical applicability, we calculated the mean BP values of individual patients for both methods and compared the agreement for overall, daytime, and nocturnal values. Further, we calculated how many of the patients identified as “non-dippers” (nocturnal systolic BP decrease of less than 10%) by the oscillometric device were also classified as such by the cuff-less device. For both analyses of intraindividual mean BP values we only included patients with at least three valid measurement pairs (for 24-h, daytime and nocturnal values, respectively). For the analysis of singular measurement accuracy (Bland–Altman analysis), all valid measurements were considered. We performed all analyses in Python 3, using the SciPy statistics library [Citation23].
Results
Dataset composition
In the analysis, 90 of 100 measured subjects (age 5–17) were included (). The measurement in 10 subjects did not meet the defined criteria of 6 valid measurement pairs which we therefore excluded. Of the 90 subjects, 78 (86.7%) were under antihypertensive medication in treatment of their primary hypertension. 54 (60.0%) were under singular ACE-Inhibitor therapy, 18 (20.0%) were under an ACE-Inhibitor + Ca-Channel-Blocker therapy, 4 (6.7%) under an AT1-Receptor-Blocker, 1 (1.1%) with an additional Ca-Channel-Blocker and 1 (1.1%) with only a Ca-Channel-Blocker.
Table 1. Dataset composition.
Identified artefacts
After screening for artefact criteria, we identified 330 instances of artefacts to be excluded from analysis. This resulted in 1218 valid measurement pairs (78.7%) ().
Table 2. Artefact prevalence; individual percentages add up to more than 100% because measurements can fulfill more than one artefact criteria.
Measurement deviation between the oscillometric and cuff-less device
For all measurements, the cuff-less device scored a systolic and diastolic mean deviation of 0.99 mmHg and 3.03 mmHg, respectively. All limits of agreement were within ±25 mmHg of the mean deviation. There was no indication of proportional bias as there was no correlation between the mean BP and differences between the devices for systolic (r = −0.03, p = 0.31) or diastolic (r = 0.00, p = 0.95) BP ( and ). Splitting the data in daytime and nocturnal measurements allowed to assess the measurement uncertainty in more detail ( and ).
Figure 4. LoA: Limits of agreement; Bland-Altman plot comparing the cuff-based and cuff-less device. For systolic BP measurements, the mean difference is 0.99 mmHg with limits of agreement of 21.44 and -19.46. For diastolic BP measurements, the mean difference is 3.03 mmHg, with limits of agreement of 24.37 and −18.31.
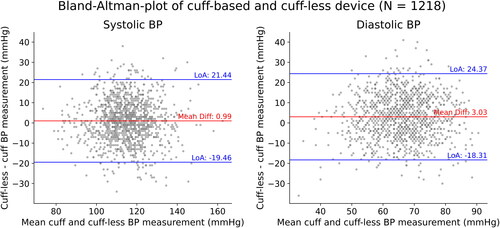
Figure 5. LoA: Limits of agreement; Bland–Altman plot comparing the cuff-based and cuff-less device split up into daytime (upper panel) and nocturnal (lower panel) measurements. For daytime measurements, the systolic mean deviation is 0.92 mmHg with limits of agreement of 21.34 and −19.51 while the diastolic mean deviation is 2.63 mmHg with limits of agreement of 23.61 and −18.36. For nocturnal measurements, the systolic mean deviation is 1.24 mmHg with limits of agreement of 21.83 and −19.35 while the diastolic mean deviation is 4.41 mmHg with limits of agreement of 26.76 and –17.93.
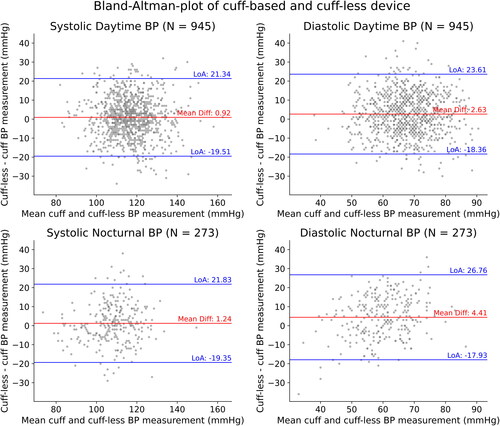
Table 3. Mean of BP values for the oscillometric and cuff-less device for all, daytime, and nocturnal measurements.
B-Score
The device scored a B-Score of 0.084 for systolic and <0.0 for diastolic values. The systolic T-RMSE was 10.47 mmHg, which outperformed the base performances of interindividual variability (B1-RMSE: 12.48 mmHg), intraindividual variability (B2-RMSE: 12.94 mmHg) and the base Deep Learning model (M-RMSE: 10.49 mmHg) as defined in the B-Score’s original publication [Citation22]. (Supplement 1).
Comparison of clinically important individual mean BP values and dipping classification agreement
Comparison of mean values for cuff-less and cuff-based BP measurements allowed to analyse the agreement of individual mean BP values (). The values derived from the oscillometric device identified 32 (68%) children as ‘non-dipper’. Of those 32 children, 26 (81.3%) were also identified as “non-dippers” by the cuff-less device.
Table 4. Agreement between mean cuff-based and cuff-less BP measurements.
Discussion
Children in particular are discomforted by BP measurements at day- and night-time. Devices using continuous pulse wave analysis promise accurate and reliable BP measurements in children without disruptive inflation of the cuff. Until now, clinical data about 24-h, cuff-less BP measuring was still lacking, especially for children and adolescents.
Evaluating the results of validation studies consisting of real-world, clinical 24-h BP data is challenging. The 2018 AAMI/ESH/ISO guidelines for BP measurement device validation are not suited for evaluating continuous blood pressure monitors in clinical settings. The guidelines focus on short-term, laboratory measurements and are of limited use for continuous monitors [Citation17,Citation24,Citation25]. Despite the limitations, the results of the study support the promising use of cuff-less devices for clinical BP measurement in children and adolescents, even though more research is needed to solidify the assessment.
In this study, the presented results grant a fair share of optimism towards the clinical applicability of cuff-less ambulatory BP measurement devices in detection and treatment monitoring of hypertension in the young. For systolic measurements, the device offers almost no mean deviation with limits of agreement comparable to validation studies of the same device conducted with via intraarterial measurement in adults [Citation20]. Diastolic measurements appear to be shifted, with comparable limits of agreement but a slightly increased mean deviation, especially during nocturnal measurements. We did not observe any proportional bias between the mean BP and the difference between the tested and the reference device, suggesting stability over a wide range of BP values in the device’s measurement approach.
These results retrieved from a large sample size (N = 90), are consistent with pre-existing, laboratory studies conducted on the SOMNOtouch NIBP [Citation18,Citation20]. The patient specific levels of accordance () are high as the device scores Grade B precision for systolic and Grade C precision for diastolic values (rounded values), as defined in the British Hypertension Societies classical validation protocol for BP measurement devices [Citation26]. Although the still often references protocol is outdated and designed for laboratory BP measurement at total rest, its application signifies the devices capability of providing reliable mean BP values, even under the challenging conditions of ambulatory 24-h BP measurement. Notably, these results are especially promising, as this style of analysis is only applicable for stationary, short-term validation [Citation26]. Consequently, reaching these results in the more challenging 24-h measurement environment highlights the capability of the approach.
However, the retrieved disagreements between oscillometric and cuff-less mean BP levels are non-zero with likely relevant clinical consequences. About 10% of patients showed a systolic difference of means of more than 10 mmHg. These insecurities could either be testament of remaining technical issues of the cuff-less technique, inherent measurement errors of the oscillometric device or a combination of both.
Subsequently, interpreting the present results is challenging: Unfortunately, there is no broadly agreed validation protocol for cuff-less BP measurement devices and no available reference for a minimal measurement error induced by the reference device (oscillometric). The B-Score was developed for easy comparability of results between suggested devices. In this study, the B-Score showed that the tested cuff-less device outperformed the defined base-performances for systolic values but not so for diastolic measurements. This is in-line with the B-Score calculated for the same device on another dataset. Notably, systolic estimation performance in the 24-h setting seemed to score a much lower B-Score than in a short-term comparison of monitoring patients under the highly dynamic influence of dobutamine and compared to intraarterial BP measurement displayed in a recent B-Score application [Citation22]. This highlights the difficulty of reliable long-duration applications and serves as another reason for cuff-less BP measurement devices being validated in a clinical 24-h measurement setting.
When comparing our results to published studies on 24-h cuff-less BP measurement, the device shows better agreement between cuff-based and cuff-less measurements than in a study conducted on ambulatory BP measurement in adults [Citation19]. Most importantly, we want to highlight that the correct determination of systolic BP values during the night emerges to be less of an issue [Citation19]. A recent study showed promising measurement accuracy of an alternative cuff-less device, although in adults [Citation27]. Yet again, without a broadly adopted metric of relative model performance such as the B-Score, comparing works without knowledge about dataset composition and BP variability remains impossible.
Summarising, some caveats remain: The data still highlights the issue of nocturnal determination of diastolic BP levels. Cuff-less devices depending on initialisation can only properly perform when initialised carefully and at perfect rest. Consequently, well-trained staff is needed to ensure optimal measurement and swift application in clinical routine. Further, signal stability is an issue for any device continuously recording multiple bio-signals. In this study, we often experienced missing or insufficient signal quality within the measurement, mostly due to an incorrectly worn oxygen saturation sensor. Fortunately, beat-to-beat BP recordings still allow to retrace the BP dynamic even when encountering missing data because there are thousands of measurement points even in the worst of scenarios. Unfortunately, at the beginning of the data recording, no preferred device had listed in the STRIDE BP database [Citation28]. Therefore, the oscillometric device, although in use for paediatric patients for years, is not validated for the use in children. However, the device has been successfully in use for paediatric patients for years. Further, we did not determine the interarm BP difference in this study to spare especially the younger children from the tedious and potentially stressful process of repeated and alternating BP measurement on both arms. Measuring and subsequently correcting for the given interarm difference could further improve the overall accuracy of the tested device. Albeit, by screening and excluding aortic and subclavian coarctation with echocardiography, the effect of undiscovered interarm differences in BP should be limited in our data. When conducting cuff-less ambulatory BP measurements, unequivocal instructions for the patients, physicians, and in the case of children for their parents, are needed. Lastly, the European Society of Hypertension does currently not recommend the use of cuff-less BP measurement devices in clinical practice. This is due to the lack of scientific evidence of the capability of providing reliable and accurate measurement results. We hope that this study will contribute to alleviating this lack of evidence [Citation17].
On the other hand, our data revealed important caveats for the application of oscillometric devices as well: While most subjects qualitatively did not report major discomfort during the measurement, those who did identified the cuff-device as obtrusive and sometimes painful. A relevant number of measurements was affected by artefacts, such as disturbed cuff inflation/deflation curves, movement artefacts or cuff-induced nocturnal arousal reactions. This is further supported by recent findings in ambulatory BP measurement in adults. Compared to this study, children showed a very similar total number of measurements affected by artefacts. However, measurement in children was less prone to artefacts during the day and more susceptible to artefacts during the night [Citation21]. A total of 20.3% of measurements were affected, with almost one quarter of nocturnal cuff inflations leading to BP relevant arousal reactions. It must be noted that these effects are commonly not detected in standard ambulatory blood pressure measurement – which itself could lead to large undiscovered measurement uncertainties in oscillometric BP measurement.
Lastly, we want to emphasise the advantages of cuff-less BP measurement in the young. Generally, the device offers beat-to-beat BP values, enabling the detection of short-term BP events. Additionally, the ECG and accelerometer signals allow to monitor heart rate and motor activity and therefore possibly discriminate between high BP levels due activity from such of pernicious character. Cuff-less BP measurement devices are less affected by signal-dropout as their beat-to-beat recording provides thousands of individual measurement points. Further, the improved comfort over cuff-based systems may very well enhance patient adherence. The improved comfort of cuff-less devices thus may motivate clinicians and patients alike to conduct ambulatory BP measurements more liberally and at shorter intervals.
These results are a contribution to the evidence requested by the ESH to promote cuff-less BP measurement into broad clinical application. However, the final evaluation these promising results is dependent on (a) a widely accepted validation protocol for cuff-less BP measurement devices, (b) a plausible estimation of the minimum measurement insecurity induced by the oscillometric reference device and (c) the broad adoption of a measure of relative model performance, enabling the direct comparison of multiple cuff-less devices tested on different datasets.
Conclusion
The SOMNOtouch NIBP cuff-less ambulatory BP measurement device demonstrated little measurement deviation compared to a validated and widely used cuff-based ambulatory BP measurement device during 24-h measurement in children and adolescents. The device shows even better measurement accuracy than formerly reported in adults, most notably improving on the issue of nocturnal systolic BP determination. In our study, a relevant proportion of oscillometric measurements was affected by measurement artefacts. The SOMNOtouch NIBP enables continuous (beat-to-beat) BP measurement and is a promising approach to less disturbing BP measurement, possibly improving patient adherence and willingness to conduct regular ambulatory BP measurements. These results are an important piece in the evidence requested by the ESH before cuff-less BP measurement devices can reach broad clinical application.
Supplemental Material
Download MS Word (115.8 KB)Disclosure statement
T.L.B., A.P. and N.P. advise SOMNOmedics on blood pressure measurement and received travel support. M.H-W. and B.B. have no competing interests.
Data availability statement
The data analysed in the manuscript are available from the corresponding author of this publication upon reasonable request. For further information about data availability, please contact the corresponding author.
Additional information
Funding
References
- Bell CS, Samuel JP, Samuels JA. Prevalence of hypertension in children: applying the new American academy of pediatrics clinical practice guideline. Hypertension. 2019;73(1):1–10. doi: 10.1161/HYPERTENSIONAHA.118.11673.
- Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116(13):1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243.
- Weaver DJ. Hypertension in children and adolescents. Pediatr Rev. 2017;38(8):369–382. doi: 10.1542/pir.2016-0106.
- Rao G. Diagnosis, epidemiology, and management of hypertension in children. Pediatrics. 2016;138(2):e20153616. doi: 10.1542/peds.2015-3616.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):E13–E115. doi: 10.1161/HYP.0000000000000065.
- Moore C, Dobson A, Kinagi M, et al. Comparison of blood pressure measured at the arm, ankle and calf. Anaesthesia. 2008;63(12):1327–1331. doi: 10.1111/j.1365-2044.2008.05633.x.
- Veerman DP, van Montfrans GA, Wieling W. Effects of cuff inflation on self-recorded blood pressure. Lancet. 1990;335(8687):451–453. doi: 10.1016/0140-6736(90)90676-v.
- Davies RJO, Jenkins NE, Stradling JR. Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ. 1994;308(6932):820–823. doi: 10.1136/bmj.308.6932.820.
- Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. J Am Med Assoc. 2007;298(8):874–879. doi: 10.1001/jama.298.8.874.
- Lo JC, Sinaiko A, Chandra M, et al. Prehypertension and hypertension in community-based pediatric practice. Pediatrics. 2013;131(2):e415–e424. doi: 10.1542/peds.2012-1292.
- Moin A, Mohanty N, Tedla YG, et al. Under-recognition of pediatric hypertension diagnosis: examination of 1 year of visits to community health centers. J Clin Hypertens. 2021;23(2):257–264. doi: 10.1111/jch.14148.
- Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens. 1995;13(12 Pt 1):1377–1390.
- Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51(1):55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727.
- Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the pressioni arteriose monitorate e loro associazioni (PAMELA) study. Circulation. 2005;111(14):1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B.
- Gavriilaki M, Anyfanti P, Nikolaidou B, et al. Nighttime dipping status and risk of cardiovascular events in patients with untreated hypertension: a systematic review and meta-analysis. J Clin Hypertens. 2020;22(11):1951–1959. doi: 10.1111/jch.14039.
- Stergiou GS, Mukkamala R, Avolio A, et al. Cuffless blood pressure measuring devices: review and statement by the european society of hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2022;40(8):1449–1460. doi: 10.1097/HJH.0000000000003224.
- Bilo G, Zorzi C, Ochoa Munera JE, et al. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the european society of hypertension international protocol revision 2010. Blood Press Monit. 2015;20(5):291–294. doi: 10.1097/MBP.0000000000000124.
- Socrates T, Krisai P, Vischer AS, et al. Improved agreement and diagnostic accuracy of a cuffless 24-h blood pressure measurement device in clinical practice. Sci Rep. 2021;11(1):1143. doi: 10.1038/s41598-020-80905-x.
- Patzak A, Mendoza Y, Gesche H, et al. Continuous blood pressure measurement using the pulse transit time: comparison to intra-arterial measurement. Blood Press. 2015;24(4):217–221. doi: 10.3109/08037051.2015.1030901.
- Bothe TL, Bilo G, Parati G, et al. Impact of oscillometric measurement artefacts in ambulatory blood pressure monitoring on estimates of average blood pressure and of its variability: a pilot study. J Hypertens. 2023;41(1):140–149. doi: 10.1097/HJH.0000000000003315.
- Bothe TL, Patzak A, Pilz N. The B-Score is a novel metric for measuring the true performance of blood pressure estimation models. Sci Rep. 2022;12(1):12173. doi: 10.1038/s41598-022-16527-2.
- Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0: fundamental algorithms for scientific computing in python. Nat Methods. 2020;17(3):261–272. doi: 10.1038/s41592-019-0686-2.
- Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of medical instrumentation/european society of hypertension/international organization for standardization (AAMI/ESH/ISO) collaboration statement. Hypertension. 2018;71(3):368–374. doi: 10.1097/HJH.0000000000001634.
- Pilz N, Patzak A, Bothe TL. Continuous cuffless and non-invasive measurement of arterial blood pressure—concepts and future perspectives. Blood Press. 2022;31(1):254–269. doi: 10.1080/08037051.2022.2128716.
- O’Brien E, Petrie J, Littler W, et al. The British hypertension society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11:677–679.
- Nachman D, et al. Twenty-four-hour ambulatory blood pressure measurement using a novel noninvasive, cuffless, wireless device. Am J Hypertens. 2021;34:1171–1180.
- Stergiou GS, O’Brien E, Myers M, et al. STRIDE BP: an international initiative for accurate blood pressure measurement. J Hypertens. 2020;38(3):395–399. doi: 10.1097/HJH.0000000000002289.
