Abstract
Copper–gold porphyry deposits are the world’s main source of copper and a significant source of gold. They consist of vein networks and their surrounding alteration zones. Commonly the deposits are centred on narrow intrusions (stocks), but calling these deposits ‘porphyries’ is unjustified because the name carries little descriptive or genetic value. Extensional veins were formed by hydraulic fracturing of the stocks, at depths where open spaces could not be maintained and where fluid pressure approaches lithostatic pressure. The post-crystallisation timing of the veins is important because it indicates that the host stocks could not have been the direct sources of either metals or ore-forming fluids. In the traditional magmatic model, precursor batholiths, lying at depth, are inferred to be the sources of the Cu and Au in the overlying host stocks. In this model, the batholiths are assumed to have crystallised and produced the mineralising aqueous fluids, Cu and Au. However, in many porphyry deposits, the concept of metal and fluid supply from deeper batholiths is problematic. Neither Cu nor Au is strongly enriched during the crystallisation of silicate magmas, and although hypersaline fluids are a characteristic of Cu–Au porphyry deposits globally, the source of the Cl remains unconstrained. There is little evidence that silicate magmas can release such Cl-rich fluids, and it remains unexplained how elevated levels of Cl may be achieved in a silicate magma. Therefore, the starting assumption that these deposits formed predominantly from magmatic sources and processes is questioned. This study has selectively focused on the roles of rheology, rock mechanics, vein control, metal-enrichment processes and the sources of Cl. Non-magmatic processes may be enough to facilitate strong partitioning of Cu and Au into high-temperature, oxidising, high-salinity, hydrothermal fluids to form Cu–Au porphyry deposits.
Mineralised quartz veins were introduced in fluids during hydraulic fracturing of their host intrusions when these stocks were brittle and had cooled significantly below their solidus temperatures.
The porphyry intrusions hosting Cu–Au (copper–gold) mineralisation were not the direct sources of either the fluids or the metals.
The sources of Cu and Au included large volumes of surrounding and underlying rocks up to kilometres from the sites of deposition.
Cu and Au do not become strongly concentrated during crystal fractionation in evolving silicate magmas.
Unaltered igneous rocks have relatively low Cl contents, and experiments suggest low Cl solubilities in granitic to granodioritic magmas.
It is highly unlikely that all the Cl required for metal complexing and transport was available from within silicate magmas.
KEY POINTS
Introduction
Porphyry deposits (as they are widely referred to in the literature) comprise stockwork, disseminated and breccia-hosted mineralisation, and are the world’s major sources of copper, with important gold, rhenium and molybdenum in addition (Cooke et al., Citation2005; Sillitoe, Citation2010). These deposits are very large systems that involve up to 3 km3 of ore and can extend to 2 km in depth. For example, El Teniente in Chile contains 90 Mt Cu, and Grasberg in Indonesia has 90 Moz or 2800 t Au (Phillips, Citation2022). These and other important examples are listed in with 2005 figures. More recent figures suggest that the Rio Blanco–Los Bronces district in Chile may be much larger, with 206.7 Mt Cu (Piquer et al., Citation2015). Mineralisation comprises pyrite and chalcopyrite, with lesser bornite and chalcocite in veins as well as disseminated through the alteration halo, especially in the alteration zones of pervasive development of K-feldspar and/or biotite (both called potassic alteration). Host rocks include porphyritic stocks, dykes and a multitude of immediate wall rocks (Cooke et al., Citation2005; Corbett & Leach, Citation1998; Porter, Citation2020; Singer et al., Citation2005; Sillitoe, Citation2010). All these deposits formed in metamorphic environments with high geothermal gradients and mixed metasedimentary and meta-igneous country rocks. Most deposits occur in Phanerozoic continental and island volcanic-arc subduction-boundary settings, particularly in circum-Pacific regions (Sillitoe, Citation1972). Other important regions for these deposits include China, Mongolia, Kazakhstan, Uzbekistan, Romania and Turkey (). Hydraulic quartz veins are found in all deposits (Harris & Holcombe, Citation2014; Tosdal & Dilles, Citation2020; Tosdal & Richards, Citation2001). Along with the proximal alteration, these veins comprise the mineralisation and indicate that the introduction of ore fluids, Cu and Au occurred under lithostatic conditions and into solid media. As such, the host stock had cooled significantly from its magmatic temperatures before veins formed; therefore, the ores clearly post-date their immediate hosting porphyry stocks (Phillips & Vearncombe, Citation2021).
Figure 1. Map of some of the largest Cu–Au porphyry deposits showing their concentration around the Pacific Rim and relationship to past and present orogenic belts. Many in North and South America have subordinate Au.
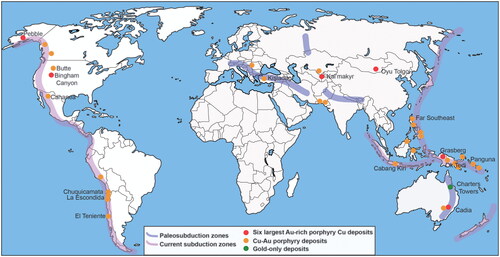
Table 1. Large Cu–Au porphyry deposits.
Several important questions arise once it is recognised that the host stocks were cold enough (relative to magmatic temperatures) to be brittle when the subsequent mineralisation was introduced, and the source of Cu, Au and ore fluids was not from within the stocks that now host much of the mineralisation. These questions include:
Why are host intrusions important when mineralisation was introduced into cool, brittle rocks? Would studies of rock rheology be useful?
If the host rocks are not the sources of the ore fluids, copper or gold, why has the eponymous porphyritic texture of some host rocks been considered so important?
Are there any definite limitations on the sources of the copper and gold; could the source be 100s of metres or even many kilometres from the host stocks and dykes?
Which, out of igneous, sedimentary and metamorphic rocks, were or could be involved as sources of the metals and fluids?
What are the sources of the Cl in the saline and hypersaline fluid inclusions (presumed ore fluids) in the giant deposits?
Are the reported meteoric waters synchronous and part of the mineralisation process, or a later and overprinting event that modifies mineralisation, or both?
Is there a clear rationale for retention of the custom terminology applied to porphyry deposits (e.g. A, B, D veins; potassic alteration, sericite alteration); does the usage reflect unique characteristics, or does this approach risk isolating the communication of porphyry deposit research from mainstream geology?
We address these questions in what represents a significant departure from the traditional magmatic model for these deposits, and we suggest that partitioning of the metals involving a silicate magma is not necessary. We can begin to answer some of these questions by introducing information from outside economic geology, especially from structural geology and hard rock (igneous and metamorphic) petrology. This study has selectively focused on the roles of rheology, rock mechanics, vein control, metal-enrichment processes and the sources of Cl. It has also proved beneficial to draw information and ideas learned from the study of other gold deposit types (e.g. gold-only deposits), which, although having some stark contrasts, inform on the general behaviour of the element gold in Earth’s crust. There is no attempt to discuss the many other anomalous elements such as Mo at this stage, as any viable model needs to adequately explain the origins of Cu and Au regardless of any other elements.
It was a comprehensive review of gold-only deposits (i.e. those lacking economic Cu) that led to this enquiry about Cu–Au porphyry deposits (Phillips, Citation2022). For the gold deposits lacking economic Cu, it has proved difficult to find examples formed by genuinely magmatic processes, with the only examples being few in number and related to immiscible sulfide melts (Holwell & Keays, Citation2014). This raised interest in understanding whether Au had been concentrated by magmatic processes in Cu–Au deposits, given that magmatic processes cannot generally explain gold in the gold-only deposits. In Archean greenstone belts, for example, many gold deposits formed in and around auriferous quartz veins in specific igneous rocks (including porphyries) but, as in Cu–Au porphyry deposits, the igneous host rocks were solidified before the mineralising event and were not the sources of the gold or the fluids. For these Archean gold deposits, there was no assumption that the mineralising fluids were magmatic in origin simply because they infiltrated an igneous rock during gold deposition. A natural extension from the Archean review becomes the current critical re-examination of the traditional genetic model for Cu–Au porphyry deposits, and any potential impacts this might have on exploration strategies.
Structural terminology
Over the last half century, a considerable literature has accumulated on the formation of hydraulic quartz veins, particularly as applied to mineral deposits, e.g. Kerrich and Allison (Citation1978) on the Yellowknife gold mine in Canada, and then Cox et al. (Citation1987), Ridley (Citation1993), Sibson (Citation1987) and Vearncombe (Citation1988). Important controls on vein formation include rock properties, relative orientations of rock units and stress fields and, most importantly, the relationship between fluid pressure (Pf) and lithostatic pressure (Pl), expressed as Pf/Pl. Two important terms in this discussion are as follows:
Rheology (sometimes termed competence) is the study of the mechanical behaviour (of rocks) during deformation, and our interest here includes rheological contrasts between juxtaposed rock units. Less competent rocks tend to flow, whereas more competent rocks are rigid. The latter can fracture in response to deformation and hence have potential to host network vein mineralisation.
Hydraulic fracture is the breakage of rock under high fluid pressure. Any contrasts in tensile strength between adjacent rock bodies will influence which rocks can hydraulically fracture. Tensile veins open through extension, where the fluid pressure exceeds the combined forces that resist breakage, and shear veins form on fault planes; both types are important in porphyry deposits.
The process of solid, brittle, igneous rocks being hydraulically fractured by high fluid pressures to form mineralised veins has been widely recognised in studies of the formation of Archean greenstone gold deposits since the 1980s, e.g. Sigma mine in Canada (Robert & Brown, Citation1986), the Yandal Belt (Vearncombe, Citation1998), the Golden Mile and Mt Charlotte in the Kalgoorlie goldfield (Boulter et al., Citation1987; Ridley & Mengler, Citation2000; Travis et al., Citation1971) and the Hunt mine at Kambalda–St Ives (Phillips & Groves, Citation1984). These gold deposits comprise networks of auriferous veins and alteration that are hosted in Archean igneous rocks that have not been considered to be the sources of fluids or metals but, instead, are generally explained as rheologically favourable sites for fluid access. The ladder-vein networks of auriferous quartz veins in hydraulically fractured diorite at the Morning Star and A1 mines in the Victorian Gold Province have been mined and documented since the 19th century without any a priori assumption that the ore fluids and gold came from the host dykes (Anderson & King, Citation2017; McAndrew, Citation1965). Some of the broader structural principles discussed here are already being applied in the study of Cu–Au porphyry deposits (Corbett, Citation1994; Skarmeta, Citation2021) but, in other studies, these principles are not being used.
Magma terminology
Magma is molten rock material that originates beneath the Earth’s surface and may be emplaced and cooled to become an igneous rock. We use the term magma to encompass the molten rock, the dissolved volatiles (gases) and the minerals crystallised from the melt or picked up from elsewhere in the crust. Magmas typically include enclaves of incorporated rocks and smaller aggregates of mineral grains. For porphyry deposits, this discussion of magma is confined to partially molten systems dominated by silicate melt components. Not all previous studies have used the term magma consistently and, to avoid uncertainty, we commonly discuss the separate components of magmas, as listed above.
Magmas contain variable proportions of silicate melt and crystals. When the crystals are volumetrically dominant, the system is commonly called an igneous mush, and there is a transition through a mushy stage as crystallisation proceeds. Both crystal-rich mushes and relatively crystal-poor magmas can supply heat and volatile fluids to surrounding rocks. The solidus reflects the temperature at which silicate melt has completely crystallised. At temperatures below the solidus, crystals and a fluid phase (normally aqueous) may remain, and heat transfer will continue.
The environment above the solidus involves igneous melts, elevated metamorphic temperatures and, potentially, aqueous fluids. Below the solidus, the environment is best described as the realm of metamorphism and hydrothermal processes until temperatures transition to those more akin to deeper weathering and ultimately surface conditions.
Meteoric waters may be active and synchronous with magmatism or may be late and post-magmatic. These include waters that originated under geologically recent near-surface conditions.
When referring to ore deposits and their genesis, the term magmatic has caused some confusion because it has undergone considerable definition creep, from requiring the critical involvement of a silicate melt to any deposit proximal to an igneous rock mass. We accept that, in economic geology at least, magmatic has a broad and flexible usage, but note that this may not be helpful for efficient communication or in deposit understanding or exploration.
Three possible roles have been invoked for magma in the traditional model for the formation of porphyry deposits—as the source of metals and fluid components, as the partitioning medium for Cu and Au into at least one of the constituents that can make up a magma, and as the source of heat energy to drive mineralising processes.
An igneous body hosting part of the mineralisation is referred to here as the stock, and a large igneous body inferred at depth is referred to as the precursor pluton or batholith ( and ). Both terms are used in the recent literature, including Sillitoe (Citation2010). The term porphyritic describes an igneous rock that contains crystals with a bimodal size distribution—larger crystals in a finer-grained groundmass.
Figure 2. Idealised cross-section showing stocks hosting Cu–Au porphyry mineralisation and a large precursor batholith inferred to lie at greater depth. There will be an even deeper zone of partial melting that represents the magma source. This study is primarily about the role of the stock, and whether postulated precursor batholiths could be the prime sources of the required large volumes of mineralising fluids and metals.
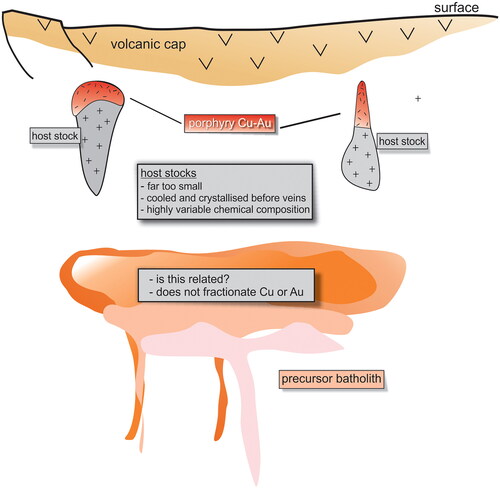
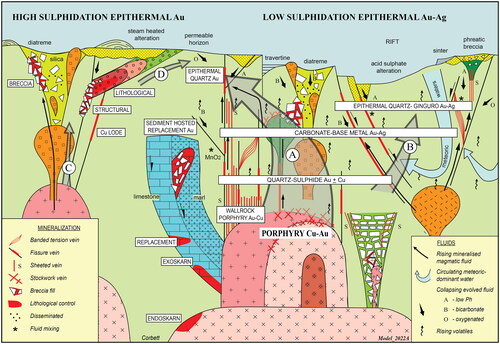
Figure 3. Schematic cross-section of many structural and host-rock settings of gold and base-metal deposits in a volcanic belt. Precursor batholiths are inferred to exist at depth. Reproduced with permission from Greg Corbett and based on his extensive fieldwork at porphyry and epithermal deposits, especially around the Pacific margins.
There will be different source areas for the different components of porphyry systems. The source of any large precursor batholith will be the zone of partial melting at several kilometres depth and significantly deeper than the emplacement depths of the batholiths. The sources of the hosting stocks will lie below their current depth but may or may not be related to any deeper batholiths. A commonly accepted depth of emplacement for these stocks is ∼6 km (Skarmeta, Citation2021). Source regions for Cu, Au and Cl may each be different, although the intimate spatial relationship between Cu and Au in these deposits suggests that these two metals, possibly associated with Cl, have a common source and pathway through the crust.
Ore-related terminology
The porphyry deposits that produce Cu also produce Au, although the latter varies from being a minor by-product that is relatively easy to extract to a major co-product that accounts for significant revenue from the mine. We address all these as porphyry Cu–Au deposits, and therefore any explanation for their origin needs to explain both the Cu and the Au. In general, the Cu–Au deposits in the Americas produce more Mo and Re, and those in the SW Pacific more Au (Corbett & Leach, Citation1998; Sillitoe, Citation1997). However, as much as possible, we avoid separating deposit types where their differences are gradational and where boundaries are primarily of commercial importance. For example, we avoid distinguishing porphyry deposits based on relative amounts or value of the Cu, Au, Mo and Re metals because some such distinctions are arbitrary, bear little genetic relevance and are ephemeral, depending on commodity prices. As the aim here is to better understand the genesis of these deposits by using knowledge of Cu and Au behaviour, we focus on the Cu–Au porphyries.
Unlike the above-mentioned gradation, there is a major distinction between Cu–Au deposits (gold-plus such as Cu–Au porphyry deposits) and gold-only deposits that are the globally predominant sources of gold (e.g. Archean greenstone gold, Witwatersrand, Carlin, and slate-belt sequences) but which are not the focus here (Phillips & Powell, Citation1993, Citation2015). The current discussion focusses on porphyry deposits that contain both Cu and Au (with or without additional metals) and not the gold-only deposits. It should be noted that there exist a smaller number of gold deposits within or associated with dykes and stocks that lack economic copper, and are sometimes called Au porphyry deposits. These are gold-only deposits and include Mt Leyshon (Wormald, Citation2017) and Kidston (Baker & Tullemans, Citation1990) in northeast Queensland and La Colosa in Colombia (Lodder et al., Citation2010). These are not discussed further and appear to be discrete from, rather than gradational to, the Cu–Au porphyry deposits.
Hydrothermal, literally hot water, can describe heated meteoric waters, metamorphic fluids, igneous fluids derived from silicate magmas and potentially other types.
Partitioning refers to the unequal distribution of one or more elements between two phases. For example, an element such as Rb will be enriched as silicate melts evolve relative to the crystallised solids, so Rb may build up to high concentrations in residual liquids. In contrast, Cu and Au will not be enriched in silicate melts and so do not build up to high concentrations in residual silicate liquids.
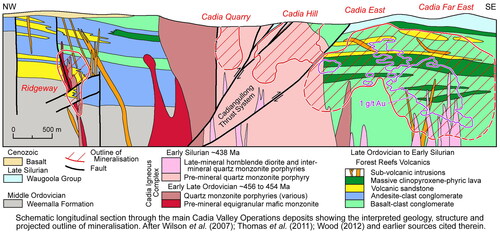
The classification of porphyry deposits is imperfect and addressed more fully below. History and context appear to play significant roles in porphyry terminology, as illustrated by the Paleozoic Cadia Valley district of central New South Wales (Hughes, Citation2017). In the Cadia Valley district, the Ridgeway Cu–Au deposit comprises mineralised veins in metasedimentary and igneous rocks, and is within 5 km of multiple Cu–Au deposits of the Cadia mineral field ( and ). Ridgeway was discovered after much of the Cadia field had been described as porphyry style, and Ridgeway therefore is itself referred to as a porphyry deposit and part of the Cadia porphyry system (Porter, Citation2017; Wilson et al., Citation2003). If the main Cadia mineral field had not been discovered first, large parts of Ridgeway might have been described as a vein deposit in mostly metasedimentary rocks, because that best describes the actual geology.
Figure 4. Stitched panorama of the main Cadia Hill Cu–Au open pit 250 km WNW of Sydney, Australia, as of 17 March 2013, soon after open cut operations had ceased. This is one of several Cu–Au vein-related deposits that comprise the Cadia Valley operations, one of the world’s largest Cu–Au porphyry deposits. Image by A Day.

Figure 5. Longitudinal section across the Cadia mineral field showing Cu–Au mineralisation in a variety of volcanic, plutonic and metasedimentary host rocks. There are numerous and varied alkaline to shoshonitic host stocks of monzonitic to dioritic composition. Igneous rocks hosting mineralisation would have cooled and been brittle at the time of hydraulic quartz veining, and so could not be the sources of either the fluids or the metals. After Porter (Citation2017) and PorterGeoscience database (2020, http://www.portergeo.com.au), with permission.
Overview of Cu–Au porphyry deposits
On a global scale, porphyry deposits are concentrated around the Pacific Rim, China through Uzbekistan and into Eastern Europe, Iran and Pakistan (). These regions coincide with convergent plate boundaries and include oceanic island arcs and continental (Andean-type) arcs. Groups of deposits are clustered within a few kilometres of one another and close to major fault zones, such as the 500 km-long Domeyko fault zone in Chile (Skarmeta, Citation2021). Multiple deposits can form in belts over 100 km long, with the dimensions of each deposit up to 10 km laterally. Some deposits are within porphyry intrusions whereas others are proximal to, or even distal from, intrusions. For example, in northern Chile, there are multiple porphyry deposits in a 14 km-long stock including Chuquicamata (over 60 Mt Cu) and Radomiro Tomic (20 Mt Cu). However, farther south in Chile, there are other examples in which mineralisation is predominantly external to the porphyry intrusion, e.g. El Teniente (Camus, Citation1975; Cannell et al., Citation2005). South of El Teniente, large porphyry deposits are lacking.
Wide alteration haloes surround porphyry deposits, with estimates that some are 10–100 km3 in volume and extend to at least 2 km depth (Kouzmanov & Pokrovski, Citation2012). Alteration zones are typically referred to in the literature as potassic, sodic–calcic, sericitic and argillic, with a chlorite–sericite zone as well. More distal alteration includes chloritic and propylitic zones. Sillitoe (Citation2010) lists the minerals in different alteration types. Proximal alteration is mapped as potassic—a term that can signify K-feldspar, biotite, muscovite, phlogopite or a mixture of these minerals. Sericitic, argillic, chloritic and propylitic alteration are less intimately associated with ore, and each of these terms can encompass a variety of minerals. Mineral lists are provided in some published descriptions (e.g. Corbett & Leach, Citation1998; Vry et al., Citation2010), but the provision of full coexisting mineral assemblages is not the norm.
As their name suggests, porphyry (i.e. porphyritic igneous rock) is a common igneous textural term associated with the host to many of these deposits, although the role of being porphyritic is not completely clear, and nor is being within a porphyritic rock a necessary characteristic of an economic porphyry deposit. The form of the igneous host may be depicted as a small, steeply plunging stock (Sillitoe, Citation2020) but, in detail, the host is commonly a narrow, steeply dipping dyke emplaced into any of volcanic or sedimentary sequences, or into underlying crystalline basement (Sillitoe, Citation2010). Large deposits have been described as comprising many sub-parallel dykes supposedly underlain, at an unknown depth, by inferred large precursor batholiths (). Mineralisation external to these stocks can be substantial, especially in carbonate host rocks where the mineralisation displays different mineralogical and geochemical characteristics owing to interactions between this type of host and the mineralising fluids ().
Vein networks are a fundamental component of porphyry deposits and have multiple orientations and cross-cutting relationships that may allow local distinction of veins by their relative ages and by the vein and wall-rock alteration mineralogy. Individual veins may be only millimetres thick, and it is the frequency and grades of such veins that are important economically. For example, mineralised veins may comprise up to 50 percent of a high-grade ore rock (Gustafson & Hunt, Citation1975). Apart from quartz, vein fillings include pyrite, chalcopyrite, bornite, chalcocite, carbonate minerals, magnetite, hematite and anhydrite.
The large sizes and abundance of fluid inclusions trapped within the mineralised quartz veins of porphyry deposits have made them particularly amenable to academic study over the last half century. It is common for suites of inclusions to contain multiple daughter minerals and to indicate the presence of a hot (mainly 300–550 °C) and oxidising, hypersaline fluid (Eastoe, Citation1978). There are also later and lower-temperature, less saline fluids that have isotopic signatures indicative of meteoric waters. The waters are associated with the formation of white micas and especially clay-mineral alteration types, and have been referred to in the literature as the phyllic and argillic zones, respectively (Kouzmanov & Pokrovski, Citation2012).
Among the ore-deposit geologists who have led the research and publishing, there appears to be considerable agreement that the genesis of Cu–Au porphyry deposits is closely linked to saline aqueous fluids and magmatic processes (Cooke et al., Citation2005; Corbett & Leach, Citation1998; Perkins et al., Citation2016; Sillitoe, Citation2010, Citation2020; Wilkinson, Citation2013) with variable and mostly later involvement of meteoric waters. This traditional magmatic model for porphyry deposits is summarised in contemporary textbooks (Ridley, Citation2013; Robb, Citation2005). Without any independent testing of alternatives, many recent publications take a magmatic origin as a given.
The large sizes of porphyry Cu–Au mining operations present special challenges for the collection of otherwise routine field data. Production imperatives of a mine that is working to a 24/7–365 schedule, whether as a large open pit or in a block-caving underground mine, mean that, for geologists, regular access to faces, benches, stopes and drive mapping may be negligible. As a result, structural and petrological studies focus on data from drill cores. This limitation is compounded by perceptions that, once the reserves of a billion-tonne porphyry deposit have been delineated, detailed geology and structural analyses are less important. As large open pits transition to major underground, automated block-caving operations, quality structural work becomes imperative, particularly to avert major rock failures. Chuquicamata mine is one example where a major structural compilation was deemed essential ahead of underground mining (Skarmeta, Citation2021), and we are aware of several other unpublished structural studies on major porphyry mines.
Scale of the ore-forming processes—ultimately large
The largest porphyry Cu–Au deposit (El Teniente; Cooke et al., Citation2005; ) exceeds 10 000 Mt of ore and contains 90 Mt of Cu in a volume of 2–3 km3. With respect to porphyry-related Au, Grasberg, and its significant wall-rock mineralisation, is the largest deposit, with 90 Moz of Au (2800 t). As shows, many other deposits have tens of Mt of Cu. The sizes of these porphyry deposits imply effective and large-scale formation processes. As a semi-qualitative example using some representative numbers, it would require 900 000 Mt of magma with a rather generous 100 ppm Cu to yield 90 Mt of Cu, and this would equate with approximately 300 km3 of magma in a precursor batholith, assuming a typical rock density around 2750 kg m−3.
Volumetric constraints on the amounts of Cu have been well appreciated, and many, but not all, studies recognise the size limitations of the host stocks and dykes for providing the required metals. This limitation has led to the advocation of large magma chambers beneath porphyry districts—the precursor batholiths of Sillitoe (Citation2010). Parallel calculations on the source requirements to account for gold in the larger porphyry deposits appear at least as challenging as for copper. A deposit of 50 Moz (1500 t) of Au or more (e.g. Grasberg, Bingham, Pebble or Kal’makyr; ) would require a large source volume. If average crustal gold concentrations of 2 ppb Au are assumed, then the source would need to have a volume of at least 250 km3, and much more if not all the gold could be effectively scavenged, transported and deposited. Options advocating magmatic and non-magmatic processes to provide the necessary enrichment of Cu and Au are discussed below.
As some of the world’s largest repositories of Cu and Au, giant porphyry deposits are unlikely to form by local or routine processes. The volumes of metal, alteration and fluid, and the partitioning of the economic elements, are all features that are likely to be extreme. The above calculations for both Cu and for Au indicate source volumes of several 100 km3. For example, a 3 km-thick slab as the source would need to be 10 by 10 km, and this implies transport distances for metals and fluids of 10 km or more, perhaps facilitated by major fault systems.
Requirement for strong and efficient metal partitioning
The large scale and two-to-three-orders-of-magnitude Cu and Au enrichment above their crustal background levels require very effective partitioning of Cu and Au (and several other geochemically similar metals). The two realistic options for this enrichment are preferential partitioning into silicate melts (rather than into crystallising minerals) and preferential partitioning into aqueous fluids (rather than remaining in silicate melts). For example, the former option (partitioning into a silicate melt) is a well-established mechanism for enrichments of elements such as the alkali metals and is largely controlled by ionic radius and valence. However, owing to the nature of the crystal liquid partition coefficients (e.g. Ewart et al., Citation1973; Ewart & Griffin, Citation1994; Luhr & Carmichael, Citation1980), when applied to Cu and Au in generic ultramafic to felsic rock types, this same mechanism of partitioning can produce only minor enrichments in residual silicate liquids, typically far less than an order of magnitude.
The second option, partitioning into an aqueous fluid, is also a well-established concept. Partitioning of metals such as Cu and Au into an aqueous fluid yields element patterns that reflect the complexing ligands in the fluid and the electronegativity of the individual metallic elements. Much greater enrichment of Cu and Au, respectively, on the order of 100 and 1000 times above the original background concentrations, is possible through highly effective complexing with ligands. This demonstrates that, for Cu and Au, partitioning into an aqueous fluid is a vastly more effective and therefore a far more likely mechanism for concentrating these elements relative to conventional igneous processes such as fractional crystallisation of a silicate melt or by low percentages of partial melting of source rocks.
Quartz veins and their timing implications for Cu and Au
Quartz-vein networks are a major component of Cu–Au porphyry deposits; they contain many of the ore minerals and provide constraints on the timing of mineralisation. As vein networks, they involve many cross-cutting relationships from which complex vein nomenclatures have been developed, such as veins 1–10, veins 1–4 (Cannell et al., Citation2005) and a further five subtypes (Vry et al., Citation2010) as well as A, B and D veins (Skarmeta, Citation2021). To avoid any terminology specific only to porphyry Cu deposits, we adopt a more general terminology of quartz veins as applicable for deposits such as New Zealand epithermal gold, Archean greenstone-hosted gold and unmineralised rocks, globally.
Veins can be described using two characteristics: (1) internal quartz textures and (2) vein and fracture network geometries. Both are informative on depth conditions, and specifically on the ratio of fluid pressure to lithostatic pressure (Pf/Pl). We summarise these here and prefer to use these characteristics rather than the A, B and D vein classification, which does not necessarily inform as to cavity, depth, load, shear stress or fluid-pressure conditions.
Vein fill
At shallow depths for ore deposits in general, vein-fills include the classic crustiform, colloform and cockade textures, as widely reported for example from NZ epithermal deposits. These textures can result from open-space filling, with the open spaces having originated through movement along rough faults. Fluids flow into such cavities by a dilational pumping motion (Sibson, Citation1987). In the literature, there are some descriptions of these textures in a small number (or in parts) of Cu–Au porphyry deposits (Harris & Holcombe, Citation2014; Penniston-Dorland, Citation2001).
In contrast, the vein textures in most porphyry deposits are generally of ‘buck’ (non-auriferous) quartz. Central seams of sulfides are common, and the mechanism of vein formation may be syntaxial, with growth by repeated fracturing along the central seam (Vearncombe, Citation1988). While we are not aware of any clear evidence for antitaxial veins, which grow by addition of vein material along the two vein–wall interfaces, we do not rule this out. Indeed, we expect such veins to be recognised once the current restrictive vein terminology is abandoned. Similarly, we are not aware of fibres that track vein-opening directions or laminated veins that may form at higher stresses, either by reactivating a structure or by deforming existing veins. However, these too may be found and described once specifically sought.
The conclusion here is that porphyry deposits form at depths greater than those at which open spaces can be maintained, and at levels deeper than where dilational pumping is the major mechanism of fluid transfer (Ridley, Citation1993).
Vein fracture geometries
The study of fracture network geometries is a valuable tool for a geologist examining veins (Harris & Holcombe, Citation2014). Commonly, these veins are in conjugate sets, but not always. The literature on porphyry deposits sometimes refers to these geometries, but by way of explanation rather than a field-based assessment. The types of fracture failure and veining that are possible within a porphyry (and in other systems) are illustrated in and . This is the conventional graph of shear stress against normal stress with the failure envelope showing that rocks are extremely weak in tension (to the left), but robust in compression (to the right). It is important to note that while the shallow failure curve, with the normal stress positive, and in pure tension (shear stress τ = 0), is constrained, there are no reliable data on the extremity of the shear stress-normal stress curve. These established network geometries, which we illustrate in and discuss below, are assessed for Cu–Au porphyry deposits from our observations and the literature. This is a widely reported vein geometry in porphyry copper systems (e.g. Skarmeta, Citation2021 for the Chuquicamata deposit) with an angle between the maximum stress and failure θ typically less than 30°.
Figure 6. (a) Conventional Andersonian faulting scenario with the Mohr circle (blue) with radius being the differential stress (ϭ1–ϭ3); this shows the maximum and minimum stresses shifted to the left by fluid pressure Pf. Where the Mohr circle intersects the failure envelope, there is conjugate faulting with an angle between the maximum stress and failure θ of about 30°. Faulting of this nature is present in Cu–Au porphyry deposits. Where this faulting is along rough surfaces, open spaces that may be mineralised occur along the fault; these deposits show some textural evidence of open space filling. (b) Mohr circle for low differential stress. The Mohr circle can be shifted to the left by the fluid pressure Pf, and the minimum stress becomes negative. Where the Mohr circle intersects the failure envelope, there is conjugate failure with dilation (Ridley, Citation1993). This appears to be the most reported vein geometry in porphyry copper systems (e.g. Skarmeta, Citation2021 for the Chuquicamata deposit) with an angle between the maximum stress and failure θ typically less than 30°. (c) Mohr circle for low differential stress (Mohr circle radius) and Pf high, the Mohr circle intersects the failure envelope with an α angle of 0°, producing flat hydraulic veins in a valve-like activity (e.g. Sibson, Citation1996). This is widely reported from porphyry systems (e.g. Cannell et al., Citation2005 for El Teniente) but is rarely dominant. Fluid conditions are of Pf/Pl = 1 or greater.
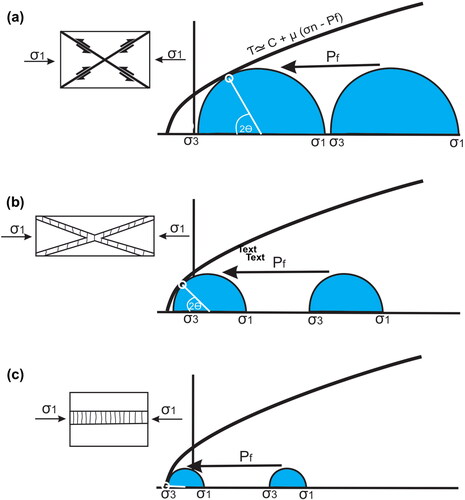
Figure 7. (a) Mohr circle for small differential stress (Mohr circle radius) and Pf high, the Mohr circle intersects the failure envelope with θ angle of 0°, producing flat hydraulic veins in a valve-like activity. (b) Mohr circle for low differential stress and the circle can be shifted to the left by the fluid pressure Pf as the minimum stress becomes negative. Where the circle intersects the failure envelope, there is conjugate failure with dilation. (c) Conventional faulting with the Mohr circle showing maximum and minimum stresses shifted to the left by fluid pressure Pf. Where the Mohr circle intersects the failure envelope, there is conjugate faulting with an angle between the maximum stress and failure θ of about 30°. (d) Mohr circle for stress-fluid conditions like those in (b) and (c) (yielding intact rock with hydraulic dilation). Such conditions may reactivate a weak but grossly misoriented fault or other weakness, such as a lithological contact. This is the widely used interpretation of the misoriented shear zone at Val D’Or Archean gold mine and is the interpretation of Piquer et al. (Citation2021) for combined vein dilation and misoriented faulting in porphyry systems of Chile, and an internal process at Chuquicamata (Skarmeta, Citation2021). Fluid conditions are of Pf/Pl = 1 or more. For clarity, details of the Mohr circle are not shown here. (e) Mohr circle diagram for high to extremely high differential stress conditions where failure may produce faults and shear zones. In upper crustal levels, where local Pf is below the regional Pf, fluids may flow into the fault (by a pump-like activity, Sibson, Citation1987). At deeper levels, where open space is not maintained, processes along the failure zone are complex and may include metamorphic reaction bands and veining owing to volume change. This is the proposed stress-driven mechanism to form laminated veins (Hobbs & Ord, Citation2023). We are not aware of these having detailed documentation, but they have been argued for in geological meetings about porphyry systems.
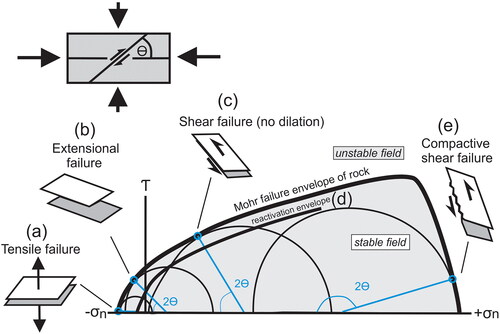
Importantly, the veins and evidence of hydraulic fracture indicate that all rocks that host mineralisation were brittle at the time of fluid infiltration. Ductile deformation with cleavages or schistosities is present in porphyry systems along faults, but most descriptions indicate that host stocks lack pervasive deformation. This implies that temperatures were significantly below any conditions of partial melting and below those where one would expect regional ductile deformation. As the host rock was solid and brittle at the time of vein mineralisation, any deposited metals and their transporting fluids must have come from outside the mineralised host rocks.
Timing
The structural features of porphyry Cu–Au deposits place important timing constraints on the introduction of mineralisation (Piquer et al., Citation2021; Skarmeta, Citation2021). In detail, the veins propagate through fractures into solid rock, from below to across the brittle–ductile transition, with local-scale controls (Skarmeta, Citation2021). Alteration accompanying the veining can be much later than the intrusions, and Skarmeta’s review of timing and the geochronology suggests that some mineralisation and alteration post-date magmatic activity by a million years. The mineralised structures within porphyries are those that are strongly misoriented for reactivation (Piquer et al., Citation2021), suggesting regional tectonic control. Similar examples include auriferous structures within Archean greenstone deposits (e.g. the Sigma deposit in Canada; Robert & Brown, Citation1986; Sibson et al., Citation1988). These results confirm that the observations of Skarmeta are more than simply local in significance. They are an important contribution to a general understanding of why mineralised vein systems occur where they do. If the structural–mechanical properties of a host stock are indeed important, then variable juxtaposed igneous host-rock types and their tensile strengths would be reflected in differing patterns of veining.
Magmatic processes and constraints
There is a good match between the traditional magmatic model for porphyry Cu–Au deposits, the general timing of igneous activity and the occurrence of high geothermal gradients. In addition, some of the geochemical signatures of the mineralisation are compatible with derivation from igneous rocks. However, much of the evidence also appears compatible with alternative explanations and may be better explained outside the traditional magmatic model. Even the traditional model has been subjected to many ad hoc modifications over time, and we discuss some of the end-member options. Our approach is to reconsider these deposits, not just in igneous terms, but within an overall metamorphic and igneous environment that involves elevated heat flows and a variety of fluid and metal sources in the Earth’s crust.
Several aspects of the traditional magmatic model are discussed: any specific and favourable igneous rock types, Cu and Au partitioning into silicate melts, theoretical and evidential support for the idea of large precursor batholiths as metal and fluid sources, aqueous fluids from crystallising magmas, and heat energy from magmatic bodies. A discussion then follows on the possible sources of the characteristic abundant Cl, as recorded in hypersaline fluid inclusions associated with the mineralisation.
Origins and chemical compositions of host stocks and dykes
A wide variety of igneous rocks host vein-related copper and gold, especially diorites, quartz diorites, monzodiorites and granodiorites, with a range from silicic to intermediate and lesser mafic rock types. This wide range of host-rock composition is evident, even within single deposits such as El Teniente (Camus, Citation1975; Cannell et al., Citation2005; Vry et al., Citation2010). The porphyritic intrusions in Cu–Au porphyry deposits are exclusively of oxidised I-type and magnetite-series affiliation (Ishihara, Citation1981), and typically metaluminous and medium-K calcalkaline, but may also fall into the high-K calcalkaline and shoshonitic or alkaline fields. Calcalkaline igneous rocks are the main hosts for porphyry Cu–Au deposits, and high-K types host the largest examples (Cooke et al., Citation2005).
In a major synthesis of deposits, Seedorff et al. (Citation2005) concluded that: ‘The compositions of igneous rocks related to porphyry deposits cover virtually the entire range observed for present-day volcanic rocks‘. The obvious conclusion here is that, apart from calc-alkaline and high-K affinity, the magma composition seems immaterial, and the host rocks for deposits even extend to alkaline types. Since fractionation is supposed to be the mechanism in traditional models for the concentration of Cu and Au, this lack of apparent relationship between porphyry silicate composition and mineralisation is troubling for the model of a magmatic origin for the metals. One cannot suggest that all these magma variations are due to fractionation of a single originally mafic magmatic system, partly because they would represent a vast range of degrees of fractionation, and in any case, different incompatible trace-element ratios between different rock types also make this unlikely. Thus, either the Cu and Au contents in a potential source magma are not much dependent on its silicate composition or the magmas are not the ultimate sources of the metals.
The host rocks for porphyry Cu–Au deposits span a variety of settings in the crust and mantle. The dominant minerals that link the main, unaltered host rocks of diorite, quartz diorite, monzodiorite and granodiorite are plagioclase, lesser K-feldspar and minor to moderate quartz contents, with additional biotite and hornblende. Granodiorites that are not associated with Precambrian trondhjemite–tonalite–granodiorite series can have a variety of origins. These origins include fractionation from enriched mantle parental magmas, such as K-rich diorites, and possibly magma mixing, although this is disputed (e.g. Frost & Mahood, Citation1987). Brown (Citation2001) pointed out that granitic magmas (granites to granodiorites) are the necessary complements to the melt-depleted granulites commonly formed in the deep crust (e.g. Arth & Hanson, Citation1972; Otamendi et al., Citation2009). For the majority of granodiorites (and other I-type granitic rocks), Clemens et al. (Citation2011) showed that the only mechanism that simultaneously explains their major-element, trace-element, isotopic and mineralogical characteristics is partial melting of pre-existing meta-igneous crustal rocks, with variable entrainment of the solid products of the melting reactions. Recent summaries of the evidence can be found in Clemens (Citation2012) and Brown (Citation2013). Some of the more mafic plutonic rocks (such as diorites and monzodiorites), which also host Cu–Au porphyry deposits, are likely to represent magmas produced largely through partial melting of enriched mantle and subsequent differentiation or hybridisation (e.g. Shaw et al., Citation1993).
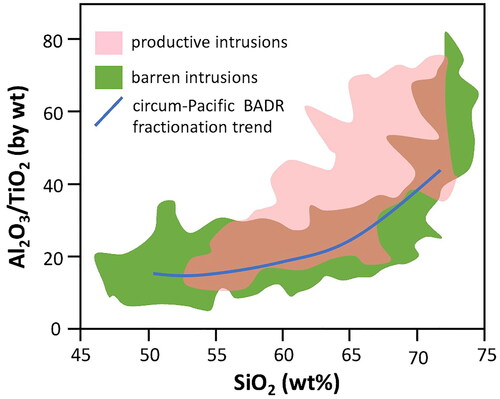
In one detailed study of vein-related deposits in intrusive rocks, but not restricted to porphyry types (Baker et al., Citation2005), the elements Si, Na and K were used as proxies for magma type. For both Cu and Au, the host igneous rocks for these vein deposits are quite variable with 50 to almost 80 wt% SiO2. A slightly different study by Loucks (Citation2014) used Al2O3/TiO2 vs SiO2 and found no special igneous composition linked to porphyry deposits (). For an important deposit class traditionally explained by rather special magmatic processes, the lack of a specific type of host rock makes this explanation problematic. This is because the ‘special magmatic processes’ would have to be common to the evolution of many different magma types that originate from different sources and evolve in very different ways. This makes a common ‘special’ magmatic process leading to the required extreme enrichment in Cu and Au highly unlikely.
Figure 8. Plot of Al2O3/TiO2 vs SiO2 for some productive and barren porphyry intrusive rocks (including an overlap area in brown). The wide range of SiO2 (∼54 to 73 wt%) in the productive intrusions suggests that specialised magma processes, such as fractionation, are unlikely to be essential features in the formation of these deposits. The blue line shows the generalised fractionation trend for a basalt–andesite–dacite–rhyolite compositional series. Primary data are from Loucks (Citation2014, ).
Porphyritic texture in medium- to fine-grained igneous rocks is a product of relatively rapid cooling. It is common in shallow plutonic as well as volcanic regimes and does not necessarily denote catastrophic decompression or fluid release. Certainly, dissolved volatiles can be flashed off in magmatic systems. This commonly leads to pressure quenching—solidification by pressure drop rather than cooling. However, the porphyritic texture is not a diagnostic signature of this process. It can also result from cooling at a variety of rates. All that is required is that the cooling rate be faster than that necessary for growing phenocryst-sized crystals. On the other hand, stocks, pipes and dykes do commonly have porphyritic textures, which are even present in some granitic batholiths. This underlines the wide range of cooling or quenching rates that can produce porphyritic rocks. The only common denominator is that, in the plutonic realm, porphyritic texture and medium to fine grainsize are signatures of shallow-crustal emplacement. This might reflect faster cooling rates for magmas intruded at shallower levels where ambient country-rock temperatures are likely to be lower than at deeper levels.
Partitioning of metals into silicate magmas
We examine the efficacy of magmatic processes to partition selected metals into residual silicate melts as the magma crystallises. Those metals enriched by this process have an ionic radius, charge or electronegativity that make substitution in newly formed crystals unfavourable, i.e. they are incompatible and, as a result, can potentially form ores in the more evolved igneous rocks. Examples include Li, Rb and U, and all three of these have very low concentrations in ultramafic rocks but increasing concentrations from mafic to intermediate to felsic rocks and ultimately to pegmatites ().
Figure 9. Average concentrations in common igneous rocks for different elements [in log10 ppm]: (top) compatible behaviour demonstrated by Cr and Ni, which are enriched in ultramafic rocks; (centre) incompatible behaviour demonstrated by Li, Th and U with enrichment in felsic rocks; (bottom) little variation with igneous rock type for Au and five of the chalcophile (affinity for sulfur) elements. Data sources include the GERM database: https://earthref.org/germrd/, Mason (Citation1966), Taylor and McLennan (Citation1985) and Wedepohl (Citation1995).
![Figure 9. Average concentrations in common igneous rocks for different elements [in log10 ppm]: (top) compatible behaviour demonstrated by Cr and Ni, which are enriched in ultramafic rocks; (centre) incompatible behaviour demonstrated by Li, Th and U with enrichment in felsic rocks; (bottom) little variation with igneous rock type for Au and five of the chalcophile (affinity for sulfur) elements. Data sources include the GERM database: https://earthref.org/germrd/, Mason (Citation1966), Taylor and McLennan (Citation1985) and Wedepohl (Citation1995).](/cms/asset/64440c25-3cc1-4a99-a514-5a8fbcc9d375/taje_a_2237105_f0009_c.jpg)
Neither Cu nor Au follows the distribution pattern of incompatible elements like Li, Rb and U, nor that of compatible elements like Ni and Cr. The global distribution of Cu and Au in igneous rocks provides minimal support for the concentration of these metals in felsic rocks, and pegmatites are not major sources of Cu or Au either (Pollard, Citation2017). Globally, the evidence does not support magmatic processes (those involving partitioning into silicate melts) as accounting for the required two-orders-of-magnitude enrichment of either Cu or Au in porphyry deposits. Cu tends to be at slightly higher levels in mafic rocks, and Au shows little systematic variation between ultramafic, mafic, intermediate and felsic rocks (Pitcairn, Citation2011).
Large precursor batholiths?
In the traditional genetic model for porphyry deposits, a common assumption has been that one or more large batholiths lie at depth and that these supplied the ore components (the precursor plutons of Sillitoe, Citation2010; ). The importance of these hypothetical batholiths in the genetic model is to accommodate the volumetric aspects of metal enrichment required to form such large porphyry deposits. Geophysics and field mapping have been rather unsuccessful in identifying large batholiths that could then be confirmed as the sources of metals or fluids to form deposits (Pritchard & Gregg, Citation2016). However, making such a link would never be easy, and geophysics and mapping would not be the preferred methods to confirm any genetic link.
The background to the widely held perception of large, hidden batholiths is explained by Cruden (Citation1998, Citation2006), who summarised some earlier literature. He suggests that ‘Historically, granitic plutons have been viewed as areally extensive intrusions with steep sides that continue to great depth in the crust (Buddington, Citation1959 …)‘, and Cruden follows this quote with several later references to others who have followed Buddington’s view.
In the broader igneous literature, thick batholiths dominated by silicate melt are not favoured (Lundstrom & Glazner, Citation2016). Instead, for felsic intrusions, small pipes or dykes are modelled as feeding relatively thin sheets of granitic rocks (Clemens & Mawer, Citation1992). The perception of enormous volumes of granitic magma forming batholiths is, to some extent, a legacy of the view that granitic magmas rise through the crust as huge, buoyant magma globules (diapirs). In reviews of this subject, Clemens and Mawer (Citation1992) and Petford et al. (Citation2000) have shown that neither small nor large diapirs can effectively transport magmas through the crust. Magmas generally fracture their own channels upward, forming dykes. Recently, Phillips et al. (Citation2022) demonstrated that at least one large pluton, the Strathbogie batholith, of approximately 2000 km2, is remarkably thin, with an aspect ratio (width to thickness) of the order of several hundred. A similar conclusion has been drawn from the even larger Donkerhuk batholith of southern Africa (Hall & Kisters, Citation2016). If this pizza shape is a common feature, then we would have no good reason to hypothesise the existence of the sorts of voluminous batholithic parent bodies to porphyry systems, as required by the igneous fractionation model for the concentration of metals in residual magmas and magmatic hydrothermal fluids.
In some models, nearly all batholiths are viewed as formed from crystal-rich mushes from which volcanic liquids were extracted (e.g. Bachmann & Bergantz, Citation2004), but, based on volumetric and compositional considerations, there is considerable doubt about the general applicability of this model for Cu–Au porphyry deposits (Clemens et al., Citation2020). Nevertheless, such bodies might exist. If they do, it is worth noting that the model of a batholithic parent to a porphyry system would not work particularly well. The large pool of silicate melt that would be required to fractionate and concentrate Cl-, Cu- and Au-rich fluids would simply not be present. It is true that, in a mush, the remaining interstitial melt might be a source of some chloride and metals. However, much of this would have been lost in the extracted volcanic liquids, and one would then have to understand how the residue might be extracted to form a mineralising fraction. One could call upon heating by new magma or gas sparging (e.g. Bachmann & Bergantz, Citation2006; Pistone et al., Citation2020). However, this still leaves the potential problem that the mooted parental batholiths may have nowhere near the volumes required to satisfy the mass-balance requirements of a magmatic fractionation model.
Role of magma in providing heat energy to drive mineralising processes
In the environments in which porphyry Cu–Au deposits formed, geothermal gradients are high, with active plutonism, volcanism and metamorphism (Bickle & McKenzie, Citation1987). The hydraulic quartz veins indicate that the host stocks had cooled enough to be sufficiently brittle for them to be susceptible to hydraulic fracturing prior to the mineralising events (see above). This means that host stocks must have relatively minor direct effects on generating high metal concentrations in introduced aqueous fluids. At the intrusive level of the host porphyry stocks, other plutons might also supply heat that could supplement the regional heating owing to the high metamorphic gradients.
The size of the largest Cu–Au porphyry deposits, and involvement of substantial volumes of ore fluids, implies a major input of heat energy from the deeper crust or upper mantle. Felsic to intermediate magmas are less effective than mafic magmas in providing the required amount of heat energy on such a large scale. In this regard, it is worth noting that basaltic liquids have almost twice the heat capacity of granitic liquids, and they are also at much higher initial temperatures around 1250 °C compared with 800 °C.
Thermal modelling of fluid flow around cooling intrusive bodies such as that employed by Cathles (Citation1997) may not be applicable to porphyry stocks if the intrusion is not penecontemporaneous with mineralisation, and in cases in which the stocks cooled below their solidus temperatures prior to mineralisation, as indicated by the structural data for the veins (see above).
It can be misleading to construe the involvement of heat energy (thermal) as necessarily caused by igneous rocks, or to consider processes as magmatic when they are more correctly characterised as part of a thermal anomaly (metamorphism).
Role and importance of Cl in the ore-forming environment
Hydrothermal processes and the partitioning of metals into aqueous fluids
A hydrothermal process (literally one involving hot water) has the capability to partition ore elements into an aqueous fluid (Helgeson, Citation1964). In general, the dissolution of metals into a hydrothermal fluid is greatly facilitated by the presence of complexing agents, i.e. ligand species (Ridley, Citation2013; Robb, Citation2005). The effective combination of metal and ligand depends upon strong bonding between the two as well as availability of that ligand in nature.
Both oxidised Cl ligands and reduced S ligands have been proposed in the formation of Cu and Au deposits generally. However, given the oxidising conditions implied by the widespread presence of hematite, anhydrite and magnetite but not pyrrhotite in porphyry Cu–Au systems, the reduced S ligand is unlikely to be the dominant agent for either of these metals. Under these oxidising conditions, the dominant S species is sulfate (SO42–), but this ligand is not known for strong bonding with Cu or Au at elevated temperatures in the crust. Hence for Cu–Au porphyry deposits, chloride (Cl–) is likely to be the dominant ligand for Cu and Au, explaining their close association in this environment. The strong partitioning of both Cu and Au into high-temperature, oxidising, high-salinity hydrothermal fluids is predicted from the aqueous geochemistry of these two metals and their affinities for complexing with the Cl– ligand (Puddephatt, Citation1978).
Saline- and hypersaline fluids in Cu–Au porphyry deposits
For half a century since the pioneering studies at Butte Montana, Bingham Canyon Utah and Climax Colorado all in the USA (Roedder, Citation1971), hypersaline fluid inclusions have been recorded in mineralised quartz veins from porphyry Cu–Au deposits. Roedder had the unusual opportunity of being able to focus on fluid inclusions within multi-disciplinary deposit studies by the US Geological Survey during the time when interest in porphyry deposits was at its zenith in the western USA. This situation gave him a keen appreciation of the geological context of his samples. Subsequent studies at the Panguna deposit on Bougainville Island and at Freda River, Papua New Guinea (Eastoe, Citation1978), Kal’makyr in Uzbekistan, Sar Cheshmeh in Iran, El Salvador in Chile, Santa Rita in New Mexico and Ely in Nevada, and several other porphyry deposits, as compiled by Roedder (Citation1984), have demonstrated that the hypersaline fluids in these deposits are global in their distribution. Most studies have also recorded additional lower-salinity fluid types, e.g. including the later groundwater incursion at Panguna (Eastoe, Citation1978).
In early studies of fluid inclusions in porphyry systems, there was a rigorous focus on the petrographic methods that might be used to identify and separate primary inclusions (trapped at the time of crystal growth) from secondary inclusions (formed along healed cracks after a crystal has grown) that sampled fluids of later origins (Roedder, Citation1967). Several diagnostic textures are provided in a detailed summary of petrographic interpretations by Roedder (Citation1979). Both Roedder’s compilation and Eastoe’s studies list many salinity values of 50–80 wt% NaCl eq. The high salinities and oxidising conditions are compatible with the common daughter minerals such as halite, sylvite, hematite and anhydrite.
A very interesting comment by Roedder (Citation1984, pp. 439 and 446) is that, despite the many differences between individual porphyry deposits, the similarities among the fluid-inclusion suites from Cu–Au porphyry deposits are striking. Roedder went on to list over 40 Cu porphyry deposits globally, with summaries for many more, all with examples of high to very high salinities among their fluid-inclusion suites (Roedder, Citation1984, table 15.5). He also recognised that the hypersaline inclusion population set these deposits apart from most other ore-deposit types (although we note not all). Later, in one economic geology textbook, Guilbert and Park (Citation1986, p. 412) summarised the porphyry fluids as 30–60 wt% NaCl eq. for earlier fluids, with later fluids being less saline.
A recent review of 60 porphyry deposits (Prokofiev & Naumov, Citation2022) confirms the earlier findings and indicates that the Cu–Au porphyry deposits include populations of saline inclusions with >50 wt% NaCl eq. The same dataset also shows much lower salinities in the inclusions of Au porphyry deposits that lack economic Cu or Mo, especially after the data are corrected for what appears to be a misclassification of the Far Southeast deposit as an Au porphyry lacking Cu; following Corbett and Leach (Citation1998), we classify this as a Cu–Au porphyry.
Overall, the hypersaline fluid inclusions of the Cu–Au porphyry deposits (commonly 50–80 wt% NaCl eq.) are extreme by the standards of seawater (3 wt% NaCl eq.), gold-only deposits (generally ≈ seawater; Ho et al., Citation1990; Ridley & Diamond, Citation2000), volcanogenic massive sulfide deposits and sedimentary-exhalative base-metal deposits. One of the few ore types with recorded salinities that match those of the Cu–Au porphyry deposits is the IOCG (iron oxide–copper–gold) deposits, such as those in the Proterozoic of western Queensland (Williams et al., Citation2005). That these are also Cu–Au deposits is intriguing.
Hypersaline fluids appear to be an essential (rather than accidental) component of the Cu–Au porphyry-forming process, just as their absence is interpreted as an essential characteristic of gold-only deposits. This contrast supports the more generic twofold division between Cu–Au deposits, having populations of high-salinity fluids, contrasting with all gold-only deposits, which lack economic Cu and are not associated with hypersaline fluids (Phillips & Powell, Citation1993, Citation2010). Accordingly, the less common Au porphyry deposits (without economic Cu) also have low-salinity fluid inclusions.
The occurrence of high chloride contents measured in fluid inclusions from Cu–Au porphyries is important because it appears to be one of the few unifying features shared by porphyry Cu–Au deposits globally (together with their tectonic setting). Although such high Cl contents provide an elegant mechanism for solubilisation and mobility of Au and Cu, hypersaline fluid inclusion assemblages have also been explained as products of phase separation and boiling mechanisms, which are suggested for ore localisation in high-level porphyry systems (e.g. Audétat, Citation2019, and references therein).
Behaviour of Cl in sedimentary and metamorphic systems
The behaviour of Cl is discussed both for crustal sequences that lack evaporites and for evaporite-bearing sequences. At their times of burial, non-evaporite sedimentary sequences can contain considerable Cl incorporated in clastic, chemical and interstitial components (Yardley & Graham, Citation2002). The Cl partitions strongly into pore waters, and many petroleum-industry studies have described oilfield brines with salinities considerably higher than that of seawater. As porosity is reduced in the transition from diagenesis to metamorphism, the pore waters are expelled from the rock masses and transport the dissolved Cl out of the system. The earlier discussion of hydraulic veins lays out permissible timing for Cu–Au mineralisation and hydraulic fracturing. These critical processes occur during metamorphism when there is negligible rock permeability.
Once porosity is lost, it is rare for metamorphic sequences to contain halite, i.e. we do not find halite listed in mineral assemblages in metamorphic textbooks. For most crustal rocks, the Cl contents are ≤300 ppm. Biotite, hornblende and apatite can accommodate Cl substituting for OH in their structures and could contain up to 1 wt% Cl locally, although this is not the global norm (Finch & Tomkins, Citation2017); serpentinite minerals may also contain some Cl (Huang et al., Citation2018; Miura et al., Citation1981).
Evaporites as a source of Cl
Evaporites are an exception to the generally low Cl concentrations in crustal rocks, as they contain halite (NaCl; 61 wt% Cl) as their dominant mineral (typically 80% modal abundance), with subordinate Mg-chloride salts (∼15 vol%), gypsum (4 vol%; CaSO4·2H2O) and minor carbonates and K salts (Livesey, Citation2017; Warren, Citation2010). In nature, sequences of evaporites can exceed 100 m thickness of dominantly halite. An illustration of the potential importance of evaporites as a source for Cl in the crust is provided by the calculation of the Cl contained in a 10 m-thick bed of pure halite (or an equivalent volume of halite in a thicker evaporite layer). The 10 m of halite would have as much Cl as 20 000 m of average crustal rocks with a generous 300 ppm Cl, or over 500 m of a hypothetical pure biotite rock with 1 wt% Cl.
During metamorphism, evaporitic units are modified but can retain Cl-bearing minerals such as scapolite in medium-grade rocks (Almeida & Jenkins, Citation2017; De Jong et al., Citation1997; Oliver et al., Citation1992; Phillips et al., Citation1994; Yardley & Graham, Citation2002); and biotite and hornblende have been reported with 5 wt% Cl in meta-exhalites (Oen & Lustenhouwer, Citation1992). The composition of scapolite can be simplified to a calcic end-member meionite 3(CaAl2Si2O8).CaCO3 and a sodic end-member marialite 3(NaAlSi3O8).NaCl. Heating of evaporite-bearing sequences can yield metamorphic fluids of high salinity, and limited field evidence suggests that scapolite in these assemblages breaks down before the onset of partial melting. Links have been established between evaporitic rocks and some iron oxide–apatite deposits (e.g. Duan et al., Citation2021), so it would be surprising if such field relations involving scapolite had been missed by researchers working on Cu–Au porphyry deposits. In any case, despite being a major crustal reservoir of Cl (along with oceans and some diagenetic basins), at this stage we lack evidence to link evaporites to all or even most porphyry Cu–Au deposits.
Cl in common silicate magmas
The mechanisms by which Cl can be incorporated into silicate magmas appear limited. A source of Cl entering a magma at the time of partial melting and magma formation in the quantities required for eventual formation of hypersaline fluid seems unlikely, given the absence of any modally abundant minerals with significant Cl in amphibolite- and granulite-facies metamorphic rocks. The exceptions here are halite or scapolite from evaporites involved in partial melting, but given over a century of extensive, systematic igneous petrology, any involvement of such components in an igneous process would have already been identified. No such involvement has been identified in the formation of diorites, quartz diorites, monzonites and granodiorites.
Granodiorite is one of the igneous rock types common in porphyry Cu–Au systems. Crustally derived magmas with this composition form through the melting of quartz- and feldspar-bearing assemblages at temperatures of 700°C upward. This means that the potential sources for Cl are mostly the minor amounts in the biotite and amphibole involved in the melting reactions. Aqueous fluids are negligible in volume at the onset of high-temperature partial melting in the deeper parts of the crust, which occurs mostly under effectively fluid-absent conditions (e.g. Clemens, Citation2012). Cl would be expected to partition into an aqueous fluid (if there was one) relative to a coexisting magma rich in silicate liquid.
An alternative mechanism for the incorporation of Cl into a silicate magma, by inclusion of xenoliths of evaporites, is similarly challenging. Abundant halite in evaporitic xenoliths is unlikely to be incorporated into magmas without leaving any geochemical and mineralogical signs. We are not aware of any studies that have inferred evaporitic components in the stocks that host Cu–Au porphyry deposits.
The reported strong partitioning of Cu, Au and Cl into the aqueous fluids in silicate magma–fluid systems is not especially significant to this discussion because it is only the aqueous fluid here that plays an important role; a rock–fluid system would similarly partition metals and Cl into the aqueous fluid, as occurs in diagenetic basins.
Evolution of an aqueous fluid from crystallising magma
Experimental work on H2O solubility in silicate liquids (e.g. Goranson, Citation1931; Hamilton et al., Citation1964; Tamic et al., Citation2001) provides information on maximum, theoretical H2O contents. However, crustal melting typically produces magmas with only a few per cent of H2O in the melt, perhaps up to 6 wt% in rare cases (Clemens et al., Citation2020; Clemens & Watkins, Citation2001). Fluid saturation may occur as the proportion of silicate melt decreases during cooling and crystallisation of the magma. At this stage, partitioning of different metals can occur, with some enriched in the silicate magma and others in the aqueous fluid, depending upon the fluid composition. For example, Cl is predicted to partition into the aqueous fluid relative to the silicate magma (Candela & Piccoli, Citation2005). The composition of the aqueous fluid reflects those elements from the magma that are prone to partition into a fluid, together with any other elements that the fluid can dissolve from country rocks. Even with the effective partitioning of any Cl into the aqueous fluid, there is a counterbalance between a small volume of fluid potentially having high Cl or a larger volume of fluid necessarily having a lower concentration of Cl. This is analogous to the concept of an R-factor in orthomagmatic Cu–Ni(PGE-) deposits. Consequently, there is still a considerable challenge to explain the large volumes of hypersaline fluids associated with giant ore systems such as El Teniente.
On cooling to below the critical point, an aqueous fluid will separate into coexisting phases of vapour and liquid. The temperature of the critical point is 374°C for pure H2O and 407°C for seawater, so the critical point is well below silicate melt solidus temperatures unless fluid salinities are much higher than seawater. In any phase-separation process, S species will partition into the vapour phase and Cl into aqueous liquid (Kouzmanov & Pokrovski, Citation2012; ). Given the limited options for Cl to enter a magma, either at the point of partial melting or subsequently by xenolith incorporation, there are significant limitations on the general process of highly saline fluids being evolved from silicate magmas. It is also unclear why a hypothesised porphyry-mineralising fluid might be so much more saline than many other fluids evolved from crystallising igneous melts with common compositions such as granodiorites, diorites and monzonites.
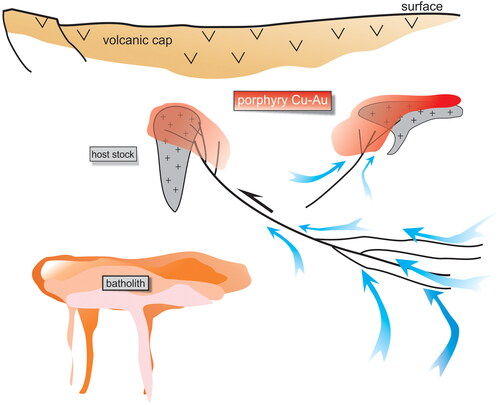
Figure 10. Conceptual model showing hot, oxidising Cu–Au fluids derived from large volumes of country rock and migrating along shear zones. Batholiths at depth may be involved in heat transfer, but they are not necessary. Host stocks contrast rheologically with their surrounds but are not necessarily of porphyritic texture. The stocks have solidified by the time the infiltrating fluids have caused fracturing and metal precipitation.
Discussion and conclusions
The traditional model for porphyry Cu–Au deposits explains their formation in terms of magmatic processes, and there has been a high degree of confidence in and dependence on this model over many decades. However, in the light of modern developments in fields such as structural geology, thermodynamics, aqueous geochemistry of hydrothermal systems, and igneous and metamorphic petrology, we review the basis of this model and the appropriate degree of certitude in it.
Evaluation of traditional magmatic model(s) for porphyry deposits
Within the economic geology community, there is a considerable degree of certainty surrounding the genesis of porphyry Cu–Au deposits. Some idea of the extent of this can be gleaned from a recent quote: ‘It is now universally accepted that mineralisation and potassic alteration are driven by the focused, commonly periodic, release of large volumes of magmatic fluids into the shallow crust …‘ (Pacey et al., Citation2020). This confidence is not a recent development but has been reinforced through at least half a century of reiteration of what might be called a magmatic mantra ().
Table 2. Quotes regarding the origin of some porphyry deposits.
These statements might reflect confidence among ore-deposit geologists, but that confidence is not universal among practitioners of many other disciplines. For example, the statements of Stanton (Citation1972) and Guilbert and Park (Citation1986) are contradictory in implicating stocks and batholiths, respectively. We examine the features of porphyry Cu–Au deposits more carefully and suggest there could be a different level of confidence. A selection of important observations and conclusions includes:
In every example, the igneous host stock had crystallised well before the mineralising event. Hence the host rock for the porphyry deposit was not the direct source of the fluid, Cu or Au.
Fractional crystallisation of silicate magma leads to only small enrichments in mafic rocks for Cu, and virtually no enrichment in Au across the span of ultramafic to felsic rocks. Any such trends are minuscule compared with what is required to accumulate the approximately 50 Mt of Cu found in the largest porphyry deposits.
Batholiths, up to 300 km3, beneath porphyry deposits are inferred in the traditional magmatic porphyry Cu–Au model (Sillitoe, Citation2020), and many orogenic belts will certainly have batholiths at depth. However, a genetic connection from a deeper batholith to a host porphyry stock and to mineralisation is rarely established, despite the obvious importance for both scientific enquiry and practical exploration.
Hydrothermal processes involving partitioning of Cu and Au into hot aqueous fluids can more easily explain the two-orders-of-magnitude enrichment of these metals required to form a porphyry Cu–Au deposit.
High salinity of an aqueous fluid facilitates effective complexing and transport of Cu and Au.
Partial melting of crustal rocks is a poor candidate for the generation of Cl-rich magmas and Cl-rich fluids on the scales required to form these deposits.
A credible source for voluminous Cl remains elusive, and we find no evidence that enough can come from a silicate magma.
Magmatic processes sensu stricto (i.e. involving metal partitioning into a silicate melt) appear unlikely to be critical in the formation of Cu–Au porphyry deposits. The traditional magmatic model becomes difficult to justify.
We do not assume that the source of Cl is from a magma just because hypersaline fluids are recorded from veins within and adjacent to igneous rocks. Instead, we begin to constrain possible sources of significant Cl in the crust and then investigate ways by which it may have been incorporated into metal-transporting hydrothermal fluids and ultimately the host porphyry stocks.
On closer inspection, there are at least three different explanations for the source of the metals and fluids, and these are discussed here as options A, B and C. The metals and fluids are derived from the host stocks (Option A), from magmatic processes in an inferred deep precursor batholith (Option B) and/or from hydrothermal processes that involve a fluid that may or may not be derived from a magma (Option C). Although these three options are not mutually exclusive, they cannot all be correct, and viewed together, they belie some of the statements of Stanton (Citation1972) suggesting that this ore-deposit type is so well understood.
Option A: ore metals and fluid derived from a host stock
This model implicates the host stock as the source of the mineralising components, and its basis can be traced back to the early 20th century (Lindgren, Citation1913; Spurr, Citation1923). A quote by Stanton from his textbook (Stanton, Citation1972; ) illustrates his belief in the near-universal acceptance of this idea at the time of his writing. This option is still widely supported today and, if not explicitly stated, then it is certainly implicit in the design of the extensive research studies of the trace-element igneous geochemistry of host stocks, dating studies of host stocks designed to inform about mineralisation age, and thermal modelling of heat and fluid flow around igneous intrusions (Cathles, Citation1997).
This option is incompatible with the structural information provided by the mineralised veins themselves. The nature of the mineralised quartz veins in porphyry Cu–Au deposits indicates that the host stocks had cooled sufficiently to be brittle at the time of mineralisation. Thus, the ore components must have been derived from sources external to the stock. Petrogenesis of the host stock and specifically models based on trace-element geochemistry of the igneous rocks become of subordinate interest, and host-rock rheology should assume greater importance. Absolute ages of host stocks do not provide ages for mineralisation but rather provide an older igneous age. Models of the thermal fields around intrusions may be less relevant to porphyry evolution if the stock has cooled below magmatic temperatures prior to mineralisation. Nevertheless, the statement about the genesis of the Escondida deposit (Jensen et al., Citation2022) confirms that Option A is still widely supported ().
Option B: magmatic processes involving large precursor batholiths
This model implicates very large precursor batholiths lying at some depths below the stocks that commonly host porphyry Cu–Au deposits. With the host stocks being up to 6 km beneath the paleosurface (e.g. Skarmeta, Citation2021), presumably the precursor batholiths would be close to 10 km deep. The batholiths are suggested as the sources of ore metals, Cl and aqueous fluids (Sillitoe, Citation2010, Citation2020; Wilkinson, Citation2013). Enrichment of Cu, Au and Cl is attributed to magmatic processes within the batholith, i.e. suggested strong partitioning of Cu and Au into the silicate melt phase.
Option B addresses the timing evidence that indicates host-stock solidification and cooling prior to mineralisation but retains an underlying magmatic explanation for porphyry deposits (Guilbert & Park, Citation1986). However, the virtually complete unanimity in support of Option A in 1972 is incompatible with the ‘inescapable evidence’ for Option B in 1986. This rather fundamental shift away from mineralisation derived from the host stock probably deserved more attention in the literature at the time. In the light of the advances in geoscience knowledge, particularly beyond the field of economic geology, which occurred in the 20th and 21st centuries, the shift from Option A to Option B would have represented a good time for a complete re-assessment of the magmatic tenet. With such a division regarding the metal source being the host or precursor, an additional question might have been whether either is implicated.
Option B relies upon deeper precursor batholiths to provide the ore components, but there is little theoretical or observational support for it. This option also relies upon strong enrichment of Cu and Au through magmatic processes, for which there is little direct evidence either in the rocks themselves or in laboratory experimental studies.
Option C: hydrothermal process involving highly saline fluid
Option C implicates hydrothermal fluids in deposit formation through the partitioning of Cu and Au into aqueous fluids. It builds upon the documentation of hypersaline fluid inclusions in the quartz veins of porphyry Cu–Au deposits. Strong partitioning necessitates adequate supply of an effective ligand to complex with the metals, and Cl is implicated here, based upon laboratory experiments and the presence of populations of fluid inclusions with very high concentrations of Cl. Enrichment calculations show that the source of the mineralising fluid would be well removed from the host stock, almost certainly involving volumes of several cubic kilometres and transport distances of hundreds of metres to kilometres (at least; ).
As already discussed, the source of the elevated Cl is unlikely to be from silicate magmas. If the source lies at a distance from the host (an obvious implication of the enrichment calculations) and not a large precursor batholith, then there are many potential source regions including sedimentary, igneous and metamorphic rocks. However, only a limited range of these rock packages have sufficient Cl to produce hypersaline fluids or the mineral assemblages necessary to provide oxidising fluids. Greater understanding of the total crustal package around these deposits might help to comprehend their genesis; this would include, but not be restricted to, the igneous rocks.
In this model, still hot but solid igneous rocks may contribute thermal energy or act as rheologically contrasting host rocks. However, silicate magmas cannot be the media for the strong partitioning of Cu or Au.
Overview of common options for the formation of porphyry Cu–Au deposits
Over the last 100 years, as geoscience has progressed, much more has been understood about magmatic processes. From this greater understanding, we might have expected greater successes from a magmatic model in addressing the problems listed above. During this same period, there has been a century of advances in the recognition of many alternative ways to form ore deposits beyond the magmatic representation (Lindgren, Citation1913). Even where contemporary data might be compatible with a magmatic model, it is rare to see questions asked about whether the same data might also be compatible with alternative genetic models that might lead to increased exploration success.
Potential to advance genetic understanding and exploration success
Improved understanding of the porphyry deposits will only come about after the rise of dissatisfaction with current models and with exploration success rates. Our intention here is to suggest that there may already be some cause for dissatisfaction. An example warranting closer scrutiny is the Yerington copper system in southern Nevada USA and whether it is as relevant or diagnostic as has been suggested. This is a Cu porphyry system inferred to have been tilted onto its side to expose a 6-km crustal section (Dilles, Citation1987; Dilles & Einaudi, Citation1992; Proffett, Citation1977). Detailed mapping illustrates the spatial relationships between various igneous rock types and mineralisation. The quality of the exposure and mapping of the Yerington example have demonstrated the spatial relationships between an intrusive body and some vein mineralisation. However, spatial relationships provide no information on how a silicate magma could become enriched in either Cu or Au by at least two orders of magnitude, nor what mineral assemblage might be involved in partial melting to produce a silicate magma with the elevated Cl levels required to transport the metals long distances throughout the vast mineralising vein networks that characterise these deposits. These are the critical questions that we have tried to address in the present work.
The development of any new model naturally follows dissatisfaction with the existing one and will most likely require additional information from fields of geoscience such as modern igneous petrology, rheology and rock mechanics, structural geology, and experimental and theoretical thermodynamics of the aqueous geochemistry of hydrothermal systems. Some early steps that may facilitate a new genetic model would include:
integration of modern metamorphic petrological ideas into porphyry copper research, and
expansion of the scale of thinking about deposit classification, to consider both the source and sink for mineralisation rather than different classifications for each variety found in the sink area as in .
This statement does not diminish the importance of careful mapping and descriptive studies of the sink environments (e.g. Corbett & Leach, Citation1998), which is fundamental for exploration, but when studying how these deposits form, a deposit site represents only the end stage in its genesis.
Metamorphic environment of porphyry deposit formation
Most Cu–Au porphyry deposits formed in metamorphic environments. This does not lessen the importance of igneous and sedimentary processes, but it does mean that prolonged periods of elevated pressure and temperature would have yielded new mineral assemblages, hydrous minerals would have undergone devolatilisation, and partial melting may well have occurred in deeper parts of the crust.
The transformation of mineral assemblages on heating is well quantified, and thermodynamic datasets now include silicate melts, which appear at around 670 °C in common crustal rocks such as those with quartz, two feldspars and mica (White et al., Citation2001). After diagenesis, and prior to the onset of any partial melting, hydrous minerals (clays, chlorites, some micas and amphiboles) break down upon heating to release aqueous metamorphic fluids. Heating of rocks containing carbonate and sulfide minerals similarly yields CO2 and S species (Evans & Tomkins, Citation2020). Importantly, dehydration, decarbonation and desulfidation reactions do not occur independent of one another, but tend to be coupled (Ferry, Citation1981). Thus, polymineralic mineral assemblages can devolatilise at temperatures around the upper greenschist facies to yield metamorphic fluids in the system C–O–H–S. For metamorphism of most sedimentary and igneous rock sequences, these fluids generally have low salinities and so do not match those of the hypersaline fluid inclusions found in Cu–Au porphyry deposits. Notwithstanding this mismatch, some such low-salinity metamorphic fluids should be expected as a significant feature of the environments that host porphyry deposits.
Within classic Cu–Au porphyry studies, it is rarely, if ever, asked whether the assembled data might be compatible with a metamorphic source for the mineralising fluids. Studies do not specifically identify any metamorphic fluid component in the porphyry environment. However, given the overall metamorphic environments of these deposits, it would be quite extraordinary if evidence for the presence of such fluids were not present somewhere in the porphyry belts. The point here is not so much about advancing, at this time, the case for metamorphic fluid involvement, but to demonstrate how a magmatic model that has been assumed to be valid for over a century (Lindgren, Citation1913) has not been rigorously reviewed, tested and updated in the light of more recent developments in geoscience (e.g. Bickle & McKenzie, Citation1987; Ferry, Citation1980, Citation2016; Froese, Citation1969, Citation1971; Fyfe et al., Citation1978; Holland & Powell, Citation2004; Oliver et al., Citation1990; Powell & Holland, Citation1988; Ridley, Citation1993; White, Citation1974).
Metamorphic waters, as the term has been used in parts of the porphyry community (e.g. Guilbert & Park, Citation1986, p. 46), describes connate and meteoric waters set into circulation by heat and pressure gradients. This description illustrates a misunderstanding as to the origin, composition and ore-forming roles of metamorphic fluids (Evans & Tomkins, Citation2020; Fyfe et al., Citation1978) and might explain why they are rarely considered in porphyry studies. In its modern usage, the term ‘metamorphic fluids’, applies to those formed through devolatilisation during metamorphism that involves hydrous, carbonate- and sulfide-bearing mineral assemblages, and specifically does not include diagenetic or meteoric waters.
Classification of Cu–Au porphyry deposits
In the case of gold deposits, and probably of much wider utility, it has been suggested that an effective classification scheme for a deposit needs to:
be easy and unambiguous to apply, and
have a sound scientific basis (Killick, Citation2003; Phillips & Powell, Citation2015).
These two criteria for a successful classification system are not fulfilled by the traditional classification applied to porphyry deposits. With respect to being practical to apply, the definition of what constitutes a porphyry deposit has led to a variety of classifications for individual deposits and considerable uncertainty about whether some are high-sulfidation epithermal deposits, porphyry deposits or something else. This has led to the broadening of definitions and postulation of a continuum between different types; see and .
With respect to a good classification system having a sound theoretical basis, we have identified significant shortcomings in some aspects of the traditional magmatic model for Cu–Au porphyry deposits, and this is illustrated by the ad hoc modifications, definition-broadening and the emergence, over time, of some incompatible models in porphyry Cu–Au studies.
For mining and metallurgy, the host rocks for Cu–Au ores are very important, and different techniques are necessary to process ores contained within calc-silicate, andesite, granodiorite or black shale, for example. These variations are well encapsulated in the classic schematic cross-sections that show the juxtaposition of many ore-deposit types (Corbett & Leach, Citation1998), but the classifications are not ideal for determining genesis.
To understand the genesis of an ore deposit or deposit type, it is important to separate features related to the area of deposition (i.e. the sink area or host rock) from those more related to the source (Phillips & Powell, Citation1993, Citation2010, Citation2015). The enrichment of metals indicates a large-scale process with source and sink separated by hundreds of metres at least, and more likely several kilometres. The sink is characterised by many different styles of mineralisation (), whereas the features of primary mineralisation that are inherited from the source, such as ore fluid type, tend to be more uniform.
The designation of this group of deposits as being porphyry may not be helpful because the term is simply not an accurate description of the deposit type. In medium- to fine-grained igneous rocks, porphyritic texture is extremely common over a wide compositional range, from a variety of tectonic settings and over a wide range of emplacement styles, from lavas and pyroclastic rocks to shallow-level plutons. Being porphyritic is not a special characteristic. As already mentioned in the introduction, some ‘porphyry’ Cu–Au deposits are not in porphyritic igneous rocks, or even in igneous rocks at all. However, this diversity of settings can make sense if the role of the mineralised host is simply that of a passive structure having mechanical characteristics that cause it to undergo preferential hydraulic fracturing once it is rheologically in the solid state.
It is then interesting to speculate what might result if a similar mineralising process were to occur in a terrain devoid of porphyritic rocks. Such deposits could not be called porphyry deposits, but they could bear many similarities with respect to timing, host-rock rheology contrasts, veins, ore mineralogy and alteration assemblages; with a different classification, many similarities may be overlooked. To the question of ‘Why are Cu–Au porphyry deposits predominantly in or near porphyry stocks?‘ the answer might be as simple as ‘They would be allocated a different name if there were no porphyries nearby‘. Given the size of porphyry deposits, it appears that the scale of thinking may be too small; the porphyry stock is the end of the line for metals and not the start.
An example from the Archean Kalgoorlie goldfield of Western Australia (Mueller, Citation2017; Phillips et al., Citation2017) highlights a global inconsistency in labelling these deposits. The small Mt Percy deposit in the Kalgoorlie goldfield comprises auriferous quartz veins in a feldspar porphyry (Dunga et al., Citation2021). The Mt Percy deposit is part of the Kalgoorlie goldfield in the Archean Yilgarn Craton and lies within 10 km of the much larger Golden Mile and Mt Charlotte deposits. Mt Percy was discovered decades after the main Kalgoorlie goldfield and is not referred to as being porphyry style (noting that Kalgoorlie was never described as being porphyry style). If Mt Percy had been discovered in isolation from Kalgoorlie, perhaps in the NE Queensland Charters Towers Gold Province, it might well have been described as an Au porphyry deposit.
There are many gold-plus (Cu–Au) styles of mineralisation near porphyry Cu–Au deposits, but these are hosted in the wall rocks around the intrusions. These nearby deposits are given different names such as skarn, carbonate replacement and high-sulfidation epithermal, but some might simply be the expression of the same metal and fluid sources, and similar mineralising processes, but within different host rocks from the main porphyry deposit. The distinctions are important for mining but need to be combined with a larger scale of processes in any genetic interpretation.
Final word
We have taken an unusual approach of not starting with the assumption that magmatic processes are necessary for providing the metals and fluids that lead to Cu–Au porphyry deposits. Instead, we take a broader view and consider the overall metamorphic—igneous environment in which these deposits have formed. We also consider the behaviour of the element gold, regardless of the deposit classifications that researchers might assign. The study has been selective as to what information we have focused on, such as rheology, rock mechanics, vein control, metal-enrichment processes and the source of Cl. This has not answered how Cu–Au porphyry deposits form but has revealed several sub-fields of geoscience that might contribute to answering some of the more intractable questions, which will ultimately benefit scientific understanding and exploration success. Hydrothermal processes may be important in forming these deposits given the strong partitioning of both Cu and Au into high-temperature, oxidising, high-salinity hydrothermal fluids. We have found no essential role for magmatic processes in partitioning and enriching Cu or Au into a silicate magma. There is little evidence for ‘special magmatic processes’ that are common to the evolution of many different magma types that originate from different sources and evolve in different ways.
Acknowledgements
The authors particularly thank Caitlin Jones for her contributions to discussions and the manuscript, and Martin Hughes for sharing his wealth of knowledge of economic geology, particularly on ore fluids and copper deposits. Previous discussions with Andrew Tomkins, Nick Oliver, Greg Corbett, Tim Baker, Nick Lindsay, Peter Pollard, Mike Porter, Roger Powell, Andy Rankin, Francois Robert, Gerard Tripp and the late Patrick Williams have been much appreciated. Critical review comments by Dave Craw and two anonymous reviewers were very useful and appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Almeida, K. M. F., & Jenkins, D. M. (2017). Stability field of the Cl-rich scapolite marialite. American Mineralogist, 102(12), 2484–2493. https://doi.org/10.2138/am-2017-6132
- Anderson, J. A. C., & King, S. (2017). Walhalla—Woods Point goldfield. In G. N. Phillips (Ed.), Australian Ore Deposits (pp. 811–812). Monograph 32, Australasian Institute Mining Metallurgy.
- Arth, J. G., & Hanson, G. N. (1972). Quartz diorites derived by partial melting of eclogite or amphibolite at mantle depths. Contributions to Mineralogy and Petrology, 37(2), 161–174. https://doi.org/10.1007/BF00371074
- Audétat, A. (2019). The metal content of magmatic-hydrothermal fluids and its relationship to mineralization potential. Economic Geology, 114(6), 1033–1056. https://doi.org/10.5382/econgeo.4673
- Bachmann, O., & Bergantz, G. W. (2004). On the origin of crystal-poor rhyolites: Extracted from batholithic crystal mushes. Journal of Petrology, 45(8), 1565–1582. https://doi.org/10.1093/petrology/egh019
- Bachmann, O., & Bergantz, G. W. (2006). Gas percolation in upper-crustal silicic crystal mushes as a mechanism for upward heat advection and rejuvenation of near-solidus magma bodies. Journal of Volcanology and Geothermal Research, 149(1-2), 85–102. https://doi.org/10.1016/j.jvolgeores.2005.06.002
- Baker, E. M., & Tullemans, F. J. (1990). Kidston gold deposit. In F. E. Hughes (Ed.), Geology of the mineral deposits of Australia and Papua New Guinea (pp. 1461–1465). The Australasian Institute of Mining and Metallurgy.
- Baker, T., Pollard, P. J., Mustard, R., Mark, G., & Graham, J. L. (2005). A comparison of granite-related tin, tungsten, and gold–bismuth deposits implications for exploration. SEG Discovery, 61(61), 5–17. https://doi.org/10.5382/SEGnews.2005-61.fea
- Bickle, M. J., & McKenzie, D. (1987). The transport of heat and matter by fluids during metamorphism. Contributions to Mineralogy and Petrology, 95(3), 384–392. https://doi.org/10.1007/BF00371852
- Boulter, C. A., Fotios, M. G., & Phillips, G. N. (1987). The Golden Mile, Kalgoorlie: A giant gold deposit localized in ductile shear zones by structurally induced infiltration of auriferous metamorphic fluid. Economic Geology, 82(7), 1661–1678. https://doi.org/10.2113/gsecongeo.82.7.1661
- Brown, M. (2001). Crustal melting and granite magmatism: Key issues. Physics and Chemistry of the Earth Part A—Solid Earth and Geodesy, 26(4-5), 201–212. https://doi.org/10.1016/S1464-1895(01)00047-3
- Brown, M. (2013). Granite: From genesis to emplacement. Geological Society of America Bulletin, 125(7-8), 1079–1113. https://doi.org/10.1130/B30877.1
- Buddington, A. K. (1959). Granite emplacement with special reference to North America. Geological Society of America Bulletin, 70(6), 671–747. https://doi.org/10.1130/0016-7606(1959)70[671:GEWSRT2.0.CO;2]
- Camus, F. (1975). Geology of the El Teniente orebody with emphasis on wall rock alteration. Economic Geology, 70(8), 1341–1372. − https://doi.org/10.2113/gsecongeo.70.8.1341
- Candela, P. A., & Piccoli, P. M. (2005). Magmatic processes in the development of porphyry-type ore systems. Economic Geology, 100th Anniversary Volume, 25–37. https://doi.org/10.5382/AV100.03
- Cannell, J., Cooke, D. R., Walshe, J. L., & Stein, H. (2005). Geology, mineralization, alteration, and structural evolution of the El Teniente porphyry Cu–Mo deposit. Economic Geology, 100(5), 979–1003. https://doi.org/10.2113/gsecongeo.100.5.979
- Cathles, L. M. (1997). Thermal aspects of ore formation. In H. L. Barnes (Ed.), Geochemistry of Hydrothermal Ore Deposits (3rd ed., pp. 125–190). John Wiley.
- Clemens, J. D. (2012). Granitic magmatism, from source to emplacement: A personal view. Applied Earth Science, 121(3), 107–136. https://doi.org/10.1179/1743275813Y.0000000023
- Clemens, J. D., Bryan, S. E., Stevens, G., Mayne, M. J., & Petford, N. (2020). How are silicic volcanic and plutonic systems related, and what are the roles of mafic magmas and crystal mushes? Earth-Science Reviews, 200.
- Clemens, J. D., & Mawer, C. K. (1992). Granitic magma transport by fracture propagation. Tectonophysics, 204(3-4), 339–360. https://doi.org/10.1016/0040-1951(92)90316-X
- Clemens, J. D., Stevens, G., & Bryan, S. E. (2020). Conditions during the formation of granitic magmas by crustal melting—hot or cold; drenched, damp or dry? Earth-Science Reviews, 200, 102982. https://doi.org/10.1016/j.earscirev.2019.102982
- Clemens, J. D., Stevens, G., & Farina, F. (2011). The enigmatic sources of I-type granites and the clinopyroxene–ilmenite connexion. Lithos, 126(3-4), 174–181. https://doi.org/10.1016/j.lithos.2011.07.004
- Clemens, J. D., & Watkins, J. M. (2001). The fluid regime of high-temperature metamorphism during granitoid magma genesis. Contributions to Mineralogy and Petrology, 140(5), 600–606. https://doi.org/10.1007/s004100000205
- Cooke, D. R., Hollings, P., & Walshe, J. L. (2005). Giant porphyry deposits: Characteristics, distribution, and tectonic controls. Economic Geology, 100(5), 801–818. https://doi.org/10.2113/gsecongeo.100.5.801
- Corbett, G. J. (1994). Regional structural control of selected Cu/Au occurrences in Papua New Guinea. In R. Rogerson (Ed.), Geology, Exploration and Mining Conference, June 1994, Lae, Papua New Guinea Proceedings (pp. 125–129). The Australasian Institute of Mining and Metallurgy.
- Corbett, G. J., & Leach, T. M. (1998). Southwest Pacific Rim gold–copper systems: Structure, alteration, and mineralization. Society of Economic Geologists, Special Publication, 6, 237. https://doi.org/10.5382/SP.06
- Cox, S. F., Etheridge, M. A., & Wall, V. J. (1987). The role of fluids in syntectonic mass transport, and the localization of metamorphic vein-type ore deposits. Ore Geology Reviews, 2(1-3), 65–86. https://doi.org/10.1016/0169-1368(87)90024-2
- Cruden, A. R. (1998). On the emplacement of tabular granites. Journal of the Geological Society, 155, 853–862. https://doi.org/10.1144/gsjgs.155.5.0853
- Cruden, A. (2006). Emplacement and growth of plutons: Implications for rates of melting and mass transfer in continental crust. In M. Brown & T. Rushmer (Eds.), Evolution and Differentiation of the Continental Crust (pp. 455–519). Cambridge University Press.
- De Jong, G., Rotherham, J., Phillips, G. N., & Williams, P. J. (1997). Mobility of rare-earth elements and copper during shear-zone-related retrograde metamorphism. Geologie en Mijnbouw (Geology and Mining), 76(4), 311–319. https://doi.org/10.1023/A:1003232119010
- Dilles, J. H. (1987). The petrology of the Yerington batholith, Nevada: Evidence for the evolution of porphyry copper ore fluids. Economic Geology, 82(7), 1750–1789. https://doi.org/10.2113/gsecongeo.82.7.1750
- Dilles, J. H., & Einaudi, M. T. (1992). Wall-rock alteration and hydrothermal flow paths about the Ann-Mason porphyry copper deposit, Nevada—a 6-km vertical reconstruction. Economic Geology, 87(8), 1963–2001. https://doi.org/10.2113/gsecongeo.87.8.1963
- Duan, C., Li, Y., Mao, J., Zhu, Q., Xie, G., Wan, Q., Jian, W., & Hou, K. (2021). The role of evaporite layers in the ore-forming processes of iron oxide–apatite and skarn Fe deposits: Examples from the middle–lower Yangtze River metallogenic Belt, East China. Ore Geology Reviews, 138, 104352. https://doi.org/10.1016/j.oregeorev.2021.104352
- Dunga, G., Sully, D., Hagemann, S. G., Duuring, P., & Danyushevsky, L. (2021). Structural setting, wall rock alteration and gold mineralisation of the Mt Percy gold deposit, Kalgoorlie, Western Australia. Mineralium Deposita, 56(8), 1449–1470. https://doi.org/10.1007/s00126-020-00993-7
- Eastoe, C. J. (1978). A fluid inclusion study of the Panguna porphyry copper deposit, Bougainville, Papua New Guinea. Economic Geology, 73(5), 721–748. https://doi.org/10.2113/gsecongeo.73.5.721
- Evans, K. A., & Tomkins, A. (2020). Metamorphic fluids in orogenic settings. Elements, 16(6), 381–387. https://doi.org/10.2138/gselements.16.6.381
- Ewart, A., Bryan, W. B., & Gill, J. B. (1973). Mineralogy and geochemistry of the younger volcanic islands of Tonga, S. W. Pacific. Journal of Petrology, 14(3), 429–465. https://doi.org/10.1093/petrology/14.3.429
- Ewart, A., & Griffin, W. L. (1994). Application of proton-microprobe data to trace-element partitioning in volcanic rocks. Chemical Geology, 117(1-4), 251–284. https://doi.org/10.1016/0009-2541(94)90131-7
- Ferry, J. M. (1980). A case study of the amount and distribution of heat and fluid during metamorphism. Contributions to Mineralogy and Petrology, 71(4), 373–385. https://doi.org/10.1007/BF00374708
- Ferry, J. M. (1981). Petrology of graphitic sulfide-rich schists from south-central Maine: An example of desulfidation during prograde regional metamorphism. American Mineralogist, 66, 908–930.
- Ferry, J. M. (2016). Fluids in the crust during regional metamorphism: Forty years in the Waterville limestone. American Mineralogist, 101(3), 500–517. https://doi.org/10.2138/am-2016-5118
- Finch, E. G., & Tomkins, A. G. (2017). Fluorine and chlorine behaviour during progressive dehydration melting: Consequences for granite geochemistry and metallogeny. Journal of Metamorphic Geology, 35(7), 739–757. https://doi.org/10.1111/jmg.12253
- Froese, E. (1969). Metamorphic rocks from the Coronation Mine and surrounding areas. Geological Survey of Canada Paper, 68(5), 57–77.
- Froese, E. (1971). The graphical representation of sulfide-silicate phase equilibria. Economic Geology, 66(2), 335–341. https://doi.org/10.2113/gsecongeo.66.2.335
- Frost, T. P., & Mahood, G. A. (1987). Field, chemical, and physical constraints on mafic–felsic magma interaction in the Lamark Granodiorite, Sierra Nevada, California. Geological Society of America Bulletin, 99(2), 272–291. https://doi.org/10.1130/0016-7606(1987)99<272:FCAPCO>2.0.CO;2
- Fyfe, W. S., Price, N. J., & Thompson, A. B. (1978). Fluids in the Earth’s Crust. Elsevier.
- Goranson, R. W. (1931). The solubility of water in granite magmas. American Journal of Science, s5-22(132), 481–502. https://doi.org/10.2475/ajs.s5-22.132.481
- Guilbert, J. M., & Park, C. F. (1986). The Geology of Ore Deposits. Waveland Press, Inc.
- Gustafson, L. B., & Hunt, J. P. (1975). The porphyry copper deposit at El Salvador, Chile. Economic Geology, 70(5), 857–912. https://doi.org/10.2113/gsecongeo.70.5.857
- Hall, D., & Kisters, A. (2016). From steep feeders to tabular plutons—emplacement controls of syntectonic granitoid plutons of the Damara Belt, Namibia. Journal of African Earth Sciences, 113, 51–64. https://doi.org/10.1016/j.jafrearsci.2015.10.005
- Hamilton, D. L., Burnham, C. W., & Osborn, E. F. (1964). The solubility of water and effects of oxygen fugacity and water content on crystallization of mafic magmas. Journal of Petrology, 5(1), 21–39. https://doi.org/10.1093/petrology/5.1.21
- Harris, A. C., & Holcombe, R. J. (2014). Quartz vein emplacement mechanism at the E26 Porphyry Cu–Au Deposit, New South Wales. Economic Geology, 109(4), 1035–1050. https://doi.org/10.2113/econgeo.109.4.1035
- Helgeson, H. C. (1964). Complexing and Hydrothermal Ore Deposition. McMillan Co.
- Ho, S. E., Groves, D. I., & Phillips, G. N. (1990). Fluid inclusions in quartz veins associated with Archaean gold mineralization: Clues to ore fluids and ore depositional conditions and significance to exploration. In H. K. Herbert & S. E. Ho (Eds.), Proceedings of the Conference on Stable Isotopes and Fluid Processes in Mineralization (pp. 35–50). Geology Department and Extension, University of Western Australia Publication 23.
- Hobbs, B. E., & Ord, A. (2023). An alternative to the fault valve model. Australian Journal of Earth Sciences, 70(7), 957–970. https://doi.org/10.1080/08120099.2023.2218452
- Holland, T. J. B., & Powell, R. (2004). An internally consistent thermodynamic dataset for phases of petrological interest. Journal of Metamorphic Geology, 16(3), 309–343. https://doi.org/10.1111/j.1525-1314.1998.00140.x
- Holwell, D. A., & Keays, R. R. (2014). The formation of low-volume, high-tenor magmatic PGE–Au sulfide mineralization in closed systems: Evidence from precious and base metal geochemistry of the Platinova Reef, Skaergaard Intrusion, East Greenland. Economic Geology, 109(2), 387–406. https://doi.org/10.2113/econgeo.109.2.387
- Huang, R., Ding, X., Lin, C-T., Zhan, W., & Ling, M. (2018). Effect of saline fluids on chlorine incorporation in serpentine. Solid Earth Sciences, 3(3), 61–66. https://doi.org/10.1016/j.sesci.2018.04.001
- Hughes, M. J. (2017). Mineralisation of the Lachlan Orogen. In G. N. Phillips (Ed.), Australian Ore Deposits (pp. 729–738). Monograph 32, Australasian Institute Mining Metallurgy.
- Ishihara, S. (1981). The granitoid series and mineralization. Economic Geology Anniversary, 75, 458–484. https://doi.org/10.5382/AV75.14
- Jensen, K. R., Campos, E., Wilkinson, J. J., Wilkinson, C. C., Kearsley, A., Miranda-Díaz, G., & Véliz, W. (2022). Hydrothermal fluid evolution in the Escondida porphyry copper deposit, northern Chile: Evidence from SEM-CL imaging of quartz veins and LA-ICP-MS of fluid inclusions. Mineralium Deposita, 57(2), 279–300. https://doi.org/10.1007/s00126-021-01058-z
- Kerrich, R., & Allison, I. (1978). Vein geometry and hydrostatics during Yellowknife mineralisation. Canadian Journal of Earth Sciences, 15(10), 1653–1660. https://doi.org/10.1139/e78-169
- Killick, A. M. (2003). Fault rock classification: An aid to structural interpretation in mine and exploration geology. South African Journal of Geology, 106(4), 395–402. https://doi.org/10.2113/106.4.395
- Kouzmanov, K., & Pokrovski, G. S. (2012). Hydrothermal controls on metal distribution in porphyry Cu (–Mo–Au) systems. Economic Geology, Special Publication, 16, 573–618. https://doi.org/10.5382/SP.16.22
- Lindgren, W. (1913). Mineral Deposits. McGraw-Hill.
- Livesey, J. (2017). Chandler salt deposit. In G. N. Phillips (Ed.), Australian Ore Deposits (pp. 575–576). Monograph 32, Australasian Institute Mining Metallurgy.
- Lodder, C., Padilla, R., Shaw, R., Garzon, T., Palacio, E., & Jahoda, R. (2010). Discovery history of the La Colosa Gold Porphyry Deposit, Cajamarca, Colombia. Economic Geology, Special Publication, 15, 19–28. https://doi.org/10.5382/SP.15.1.03
- Loucks, R. R. (2014). Distinctive composition of copper-ore-forming arc magmas. Australian Journal of Earth Sciences, 61(1), 5–16. https://doi.org/10.1080/08120099.2013.865676
- Luhr, J. F., & Carmichael, I. S. E. (1980). The Colima volcanic complex, Mexico. I: Post-caldera andesites from Volcan Colima. Contributions to Mineralogy and Petrology, 71(4), 343–372. https://doi.org/10.1007/BF00374707
- Lundstrom, C. C., & Glazner, A. F. (2016). Silicic magmatism and the volcanic—plutonic connection. Elements, 12(2), 91–96. https://doi.org/10.2113/gselements.12.2.91
- Mason, B. (1966). Principles of Geochemistry (3rd ed., 329 pp). Wiley.
- McAndrew, J. (1965). Gold deposits of Victoria. In J. McAndrew (Ed.), Geology of Australian Ore Deposits (2nd ed., pp. 450–456). Australasian Institute of Mining and Metallurgy.
- Miura, Y., Rucklidge, J., & Nord, G. L. (1981). The occurrence of chlorine in serpentine minerals. Contributions to Mineralogy and Petrology, 76(1), 17–23. https://doi.org/10.1007/BF00373679
- Mueller, A. G. (2017). Mount Charlotte deposit–Kalgoorlie Goldfield. In G. N. Phillips (Ed.), Australian Ore Deposits (pp. 195–198). Monograph 32, Australasian Institute of Mining and Metallurgy.
- Oen, I. S., & Lustenhouwer, W. J. (1992). Cl-rich biotite, Cl–K hornblende, and Cl-rich scapolite in meta-exhalites: Nora, Bergslagen, Sweden. Economic Geology, 87(6), 1638–1648. https://doi.org/10.2113/gsecongeo.87.6.1638
- Oliver, N. H. S., Valenta, R. K., & Wall, V. J. (1990). The effect of heterogeneous stress and strain on metamorphic fluid flow, Mary Kathleen, Australia, and a model for large-scale fluid circulation. Journal of Metamorphic Geology, 8(3), 311–331. https://doi.org/10.1111/j.1525-1314.1990.tb00475.x
- Oliver, N. H. S., Wall, V. J., & Cartwright, I. (1992). Internal controls on fluid compositions in amphibolitic-facies scapolitic calc-silicates, Mary Kathleen, Australia. Contributions to Mineralogy and Petrology, 111(1), 94–112. https://doi.org/10.1007/BF00296581
- Otamendi, J. E., Ducea, M. N., Tibaldi, A. M., Bergantz, G. W., de la Rosa, J. D., & Vujovich, G. I. (2009). Generation of tonalitic and dioritic magmas by coupled partial melting of gabbroic and metasedimentary rocks within the deep crust of the Famatinian magmatic arc, Argentina. Journal of Petrology, 50(5), 841–873. https://doi.org/10.1093/petrology/egp022
- Pacey, A., Wilkinson, J. J., Boyce, A. J., & Millar, I. L. (2020). Magmatic fluids implicated in the formation of propylitic alteration: Oxygen, hydrogen, and strontium isotope constraints from the Northparkes porphyry Cu–Au district, New South Wales, Australia. Economic Geology, 115(4), 729–748. https://doi.org/10.5382/econgeo.4732
- Penniston-Dorland, S. C. (2001). Illumination of vein quartz textures in a porphyry copper ore deposit using scanned cathodoluminescence: Grasberg Igneous Complex, Irian Jaya, Indonesia. American Mineralogist, 86(5-6), 652–666. https://doi.org/10.2138/am-2001-5-606
- Perkins, J. P., Ward, K. M., Shanaka, L., de Silva, G. Z., Beck, S. L., & Finnegan, N. J. (2016). Surface uplift in the Central Andes driven by growth of the Altiplano-Puna magmatic body. Nature Communications, 7, 13185. https://doi.org/10.1038/ncomms13185
- Petford, N., Cruden, A. R., McCaffrey, K. J. W., & Vigneresse, J-L. (2000). Granite magma formation, transport and emplacement in the Earth’s crust. Nature, 408(6813), 669– 673. https://doi.org/10.1038/35047000
- Phillips, G. N. (2022). Formation of Gold Deposits. Springer.
- Phillips, G. N., & Groves, D. I. (1984). Fluid access and fluid-wall rock interaction in the genesis of the Archaean gold-quartz vein deposit at Hunt mine, Kambalda. Western Australia. In R. P. Foster, (Ed.), Gold 82. (pp. 389–416)Balkema.
- Phillips, G. N., Kisters, A. F. M., & Clemens, J. D. (2022). The tabular Strathbogie batholith in central Victoria. Australian Journal of Earth Sciences, 69(6), 776–800. https://doi.org/10.1080/08120099.2022.2032340
- Phillips, G. N., & Powell, R. (1993). Link between gold provinces. Economic Geology, 88(5), 1084–1098. https://doi.org/10.2113/gsecongeo.88.5.1084
- Phillips, G. N., & Powell, R. (2010). Formation of gold deposits: A metamorphic devolatilization model. Journal of Metamorphic Geology, 28(6), 689–718. https://doi.org/10.1111/j.1525-1314.2010.00887.x
- Phillips, G. N., & Powell, R. (2015). A practical classification of gold deposits, with a theoretical basis. Ore Geology Reviews, 65, 568–573. https://doi.org/10.1016/j.oregeorev.2014.04.006
- Phillips, G. N., Hergt, J., & Powell, R. (2017). Kalgoorlie goldfield—petrology, alteration and mineralisation of the Golden Mile Dolerite. In G. N. Phillips (Ed.), Australian Ore Deposits (pp. 185–194). Monograph 32, Australasian Institute of Mining and Metallurgy.
- Phillips, G. N., & Vearncombe, J. R. (2021). The humble quartz vein. Australian Institute of Geoscientists News, June 2021, 49–50.
- Phillips, G. N., Williams, P. J., & De Jong, G. (1994). Nature of metamorphic fluids and significance for metal exploration. Geological Society, London, Special Publications, 78(1), 55–68. https://doi.org/10.1144/GSL.SP.1994.078.01.06
- Pistone, M., Baumgartner, L. P., Bégué, F., Jarvis, P. A., Bloch, E., Robyr, M., Müntener, O., Sisson, T. W., & Blundy, J. D. (2020). Felsic melt and gas mobilization during magma solidification: An experimental study at 1.1 kbar. Frontiers in Earth Science, 8, 175. https://doi.org/10.3389/feart.2020.00175
- Piquer, J., Skarmeta, J., & Cooke, D. R. (2015). Structural evolution of the Rio Blanco–Los Bronces district, Andes of Central Chile: Controls on stratigraphy, magmatism, and mineralization. Economic Geology, 110(8), 1995–2023. https://doi.org/10.2113/econgeo.110.8.1995
- Piquer, J., Sanchez-Alfaro, P., & Pérez-Flores, P. (2021). A new model for the optimal structural context for giant porphyry copper deposit formation. Geology, 49(5), 597–601. https://doi.org/10.1130/G48287.1
- Pitcairn, I. K. (2011). Background concentrations of gold in different rock types. Applied Earth Science, 120(1), 31–38. https://doi.org/10.1179/1743275811Y.0000000021
- Pollard, P. J. (2017). Australian rare element granitic pegmatites. In G. N. Phillips (Ed.), Australian Ore Deposits. (pp. 67–74). Monograph 32, Australasian Institute Mining Metallurgy.
- Porter, T. M. (2017). Cadia gold–copper deposits. In G. N. Phillips (Ed.) Australian Ore Deposits (pp. 755–758). Monograph 32, Australasian Institute Mining Metallurgy.
- Porter, M. (2020). Regular use was made of the comprehensive database of Porter Consulting. http://www.portergeo.com.au/database/mineinfo.asp?mineid=mn053
- Powell, R., & Holland, T. J. B. (1988). An internally consistent thermodynamic dataset with uncertainties and correlations: 3. Application, methods, worked examples and a computer program. Journal of Metamorphic Geology, 6(2), 173–204. https://doi.org/10.1111/j.1525-1314.1988.tb00415.x
- Pritchard, M. E., & Gregg, P. M. (2016). Geophysical evidence for silicic crustal melt in the continents: Where, what kind, and how much? Elements, 12(2), 121–127. https://doi.org/10.2113/gselements.12.2.121
- Proffett, J. M. Jr (1977). Cenozoic geology of the Yerington district, Nevada and implications for the nature and origin of Basin and Range faulting. Geological Society of America Bulletin, 88(2), 247–266. https://doi.org/10.1130/0016-7606(1977)88<247:CGOTYD>2.0.CO;2
- Prokofiev, V. Y., & Naumov, V. B. (2022). Ranges of physical parameters and geochemical features of mineralizing fluids at porphyry deposits of various types of the Cu–Mo–Au system: Evidence from fluid inclusions data. Minerals, 12(5), 529. https://doi.org/10.3390/min12050529
- Puddephatt, R. J. (1978). The Chemistry of Gold. Elsevier.
- Ridley, J. (1993). The relations between mean rock stress and fluid flow in the crust: With reference to vein- and lode-style gold deposits. Ore Geology Reviews, 8(1-2), 23–37. https://doi.org/10.1016/0169-1368(93)90026-U
- Ridley, J. (2013). Ore Deposit Geology. Cambridge University Press.
- Ridley, J. R., & Diamond, L. W. (2000). Fluid chemistry of orogenic lode gold deposits and implications for genetic models. Economic Geology Reviews, 14, 141–162. https://doi.org/10.5382/Rev.13.04
- Ridley, J., & Mengler, F. (2000). Lithological and structural controls on the form and setting of vein stockwork orebodies at the Mt Charlotte gold deposit. Economic Geology, 95(1), 85–98. https://doi.org/10.2113/gsecongeo.95.1.85
- Robb, L. (2005). Introduction to Ore-forming Processes. Blackwell Publishing. https://doi.org/10.1144/1467-7873/05-073
- Robert, F., & Brown, A. C. (1986). Archean gold-bearing quartz veins at the Sigma mine, Abitibi greenstone belt, Quebec: Part I. Geologic relations and formation of the vein system. Economic Geology, 81(3), 578–592. https://doi.org/10.2113/gsecongeo.81.3.578
- Roedder, E. (1967). Fluid inclusions as samples of ore fluids. In H. L. Barnes (Ed.), Geochemistry of Hydrothermal Ore Deposits (1st edn., pp. 515–574). Holt, Reinhart and Winston.
- Roedder, E. (1971). Fluid inclusion studies on the porphyry-type ore deposits at Bingham, Utah, Butte, Montana, and Climax, Colorado. Economic Geology, 66(1), 98–118. https://doi.org/10.2113/gsecongeo.66.1.98
- Roedder, E. (1979). Fluid inclusions as samples of ore fluids. In H. L. Barnes (Ed.), Geochemistry of hydrothermal ore deposits (2nd ed., pp. 684–737). Wiley.
- Roedder, E. (1984). Reviews in Mineralogy. Volume 12: Fluid inclusions. Mineralogical Society of America.
- Seedorff, E., Dilles, J. H., Proffett, J. M., Einaudi, M. T., Zurcher, L., Stravast, W. J. A., Johnson, D. A., & Barton, M. D. (2005). Porphyry deposits: Characteristics and origin of hypogene features. Economic Geology, 100th Anniversary Volume, 251–298. https://doi.org/10.5382/AV100.10
- Shaw, A., Downes, H., & Thirlwall, M. F. (1993). The quartz-diorites of Limousin: Elemental and isotopic evidence for Devono-Carboniferous subduction in the Hercynian belt of the French Massif Central. Chemical Geology, 107(1-2), 1–18. https://doi.org/10.1016/0009-2541(93)90098-4
- Sibson, R. H. (1987). Earthquake rupturing as a hydrothermal mineralising agent. Geology, 15(8), 701–704. https://doi.org/10.1130/0091-7613(1987)15<701:ERAAMA>2.0.CO;2
- Sibson, R. H. (1996). Structural permeability of fluid-driven fault-fracture meshes. Journal of Structural Geology, 18(8), 1031–1042. https://doi.org/10.1016/0191-8141(96)00032-6
- Sibson, R. H., Robert, F., & Poulsen, K. H. (1988). High-angle reverse faults, fluid-pressure cycling, and mesothermal gold–quartz deposits. Geology, 16(6), 551–555. https://doi.org/10.1130/0091-7613(1988)016<0551:HARFFP>2.3.CO;2
- Sillitoe, R. H. (1972). A plate tectonic model for the origin of porphyry copper deposits. Economic Geology, 67(2), 184–197. https://doi.org/10.2113/gsecongeo.67.2.184
- Sillitoe, R. H. (1997). Characteristics and controls of the largest porphyry copper–gold and epithermal gold deposits in the circum-Pacific region. Australian Journal of Earth Sciences, 44(3), 373–388. https://doi.org/10.1080/08120099708728318
- Sillitoe, R. H. (2010). Porphyry copper systems. Economic Geology, 105(1), 3–41. https://doi.org/10.2113/gsecongeo.105.1.3
- Sillitoe, R. H. (2020). Gold deposit types: An overview. Society of Economic Geologists Special Publications, 23, 1–28. https://doi.org/10.5382/SP.23.01
- Singer, D. A., Berger, V. I., Menzie, W. D., & Berger, B. R. (2005). Porphyry copper deposit density. Economic Geology, 100(3), 491–514. https://doi.org/10.2113/gsecongeo.100.3.491
- Skarmeta, J. (2021). Structural controls on alteration stages at the Chuquicamata copper–molybdenum deposit, Northern Chile. Economic Geology, 116(1), 1–28. https://doi.org/10.5382/econgeo.4769
- Spurr, J. E. (1923). The Ore Magmas. McGraw Hill.
- Stanton, R. L. (1972). Ore Petrology. McGraw-Hill.
- Tamic, N., Behrens, H., & Holtz, F. (2001). The solubility of H2O and CO2 in rhyolitic melts in equilibrium with a mixed CO2–H2O fluid phase. Chemical Geology, 174(1-3), 333–347. https://doi.org/10.1016/S0009-2541(00)00324-7
- Taylor, S. R., & McLennan, S. (1985). The Continental Crust: Its Composition and Evolution. Blackwell Scientific Publishers. Oxford.
- Tosdal, R. M., & Dilles, J. H. (2020). Creation of permeability in the porphyry Cu environment. Reviews in Economic Geology, 21, 173–204. https://doi.org/10.5382/rev.21.05
- Tosdal, R. M., & Richards, J. P. (2001). Magmatic and structural controls on the development of porphyry Cu ± Mo ± Au deposits. Reviews in Economic Geology, 14, 157–181. https://doi.org/10.5382/Rev.14.06
- Travis, G. A., Woodall, R., & Bartram, G. D. (1971). The geology of the Kalgoorlie goldfield. In J. E. Glover (Ed.), Symposium on Archaean Rocks, Special Publication (3rd ed.). Geological Society of Australia.
- Vearncombe, J. R. (1988). Fibrous vein geometry and gold mineralization. In S. E. Ho & D. I. Groves (Eds.), Advances in Understanding Precambrian Gold Deposits (Vol. II, pp. 41–61). The University of Western Australia.
- Vearncombe, J. R. (1998). Shear zones, fault networks and Archean gold. Geology, 26(9), 855–858. https://doi.org/10.1130/0091-7613(1998)026<0855:SZFNAA>2.3.CO;2
- Vry, V. H., Wilkinson, J. J., Seguel, J., & Millan, J. (2010). Multistage intrusion, brecciation, and veining at El Teniente, Chile: Evolution of a nested porphyry system. Economic Geology, 105(1), 119–153. https://doi.org/10.2113/gsecongeo.105.1.119
- Warren, J. K. (2010). Evaporites through time: Tectonic, climatic, and eustatic controls in marine and nonmarine deposits. Earth-Science Reviews, 98(3-4), 217–268. https://doi.org/10.1016/j.earscirev.2009.11.004
- Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59, 1217–1232. https://doi.org/10.1016/0016-7037(95)00038-2
- White, D. E. (1974). Diverse origins of hydrothermal fluids. Economic Geology, 69(6), 954–973. https://doi.org/10.2113/gsecongeo.69.6.954
- White, R. W., Powell, R., & Holland, T. J. B. (2001). Calculation of partial melting equilibria in the system Na2O–CaO–K2O–FeO–MgO–Al2O3 –SiO2–H2O (NCKFMASH). Journal of Metamorphic Geology, 19(2), 139–153. https://doi.org/10.1046/j.0263-4929.2000.00303.x
- Williams, P. J., Barton, M. D., Johnson, D. A., Fontboté, L., De Haller, A., Mark, G., Oliver, N. H. S., & Marschik, R. (2005). Iron oxide copper–gold deposits: Geology, space–time distribution, and possible modes of origin. Economic Geology, 100th Anniversary Volume, 371–406. https://doi.org/10.5382/AV100.13
- Wilkinson, J. J. (2013). Triggers for the formation of porphyry ore deposits in magmatic arcs. Nature Geoscience, 6(11), 917–925. https://doi.org/10.1038/ngeo1940
- Wilson, A. J., Cooke, D. R., & Harper, B. L. (2003). The Ridgeway gold–copper deposit: A high-grade alkalic porphyry deposit in the Lachlan Fold Belt, New South Wales, Australia. Economic Geology, 98(8), 1637–1666. https://doi.org/10.2113/gsecongeo.98.8.1637
- Wormald, P. J. (2017). Mount Leyshon gold deposit. In G. N. Phillips (Ed.) Australian Ore Deposits (pp. 697–704). Monograph 32, Australasian Institute Mining Metallurgy.
- Yardley, B. W. D., & Graham, J. T. (2002). The origins of salinity in metamorphic fluids. Geofluids, 2(4), 249–256. https://doi.org/10.1046/j.1468-8123.2002.00042.x
