Abstract
Background
Social cognitive impairment is common in schizophrenia, but it is unclear if it is present in individuals with high IQ. This study compared theory of mind (ToM) in schizophrenia participants with low or high IQ to healthy controls.
Methods
One hundred and nineteen participants (71 healthy controls, 17 high IQ (IQ ≥115), and 31 low IQ (IQ ≤95) schizophrenia participants) were assessed with the Movie for the Assessment of Social Cognition, providing scores for total, cognitive, and affective ToM, along with overmentalizing, undermentalizing, and no-mentalizing errors. IQ was measured with Wechsler Abbreviated Scale of Intelligence; clinical symptoms with the Positive and Negative Syndrome Scale.
Results
Healthy controls performed better than the low IQ schizophrenia group for all ToM scores, and better than the high IQ schizophrenia group for the total score and under- and no-mentalizing errors. The high IQ group made fewer overmentalizing errors and had better total and cognitive ToM than the low IQ group. Their number of overmentalizing errors was indistinguishable from healthy controls.
Conclusion
Global ToM impairment was present in the low IQ schizophrenia group. Overmentalizing was not present in the high IQ group and appears related to lower IQ. Intact higher-level reasoning may prevent the high IQ group from making overmentalizing errors, through self-monitoring or inhibition. We propose that high IQ patients are chiefly impaired in lower-level ToM, whereas low IQ patients also have impaired higher-level ToM. Conceivably, this specific impairment could help explain the lower functioning reported in persons with intact IQ.
1. Introduction
Cognitive impairments are considered core features of schizophrenia [Citation1–4], and both social and nonsocial cognition show inter-individual heterogeneity [Citation4–6]. The two types of cognitive impairments are related [Citation5], exemplified by social cognition serving as a mediator of the association between nonsocial cognition and functional outcome [Citation7]. Furthermore, whereas social cognitive impairments can be seen in patients with normal-range nonsocial cognition, normal-range social cognition and impaired nonsocial cognition are rarely seen together [Citation8]. This ‘single dissociation’ could suggest that normal-range nonsocial cognition is necessary, but not sufficient, for intact social cognition.
Among the social cognitive domains, theory of mind (ToM) has the greatest impact on functional outcomes [Citation9]. ToM is the ability to infer the mental states of others, considered to be a complex, higher-level cognitive process which is dependent on lower-level processes, such as emotion perception [Citation10,Citation11] and basic nonsocial cognition [Citation11,Citation12]. It can be divided into two components: cognitive ToM, attributing thoughts, knowledge and plans, and affective ToM, attributing emotional states [Citation13,Citation14]. In clinical populations, researchers often distinguish between three error-types that people can make when solving ToM tasks. These are overmentalizing, i.e. a propensity to excessively attribute mental states and intentions, undermentalizing, i.e. reduced ability to understand and attribute mental states, and lack of mentalizing (or ‘no-mentalizing’), i.e. entirely failing to attribute mental states [Citation15,Citation16].
A recent meta-analysis concluded that there are moderate associations between various domains of nonsocial cognition and ToM in schizophrenia without significant differences between domains [Citation17]. Vaskinn et al. [Citation11] found associations between ToM and a nonsocial composite score, accounting for between 8 and 18% of the variance in different ToM subscores. In a follow-up study of the specific neuropsychological tests included in that composite score, Sjølie et al. [Citation12] reported that no single neuropsychological test, including a measure of IQ, uniquely contributed to ToM.
To better account for the cognitive heterogeneity observed in schizophrenia, there have been attempts at subgrouping based on nonsocial cognitive test scores. Studies indicate that cognitive heterogeneity in schizophrenia can be described by three distinct and reliably identified nonsocial cognitive subgroups [Citation18]: a relatively intact group, with good cognitive performance, an intermediate group, with moderate levels of overall impairment, and a globally impaired group, with severe cognitive deficits. These subgroups have been identified both empirically and clinically from intelligence trajectories [Citation19–21]. Whereas some authors [Citation22,Citation23] have found that the subgroups differ only in their level of overall impairment, others [Citation20,Citation21] have concluded that they also exhibit different cognitive profiles. They find that the relatively intact group has a profile more similar to healthy controls (HC) than to other schizophrenia subgroups. In this view, it is characterized by more isolated deficits than the other groups, for instance in executive functioning and attention [Citation20], however, see also, [Citation21].
Similarly, there are also reports of social cognitive heterogeneity in schizophrenia. Studies using cluster analysis have identified three subgroups, with relatively intact, impaired or very impaired social cognition [Citation24–26]. The relatively intact groups appear to have only subtle social cognitive deficits when compared to HCs [Citation25–27], and have better results than the low-performing groups on several measures of nonsocial cognition [Citation24,Citation25].
In conclusion, subgrouping based on IQ differences has been shown to be a useful tool in examining cognitive heterogeneity [Citation18,Citation28]. However, it is not known whether such subgroups have distinct social cognitive profiles. Developing a better understanding of social cognitive heterogeneity in schizophrenia is important. This could enable us to better tailor treatments to different groups of patients. ToM is a particularly interesting target in this respect due to its strong associations with functional outcomes.
The aim of the current study is to investigate subgroup differences in ToM, including ToM components and error-types, by comparing low and high IQ schizophrenia groups with a HC group. We hypothesize group differences for all ToM variables, with HC outperforming the high IQ schizophrenia group, which in turn will perform better than the low IQ schizophrenia group. We make no predictions for group differences in specific patterns of ToM performance, due to the mixed results described in the social cognition literature above.
2. Materials and methods
2.1. Participants
Forty-eight participants (31 male, 17 female) with a DSM-IV diagnosis of schizophrenia (n = 34) or schizoaffective disorder (n = 14), selected from a larger sample [Citation11], were included, together with 71 HC (42 male, 29 female). We chose to include patients with schizoaffective disorder as their cognitive impairments are largely similar to those seen in schizophrenia [Citation29]. All were participants in the Thematically Organized Psychosis (TOP) study at the Norwegian Centre for Mental Disorders Research (NORMENT). Schizophrenia participants were recruited from in- and outpatient units at Oslo and Akershus University Hospitals.
Inclusion criteria were Norwegian as first language or all compulsory schooling in Norway and age 18–55 years. Exclusion criteria were neurological disease or having been hospitalized following a head trauma. For the current study, schizophrenia participants with an IQ of 115 or higher, as assessed with the subtests Similarities and Matrix Reasoning from Wechsler Abbreviated Scale of Intelligence (WASI) [Citation30], were selected for the high IQ schizophrenia group, whereas participants with an IQ between 71 and 95 were selected for the low IQ schizophrenia group. The upper cutoff of 95 was chosen to achieve statistical power and to adhere to our previous studies [Citation23]. HCs from the same geographical area were randomly selected from national statistical records and invited by letter. They were screened with the Primary Care Evaluation of Mental Disorders (PRIME-MD) [Citation31] interview and excluded if mental, neurological, or somatic disorder was present. Participants gave informed consent to participate in the study, which was approved by the Regional Ethical Committee. Clinical assessments were based on semi-structured interviews and chart reviews. Diagnostic assessments were conducted with the Structured Clinical Interview for DSM-IV (SCID-I) [Citation32] by medical doctors and psychologists who had completed a clinical training program from University of California, Los Angeles (UCLA) [Citation33]. Cognitive assessments were conducted by psychologists or master level psychology students, trained by an experienced clinical psychologist (AV).
2.2. ToM measure
ToM was measured with the Norwegian version of the MASC test [Citation34]. It consists of a 15-minute video of two men and two women at a dinner party. Participants are asked to answer 45 multiple choice questions about the characters’ thoughts, feelings, and intentions. The test yields six scores, including a total score, MASCtot (range 0–45). The individual items were further divided into two ToM component categories, cognitive ToM (MASCcog, 0–26) and affective ToM (MASCaff, 0–18), as described in [Citation11]. In addition, the MASC test distinguishes between three different error types (range 0–45 for each score): overmentalizing (MASCexc), undermentalizing (MASCless), and no-mentalizing (MASCno).
2.3. Symptom measure
Symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) [Citation35]. The Wallwork/Fortgang five-factor model [Citation36] was chosen, as it has been shown to provide the best fit in several studies [Citation37–39]. It includes the factors positive (PANSSpos), negative (PANSSneg), disorganized (PANSSdis), excited (PANSSexc) and depressed (PANSSdep). Ranges are listed in .
Table 1. Demographics and clinical features.
2.4. Statistical analyses
Analyses were performed using IBM SPSS Statistics 27th version (2020). The Kolmogorov–Smirnov–Lilliefors significance correction revealed non-normal scores in several cells. We therefore chose nonparametric statistics in all analyses. We applied six Kruskal Wallis H-tests to determine whether the low IQ schizophrenia, high IQ schizophrenia, and HC groups differed for any of the MASC scores, followed by a post hoc Dunn-Bonferroni comparison to identify differences between each pairing of the three groups. Effect sizes were calculated as eta squared (η2) for the Kruskal Wallis H-tests and as Hedge’s g for the post hoc tests. We adjusted the p-values for the two ToM components (p = 0.05/2 = 0.025) and the three error types (p = 0.05/3 = 0.017). The Hettmansperger and McKean method [Citation40] – a nonparametric alternative to the ANCOVA – was used to investigate whether any significant group difference in ToM between the two schizophrenia groups remained after controlling for differences in symptom load. Due to its high rate of type II errors, nonsignificant results should be interpreted with some caution.
3. Results
presents the preliminary analyses with demographics and clinical features. The low IQ schizophrenia group had shorter education than the other two groups. It also consisted of more inpatients, with more disorganized, excited and depressive symptoms (PANSS) than the high IQ schizophrenia group.
presents the group comparisons of MASC performance.
Table 2. Statistical analyses of MASC scores.
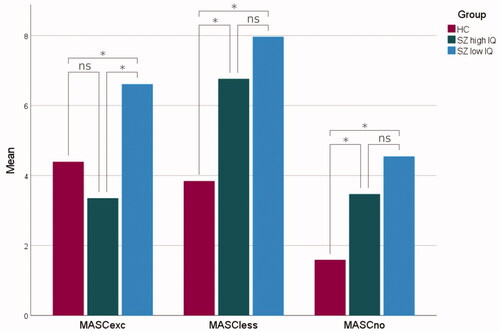
All six Kruskal–Wallis H-tests were statistically significant. The HC group had significantly better scores than the low IQ schizophrenia group for all MASC variables. Further, they significantly outperformed the high IQ schizophrenia group for MASCtot, MASCless and MASCno. The high IQ schizophrenia group performed significantly better than the low IQ schizophrenia group for three MASC variables: MASCtot, MASCcog and MASCexc.
A graphical depiction of group differences on the components is shown in . Mean differences in error types are presented in .
Figure 1. Cognitive (MASCcog) and affective (MASCaff) ToM in healthy controls (HC, red), high IQ schizophrenia (SZ high IQ, green) and low IQ schizophrenia (SZ low IQ, blue). The lines connecting individual bars indicate significant (*) and nonsignificant (ns) comparisons. Scores are presented as a percentage rate of correct answers.
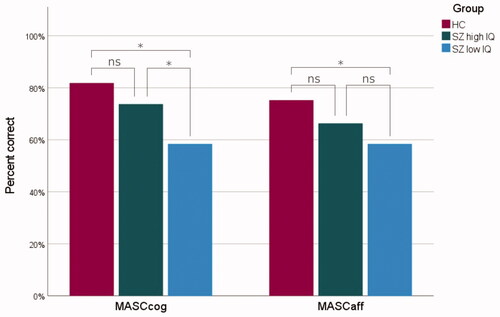
Figure 2. Overmentalizing (MASCexc), undermentalizing (MASCless) and no-mentalizing (MASCno) errors in healthy controls (HC, red), high IQ schizophrenia (SZ high IQ, green) and low IQ schizophrenia (SZ low IQ, blue). The lines connecting individual bars indicate significant (*) and nonsignificant (ns) comparisons.
The Hettmannsperger and McKean follow-up analyses yielded significant group differences between the two schizophrenia groups after controlling for PANSSdep and PANSSexc but not after controlling for PANSSdis.
4. Discussion
We investigated ToM performance using the MASC test in high and low IQ groups of patients with schizophrenia compared to a HC group. As expected, the high IQ schizophrenia group performed at an intermediate level between the HCs and the low IQ schizophrenia group for most of the measured ToM indices. However, there were some important nuances to this picture which extend previous research and may be of help in tailoring interventions to specific patient groups.
First, the reduced performance of the high IQ schizophrenia group on the overall score (large effect size) and for affective and cognitive ToM (medium-large effect sizes) indicate a generalized ToM impairment. Group differences for the two ToM components, however, did not reach statistical significance, and impairments did not appear to be due to overmentalizing. The high IQ schizophrenia group made numerically fewer such errors than HCs, with a small-moderate effect size. In contrast, the low IQ schizophrenia group had reduced scores for all ToM variables compared to HCs, indicating global ToM deficits. These were especially pronounced for the overall ToM score and for cognitive ToM.
Second, although the high IQ schizophrenia group had better overall ToM than the low IQ schizophrenia group, this was reflected primarily in better cognitive ToM and less overmentalizing. One reason for the larger difference for cognitive than affective ToM may be that MASCcog is more cognitively demanding, perhaps requiring explicit mentalizing to a larger degree than MASCaff. Complex forms of mentalizing rely more on a cognitively demanding, explicit mentalizing system [Citation41], and are indeed correlated with IQ [Citation42]. Future studies should attempt to settle if there is in fact a larger difference in cognitive than affective ToM in IQ-based subgroups.
The similar level of overmentalizing in the high IQ schizophrenia and HC groups is consistent with previous studies showing less overmentalizing than undermentalizing and lack of mentalizing in schizophrenia [Citation13,Citation34,Citation43]. However, these studies did not subgroup patients by IQ. The current findings suggest that overmentalizing in schizophrenia may be chiefly associated with lower IQ, whereas other errors may be determined by other factors. One could speculate that a superior intellect enables the high IQ schizophrenia group to evaluate the fallacious arguments involved in overmentalizing more critically. The excessive or irrational attribution of mental states seen in schizophrenia could require a ‘driver’, such as the ‘jumping to conclusions’ bias (which is also correlated with IQ; e.g. Tripoli et al. [Citation44],) or the related construct of hypersalience [Citation45]. Counteracting this tendency to overmentalize, a better self-monitoring and reasoning ability may enable the high IQ schizophrenia group to avoid making explicit overmentalizing errors. Thus, patients with lower IQ, but comparable levels of psychosis, may be as likely to generate overmentalizing responses, but less likely to inhibit them.
Undermentalizing or lack of mentalizing, on the other hand, may reflect an inability to initiate or maintain mentalizing, such as by failing to perceive or properly consider relevant social information. For example, undermentalizing and lack of mentalizing have been associated with emotion perception, while overmentalizing has not [Citation11,Citation43]. The two schizophrenia groups did not differ significantly with regard to the two former types of errors. Consequently, people with schizophrenia and high IQ could have substantial basic social cognitive impairments despite relatively intact self-monitoring (which is related to IQ in clinical populations, e.g. [Citation46,Citation47]) and higher-level reasoning ability.
Our main analyses did not take into account the IQ-level of our HCs, which may be of importance given that our high IQ schizophrenia group had a WASI IQ that was 8 points higher. With only six individuals with IQ ≤95 in the HC sample, significant power limitations make it problematic to conduct subgroup analyses. However, a follow-up analysis with four groups (high and low IQ HCs, high and low IQ schizophrenia) confirmed our findings (see Supplementary Table 1). Both schizophrenia groups had impairments compared to their IQ-matched HCs (no significant interaction effects). Thus, our findings point to the importance of social cognitive impairments in schizophrenia regardless of level of intellectual functioning. In other words, intact nonsocial cognition is not sufficient to prevent social cognitive impairment [Citation8]. Our results align well with findings from studies on nonsocial cognition that identify similar residual deficits after accounting for IQ scores [Citation23,Citation48,Citation49]. Furthermore, these specific social cognitive deficits could help to account for the marked discrepancy between nonsocial cognitive ability and functioning in patients with preserved IQ [Citation21], explaining why they may struggle in everyday life despite having an intact IQ.
When controlling for differences in levels of disorganized symptoms (PANSSdis), the two schizophrenia groups no longer differed significantly for MASCtot, MASCcog or MASCexc. Disorganized symptoms are conceptually related to nonsocial cognition, and higher scores correlate with lower scores on measures of nonsocial cognition, while the other symptom factors correlate less [Citation36,Citation50]. PANSSdis has also been shown to mediate the relationship between nonsocial cognition and functional outcomes [Citation51,Citation52]. Hence, a significant portion of what is measured by PANSSdis likely overlaps with nonsocial cognition. While we cannot completely rule out the presence of a non-cognitive component in PANSSdis, we find it likely that PANSSdis does not represent a real confounding influence on our results. Further, there were more inpatients in the low IQ schizophrenia group. This is not surprising given the strong association between IQ and functioning in schizophrenia [Citation21]. In fact, there were no inpatients in the high IQ schizophrenia group. When repeating the analyses with outpatients only, the differences in ToM between high and low IQ schizophrenia were no longer statistically significant, probably due to loss of power, although effect sizes remained largely similar (see Supplementary Table 2). For generalization purposes we argue that all participants should be included, across treatment levels.
The present study indicates that overmentalizing may be more associated with IQ than other types of mentalizing errors. Further, patients with schizophrenia and low IQ appear to have a larger impairment in cognitive than affective ToM. Such findings suggest that subgrouping methods may provide additional information about patients’ ToM functioning. This is in accordance with the conclusions drawn in IQ subgrouping studies on nonsocial cognition [Citation19,Citation21,Citation53]. Our study adds to this research by demonstrating that people with schizophrenia and high IQ have deficits in social cognition, as they do in nonsocial cognition, and that these impairments may in fact be larger than the nonsocial cognitive impairments [Citation19,Citation21].
4.1. Limitations
There are a few limitations to our study. First, several assumptions of the parametric ANCOVA were not satisfied, in particular for the fairly small high IQ schizophrenia group. All nonparametric alternatives to the ANCOVA have issues with loss of power [Citation54,Citation55]. This could have contributed to our nonsignificant results when controlling for PANSSdis. A similar study using a larger sample might have the power to ascertain some of the differences we expected. This is also particularly relevant for our follow-up analyses with four groups and when excluding inpatient participants. Second, we chose to define our low IQ group as having an IQ score ≤95, which is a higher cutoff than what is usually seen in the literature. Despite this, we still found significant differences between the patient groups which in fact strengthen our results. A final limitation of our study is the use of a 2-subtest IQ measure, which prevented us from examining verbal and nonverbal contributions to social cognition separately.
4.2. Clinical implications
The functional implications of social cognitive impairments are well documented. Thus, it is important that clinicians be aware that these are present also in high IQ schizophrenia. Although these patients do not overmentalize, they have a propensity to undermentalize or not mentalize at all. Low IQ patients have an additional susceptibility to making overmentalizing errors. To overcome the negative consequences related to these impairments, specific strategies should be considered. These may include psychoeducation directed at those afflicted as well as their caregivers. Both low and high IQ groups may also benefit from cognitive remediation strategies targeting social cognition but perhaps with differentiated foci and content.
5. Conclusions
This study identified reduced ToM in intellectually high-functioning patients with schizophrenia. This group had similar levels of undermentalizing and no-mentalizing errors as the low IQ schizophrenia group, while their performance on overmentalizing errors was on par with the HC group. We suggest that this might be explained by a dissociation between impaired basic social cognitive functions and relatively well-preserved higher-level ToM abilities in the high IQ group. These qualitative differences in ToM performance between high and low IQ groups highlight the potential of IQ subgrouping methods for studying cognitive heterogeneity in schizophrenia.
Supplemental Material
Download MS Word (23.6 KB)Supplemental Material
Download MS Word (26.6 KB)Acknowledgements
We are grateful for the effort and time provided by our research participants. We would also like to extend our thanks to our colleagues and involved research personnel at the NORMENT center.
Disclosure statement
Anja Vaskinn has received honorarium from VeraSci Inc. Ole A. Andreassen is a consultant to HealthLytics and has received speaker’s honorarium from Lundbeck and Sunovion. No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
André C. Sahl
André Sahl, PsyD from the Department of Psychology, University of Oslo. He currently works as a clinical psychologist at CRUX in Stavanger, Norway.
Henning F. Rognlien
Henning Fleischer Rognlien, PsyD from the Department of Psychology, University of Oslo. He is currently working as a clinical psychologist at Drammen District Psychiatric Centre, Vestre Viken Hospital Trust, Norway.
Ole A. Andreassen
Ole Andreassen, MD PhD, Professor in psychiatry at University of Oslo. Director of the Norwegian Centre for Mental Disorders Research (NORMENT). His research activities has involved clinical, neurocognitive, brain imaging and molecular genetics tools to identify causes and underlying pathophysiology of psychotic disorder, and develop precision medicine tools in psychiatry.
Ingrid Melle
Ingrid Melle, MD PhD, Professor of adult psychiatry, Institute of Clinical Medicine, University of Oslo. She is co-director of the Norwegian Centre for Mental Disorders Research (NORMENT). Her research focuses on the course and outcome of psychotic disorders including schizophrenia and bipolar disorders.
Torill Ueland
Torill Ueland, PhD & specialist in clinical neuropsychology. Senior Scientist at the Norwegian Centre for Mental Disorders Research, Oslo University Hospital, Norway and Associate Professor in neuropsychology at the Department of Psychology, University of Oslo, Norway. Her research focuses on cognition in schizophrenia and bipolar disorder.
Anja Vaskinn
Anja Vaskinn, PhD & specialist in clinical adult psychology. She is currently a senior researcher at the Centre for Research and Education in Forensic Psychiatry at Oslo University Hospital, Gaustad, Norway, and has been affiliated with the TOP study since its beginning in 2002 (now called the Norwegian Centre for Mental Disorders Research). Her research has primarily focused on social and nonsocial cognition in different clinical populations, notably schizophrenia and bipolar disorder.
References
- Healey KM, Bartholomeusz CF, Penn DL. Deficits in social cognition in first episode psychosis: a review of the literature. Clin Psychol Rev. 2016;50:108–137.
- Heilbronner U, Samara M, Leucht S, et al. The longitudinal course of schizophrenia across the lifespan: clinical, cognitive, and neurobiological aspects. Harv Rev Psychiatry. 2016;24(2):118–128.
- Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60(3):229–242.
- Savla GN, Vella L, Armstrong CC, et al. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39(5):979–992.
- Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18(2):146–161.
- Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136.
- Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr. Bull. 2011;37(suppl 2):S41–S54.
- Fanning JR, Bell MD, Fiszdon JM. Is it possible to have impaired neurocognition but good social cognition in schizophrenia? Schizophr Res. 2012;135(1–3):68–71.
- Fett AKJ, Viechtbauer W, Dominguez M, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588.
- Mitchell RLC, Phillips LH. The overlapping relationship between emotion perception and theory of mind. Neuropsychologia. 2015;70:1–10.
- Vaskinn A, Andersson S, Østefjells T, et al. Emotion perception, non-social cognition and symptoms as predictors of theory of mind in schizophrenia. Compr Psychiatry. 2018;85:1–7.
- Sjølie C, Meyn EK, Raudeberg R, et al. Nonsocial cognitive underpinnings of theory of mind in schizophrenia. Psychiatry Res. 2020;289:113055.
- Montag C, Dziobek I, Richter IS, et al. Different aspects of theory of mind in paranoid schizophrenia: evidence from a video-based assessment. Psychiatry Res. 2011;186(2–3):203–209.
- Shamay-Tsoory SG, Shur S, Barcai-Goodman L, et al. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res. 2007;149(1–3):11–23.
- Frith CD. 2014. The cognitive neuropsychology of schizophrenia. Hove: Psychology Press.
- Frith CD. Schizophrenia and theory of mind. Psychol Med. 2004;34(3):385–389.
- Thibaudeau É, Achim AM, Parent C, et al. A Meta-analysis of the associations between theory of mind and neurocognition in schizophrenia. Schizophr Res. 2020;216:118–128.
- Carruthers SP, Van Rheenen TE, Gurvich C, et al. Characterising the structure of cognitive hetereogenity in schizophrenia spectrum disorder: a systematic review and narrative synthesis. Neurosci Biobehav Rev. 2019;107:252–278.
- Vaskinn A, Haatveit B, Melle I, et al. Cognitive heterogeneity across schizophrenia and bipolar disorder: a cluster analysis of intellectual trajectories. J Int Neuropsychol Soc. 2020;26(9):860–872.
- Weickert TW, Goldberg TE, Gold JM, et al. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907–913.
- Wells R, Swaminathan V, Sundram S, et al. The impact of premorbid and current intellect in schizophrenia: Cognitive, symptom, and functional outcomes. NPJ Schizophr. 2015;1:15043.
- MacCabe JH, Brébion G, Reichenberg A, et al. Superior intellectual ability in schizophrenia: neuropsychological characteristics. Neuropsychology. 2012;26(2):181–190.
- Vaskinn A, Ueland T, Melle I, et al. Neurocognitive decrements are present in intellectually superior schizophrenia. Front Psychiatry. 2014;5:45.
- Bell MD, Corbera S, Johannesen JK, et al. Social cognitive impairments and negative symptoms in schizophrenia: are there subtypes with distinct functional correlates? Schizophr Bull. 2013;39(1):186–196.
- Etchepare A, Roux S, Destaillats JM, et al. What are the specificities of social cognition in schizophrenia? A cluster-analytic study comparing schizophrenia with the general population. Psychiatry Res. 2019;272:369–379.
- Rocca P, Galderisi S, Rossi A, The Italian Network for Research on Psychoses, et al. Social cognition in people with schizophrenia: a cluster-analytic approach. Psychol. Med. 2016;46(13):2717–2729.
- Hajdúk M, Harvey PD, Penn DL, et al. Social cognitive impairments in individuals with schizophrenia vary in severity. J Psychiatr Res. 2018;104:65–71.
- Carruthers SP, Van Rheenen TE, Karantonis JA, et al. Characterising demographic, clinical and functional features of cognitive subgroups in schizophrenia spectrum disorders: a systematic review. Neuropsychol. Rev. 2021;1:1–21.
- Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195(6):475–482.
- Wechsler D. 2007. Wechsler abbreviated scale of intelligence (WASI). Norwegian manual supplement. Stockholm, Sweden: Pearson Assessment.
- Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756.
- First MB, Spitzer RL, Gibbon M, et al. 1996. Structured clinical interview for DSM-IV TR axis I disorders, research version. Patient edition. New York: New York State Psychiatric Institute, Biometrics Research.
- Ventura J, Liberman RP, Green MF, et al. Training and quality assurance with the structured clinical interview for DSM-IV (SCID-I/P). Psychiatry Res. 1998;79(2):163–173.
- Fretland RA, Andersson S, Sundet K, et al. Theory of mind in schizophrenia: error types and associations with symptoms. Schizophr Res. 2015;162(1-3):42–46.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276.
- Wallwork RS, Fortgang R, Hashimoto R, et al. Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr Res. 2012;137(1–3):246–250.
- Emsley R, Rabinowitz J, Torreman M, RIS-INT-35 Early Psychosis Global Working Group, et al. The factor structure for the positive and negative syndrome scale (PANSS) in recent-onset psychosis. Schizophr Res. 2003;61(1):47–57.
- Langeveld J, Andreassen OA, Auestad B, et al. Is there an optimal factor structure of the positive and negative syndrome scale in patients with first-episode psychosis? Scand J Psychol. 2013;54(2):160–165.
- Van der Gaag M, Hoffman T, Remijsen M, et al. The five-factor model of the positive and negative syndrome scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85(1–3):280–287.
- Nakonezny PA, Shull RD. JMASM26: Hettmansperger and mckean linear model aligned rank test for the single covariate and one-way ANCOVA case (SAS). J Mod App Stat Meth. 2007;6(1):336–340.
- Apperly IA, Butterfill SA. Do humans have two systems to track beliefs and Belief-Like states? Psychol Rev. 2009;116(4):953–970.
- Bliksted V, Fagerlund B, Weed E, et al. Social cognition and neurocognitive deficits in first-episode schizophrenia. Schizophr Res. 2014;153(1–3):9–17.
- Andreou C, Kelm L, Bierbrodt J, et al. Factors contributing to social cognition impairment in borderline personality disorder and schizophrenia. Psychiatry Res. 2015;229(3):872–879.
- Tripoli G, Quattrone D, Ferraro L, EU-GEI WP2 Group, et al. Jumping to conclusions, general intelligence, and psychosis liability: findings from the multi-centre EU-GEI case-control study. Psychol. Med. 2021;51(4):623–633.
- Speechley WJ, Whitman JC, Woodward TS. The contribution of hypersalience to the “jumping to conclusions” bias associated with delusions in schizophrenia. J Psychiatry Neurosci. 2010;35(1):7–17.
- Bertollo JR, Yerys BE. More than IQ: executive function explains adaptive behavior above and beyond nonverbal IQ in youth with autism and lower IQ. Am J Intellect Dev Disabil. 2019;124(3):191–205.
- Stirling JD, Hellewell JSE, Quraishi N. Self-monitoring dysfunction and the schizophrenic symptoms of alien control. Psychol Med. 1998;28(3):675–683.
- Keefe RSE, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688–691.
- Wilk CM, Gold JM, McMahon RP, et al. No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 2005;19(6):778–786.
- Bell MD, Lysaker PH, Milstein RM, et al. Concurrent validity of the cognitive component of schizophrenia: relationship of PANSS scores to neuropsychological assessments. Psychiatry Res. 1994;54(1):51–58.
- Engelstad KN, Sundet KS, Andreassen OA, et al. Body language reading of emotion in schizophrenia: associations with symptoms and functional outcome. Scand J Psychol. 2017;58(5):359–366.
- Smelror RE, Rund BR, Lonning V, et al. Negative and disorganized symptoms mediate the relationship between verbal learning and global functioning in adolescents with early-onset psychosis. Eur Child Adolesc Psychiatry. 2020;29(12):1693–1703.
- Potter AI, Nestor PG. IQ subtypes in schizophrenia: distinct symptom and neuropsychological profiles. J Nerv Ment Dis. 2010;198(8):580–585.
- Rheinheimer DC, Penfield DA. The effects of type i error rate and power of the Ancova f test and selected alternatives under nonnormality and variance heterogeneity. J Exp Educ. 2001;69(4):373–391.
- Serlin RC, Harwell MR. More powerful tests of predictor subsets in regression analysis under nonnormality. Psychol Methods. 2004;9(4):492–509.
