ABSTRACT
Clinical Relevance
This study found 0.7% olopatadine (Pataday Once Daily Relief Extra Strength) eye drops to provide better initial comfort than 0.3% pheniramine maleate/0.025% naphazoline hydrochloride (VISINE® Allergy Eye Relief Multi-Action Antihistamine and Redness Reliever) eye drops suggesting that patients may comply better with the Pataday than VISINE.
Background
To compare the ocular comfort at instillation of Pataday and VISINE allergy eye drops.
Methods
Minimally symptomatic participants were recruited based upon Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire scores (≤3 units); they also had minimal between-eye inter-ocular comfort differences as judged by visual analogue scale scores (VAS; ≤7 units). Baseline comfort was evaluated by eye with a VAS. One drop of Pataday or VISINE was then applied to the right eye with the alternative drop being applied to the left eye. The same VAS evaluated comfort by eye at drop instillation, and then at 30 seconds, 1 minute, and 2 minutes post-instillation. Drop experience was also evaluated with Likert questions. LogMAR visual acuities and bulbar conjunctival redness were evaluated pre- and post-drop instillation.
Results
A total of 159 participants were recruited (mean ± SD age = 26.2 ± 7.5). The VAS found that eyes treated with Pataday were significantly more comfortable at instillation than eyes treated with VISINE. Likert questions indicated that participants significantly preferred Pataday drops compared to the VISINE drops at instillation with regards to overall eye comfort, eye stinging, eye burning, and foreign body sensation. There were no between drop differences in visual acuity, though eyes treated with VISINE were less red than eyes treated with Pataday.
Conclusions
Topically applied Pataday drops were more comfortable than VISINE drops.
Introduction
Allergic conjunctivitis, which affects between 15% and 40% of the population, causes significant ocular itching and redness.Citation1,Citation2 Though usually self-limiting and non-sight threatening, allergic conjunctivitis can significantly detract from a patient’s quality of life. The most common types of allergic conjunctivitis are acute (seasonal) and chronic (perennial).Citation1 When an allergen contacts the conjunctival cells, an immunoglobulin (IgE) antibody-mediated type I hypersensitivity reaction is initiated.Citation2 The inflammatory cascade begins with mast cell degranulation and release of histamine and other inflammatory mediators that produce early- and late-phase responses. Histamine binds to receptors on nerve endings and vascular endothelial cells and primarily facilitates the expected early-phase allergic response of itching and vasodilation.Citation1–3 Chemical mediators continue the reaction into the late-phase 2–24 hours after exposure with continued vasodilation causing swelling and redness, while white blood cells such as neutrophils, eosinophils, and macrophages migrate towards the affected tissues. The second wave of ocular symptoms, if untreated, may result in more serious sequelae, such as keratitis, limbal infiltrates, and ulcers.Citation1–3
Fortunately, numerous topical options are available for allergic conjunctivitis relief. When prescribing therapy, considerations should include initial comfort upon instillation as poor topical drop comfort has the potential to negatively impact compliance as patients are unlikely to maintain consistent use of a drop that causes bothersome symptoms. Top current pharmacologic options indicated for allergic conjunctivitis include medications such as olopatadine (antihistamine with mast-cell stabiliser properties) and pheniramine maleate (antihistamine) paired with naphazoline hydrochloride (ocular decongestant). These topical medications provide more immediate relief than systemic medications, in part due to the drop flushing the ocular surface of allergens and by supplementing the tear film to minimise exposure.Citation4 Naphazoline hydrochloride is an alpha adrenergic vasoconstrictor that results in decreased conjunctival hyperaemia.Citation5 When combined with pheniramine maleate, a H1-histamine antagonist, a dual action antihistamine and redness-reliever is constructed.Citation5 Olopatadine is a dual action anti-histamine and mast cell stabiliser with high H1 receptor affinity for improved anti-itch relief and effective reduction in mast cell degranulation.Citation4,Citation6 The prolonged duration of action is due to the subsequent decrease in release of other cellular mediators that upregulate the inflammatory response. While olopatadine may provide immediate relief, the optimal performance is when used consistently prior to allergen exposure.Citation5,Citation7 Although olopatadine at 0.1% and 0.2% concentrations have been in the market for a while, 0.7% olopatadine (Pataday Once Daily Relief Extra Strength; Alcon, Fort Worth, TX, USA) is a relatively new treatment that has been introduced in select markets.
The comfort of this higher concentration of olopatadine has not been fully studied, and the literature currently lacks information on how this new formulation compares to 0.3% pheniramine maleate/0.025% naphazoline hydrochloride combination drops (VISINE® Allergy Eye Relief Multi-Action Antihistamine and Redness Reliever Eye Drops, Johnson & Johnson Vision, Jacksonville, FL, USA).Citation5 Thus, this prospective, randomised, clinical study was conducted to address this knowledge gap by comparing the perceived initial ocular comfort and safety of these two drops directly after instillation. This study is clinically important because understanding patient comfort differences between drops may assist the eye care professional in selecting a topical treatment that increases patient compliance while subsequently minimising chair time by reducing patient follow-ups.
Methods
Participants
This single visit, single-masked (participants) study was conducted at the University of Alabama at Birmingham School of Optometry (Birmingham, AL, USA) and the Southern College of Optometry (Memphis, TN, USA). Participants were screened prior to the study visit with an IRB approved phone survey to evaluate study eligibility. Participants who were 18 years or older with no upper age limit and who were minimally symptomatic as determined by the Standardised Patient Evaluation of Eye Dryness (SPEED) questionnaire were recruited for this study (≤3 units).Citation8,Citation9 Participants were excluded if they had a between eye comfort difference of greater than 7 as judged with a visual analogue scale (VAS; 0–100 scale created by investigators with 100 being most comfortable),Citation10 had worn rigid gas-permeable contact lenses, were currently using any topical eye medications, had a systemic health condition known to alter the tear film, had a history of viral eye disease, had a history of diabetes, had a history of ocular surgery, had a history of severe ocular trauma, had an active ocular infection or inflammation, were currently using isotretinoin-derivatives, or were pregnant or breastfeeding.Citation11 Soft contact lens wearers were allowed to participate, but they were not allowed to wear contact lenses on the day of the study visit. This was required to avoid any tear fluctuations that may occur from contact lens removal.
Randomisation and masking
Participants who met all the study criteria were enrolled, and they were randomised to apply Pataday or VISINE to their right eye. The other drop was applied to the left eye. Eye randomisation was completed with Research Electronic Data Capture (REDCap) in real time.Citation12,Citation13 The unmasked examiner applied each drop to the patient’s eyes in the predetermined order in quick succession. The participants themselves were masked to the contents of the eye drops by over-labelling of the drop bottles. Participants were not made aware of the bottle contents until after they fully completed the study.
Clinical testing
Clinical measurements were obtained from both eyes with tests being performed in the below order. All participant demographics were collected at this time (age, sex, race, contact lens use). Participants were asked to complete a comfort VAS by eye to determine their baseline comfort and to confirm that they met the study’s VAS comfort requirements. Participants were likewise asked to complete the SPEED questionnaire for their overall comfort to ensure that they were minimally symptomatic (≤3 units). Monocular baseline visual acuity was then collected with a Bailey-Lovie high-contrast (logMAR) chart at 6 m while wearing their habitual correction. Baseline nasal and temporal bulbar and limbal redresses were next evaluated with the Keratograph 5 M (Oculus, Arlington, WA, USA). Single images of each eye were taken under normal lighting conditions. Measurements were automatically calculated with the instrument’s embedded software by region. Nasal and temporal bulbar and limbal redresses were calculated with 0-4 scales in 0.01 increments with higher scores meaning worse redness.
The investigator then readied the participants by getting them comfortable in the exam chair and fully explaining the drop procedures, so they would be prepared to complete the VAS by eye directly after drop instillation. The investigator then accessed the randomisation schedule to determine which drop would be applied in the right eye. The right eye drop was then applied, and the other drop was applied to the left eye in quick succession. Participants were allowed to wear their spectacles before answering the VAS if needed. Participants were specifically asked to complete the VAS for each eye at instillation and then at 30 seconds, 1 minute, and 2 minutes; the time was monitored with a stopwatch by the investigator. After completion of the VAS, participants were asked to immediately complete a set of 0-4 (0 = Strong Preference for First Drop; 1 = Preference for First Drop; 2 = No Drop Preference; 3 = Preference for Second Drop; 4 = Strong Preference for Second Drop) Likert scale questions for drop comfort upon instillation, stinging upon instillation, burning upon instillation, vision upon instillation and foreign body sensation. Participant responses for each eye were transformed to the relevant eye drops prior to analysis. Ocular redness and visual acuity measurements were lastly repeated just like at the beginning of the study visit to determine if the drops affected these outcomes.
Sample size and statistical analysis
The primary outcome of this study was mean difference in VAS scores between drops at instillation. Pucker et al.’ s (n = 25) data suggests that the mean VAS score for a patient who has just applied a topical artificial tear is 75.4 ± 21.6 units with higher scores being more comfortable.Citation14 Papas et al.’ s data suggest that a minimally clinically important difference for a 0–100 ocular comfort numerical rating scale is 6.8 units.Citation10 Thus, 159 participants were needed to determine if there was no difference in ocular comfort between the two drops at instillation if one assumed independent responses between eyes (power = 80%; alpha = 0.05).
All data were analysed with SAS Version 9.4 (SAS; Cary, NC, USA). Percentages and chi-square tests were used to describe the Likert questions, while means and standard deviations were used to evaluate the continuous data. Data were analysed by eye. Data were determined to meet the assumptions for parametric statistics. Since each study participant served as their own comparison, paired t-tests were used for both inter- and intra-drop statistical comparisons at specific time-points. A mixed model was used to determine if there was an interaction between treatment and time when analysing the VAS ocular comfort data.
Results
This study recruited 159 participants (mean ± SD age = 26.2 ± 7.5) with 77% of them being female. The sample was 6% Hispanic with 8%, 5%, 83%, and 4% of the participants identifying as Asian, Black or African American, White, or more than one race, respectively. A total of 58% of the participants were current contact lens wearers outside of the study. No baseline between eye differences were detected for visual acuity or bulbar or limbal redness (; All p > 0.10). No adverse events were detected during the conduct of this study.
Table 1. Between drop signs comparisons before and after drop instillation.
This study primarily evaluated between drop ocular comfort in two different ways. The first method was by using a VAS to compare eye comfort at different time points (). While there was no difference in VAS scores at baseline prior to drop instillation (p = 0.30), the drops containing Pataday were significantly more comfortable at instillation, 30 seconds, 1 minute, and 2 minutes compared to VISINE (all p < 0.001; ). This study furthermore found that there was a significant interaction between treatment and time for the VAS comfort data indicating that eyes treated with Pataday approached baseline ocular comfort faster than eyes treated with VISINE (all p < 0.001). Neither Pataday (p = 0.38) nor VISINE (p = 0.85) treated eyes reached baseline comfort within the 2-minute time frame allotted by the study.
Table 2. Comfort visual analog scale by allergy drop and instillation time.
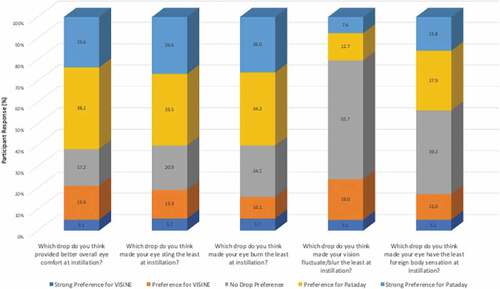
This study also compared between drop differences with an investigator-designed Likert questionnaire (). When the questions were groups as “strong preference” plus “preference” for Pataday, “no preference”, or “strong preference” plus “preference” for Visine, the participants significantly preferred Pataday over VISINE with regards to overall eye comfort at drop instillation, eye stinging at drop instillation, eye burning at drop instillation, and foreign body sensation at drop instillation (all p < 0.001; ). There was no significant between drop differences for vision fluctuation or blur at drop instillation (p = 0.71; ).
When comparing pre- and post-drop instillation differences within the same eye, there were no clinically meaningful difference in visual acuity for either drop; however, visual acuity post-drop instillation of Pataday was significantly worse compared to baseline by one acuity letter (0.02 logMAR; p < 0.001). Interestingly, instillation of either drop resulted in a significant reduction of bulbar and limbal redness (; all p < 0.001). When comparing post-drop instillation differences between the two drops, there were no significant differences in visual acuity between the two drops (p = 0.40). Alternatively, eyes treated with VISINE had significantly less bulbar and limbal redness compared to eyes treated with Pataday (; all p < 0.001), though the between drop differences noted are unlikely to represent clinically meaningful differences in ocular redness.
Discussion
Topical antihistamine efficacy has become an important research topic as new agents have become available in the market.Citation15 A well-formulated eyedrop, such as olopatadine’s dual mechanism mast-cell stabilizer/antihistamine property, should perform its primary function of symptom relief effectively without compromising tolerability.Citation15 The current study assessed differences between Pataday and Visine for ocular comfort at, and soon after, insertion.
This study specifically revealed that participants were significantly more likely to prefer Pataday containing drops over VISINE containing drops with regards to overall eye comfort, stinging, burning, and foreign body sensation. The participants of this study likewise indicated significantly better overall comfort with Pataday after instillation and at all timepoints up to 2 minutes post-drop instillation on the investigator designed VAS. However, only one of these timepoints (30 seconds post-drop instillation) is likely to be clinically relevant (mean difference >7 units).Citation10 This preference in the absence of adverse effect suggests that patients who are treated with Pataday may have better compliance and thus better relief of allergic conjunctivitis symptoms than patients who are treated with VISINE.
Participant preference for olopatadine has been previously shown in prior studies. Artal et al. compared 0.1% olopatadine hydrochloride and 0.05% ketotifen fumarate for “forced choice” comfort, and reported all participants preferred 0.1% olopatadine hydrochloride.Citation15 Furthermore, no participant reported burning on instillation with 0.1% olopatadine hydrochloride compared to 49% with 0.05% ketotifen fumarate.Citation15 Similar results to the current study have been reported in groups with allergic conjunctivitis, with a preference for 0.1% olopatadine hydrochloride compared to either 0.025% ketotifen or 0.05% ketotifen.Citation16,Citation17
Greiner and Udell subjected participants to an ocular allergen insult and used the Ocular Allergy Index (OAI) to compare 0.1% olopatadine and VISINE to a placebo at three time-points post allergen insult.Citation5 Participants reported a significantly lower OAI with either drop compared to placebo at 7-, 12-, and 20-minute post allergen insult, and a significantly lower OAI with VISINE compared to 0.1% olopatadine at 12- and 20-minute post allergen insult.Citation5 Although these findings suggest VISINE may be more effective than olopatadine at reducing short-term symptoms, the results themselves are not surprising given that olopatadine’s maximum efficacy is obtained during longer-term use.Citation5,Citation7 Like the current study, Greiner and Udell also reported a greater reduction in ocular redness with VISINE,Citation5 but it is unlikely that the current study’s redness differences are clinically meaningful since there is less than a 0.5-unit difference between drops.Citation18 Furthermore, the vast comfort preference for Pataday over VISINE in the current study is, arguably, far more important for long-term treatment compliance and overall clinical outcomes.
When prescribing topical therapy, considerations should include initial comfort upon instillation and minimum adverse reactions, in addition to convenient dosing, a rapid onset of symptom improvement, and sustained duration of action.Citation19 Poor topical drop comfort has the potential to negatively impact compliance as patients are unlikely to maintain consistent use of a drop that causes discomfort at instillation.Citation15 There is also support for better cost effectivity with treatment of Pataday because of its ocular comfort.Citation15 This is highlighted by Artal et al. who suggested a risk of increased patient costs in the form of additional office examinations or alternative therapy if an OTC drop is intolerable.Citation15 Therefore, better ocular comfort upon instillation, and not trading one symptom (itching) for an adverse event (burning or stinging) will limit unnecessary office examinations, patient cost, and healthcare dollars spent.Citation15
Prior to the present study, there has only been minimal research on Pataday comfort and especially how it compares to VISINE. As such, the results of this study are novel and needed. With that said, it is important to note that the participants in this study were in good ocular health with no anterior segment compromise or signs or symptoms of allergic conjunctivitis. This study characteristic was deemed necessary to be able to determine if there were small between drop difference. While these data provide a foundation for understanding how the study drop effects on visual acuity, ocular redness, and initial drop comfort, a future study is needed to determine how these results translate to patients with allergic conjunctivitis. An additional potential study limitation is related to the study’s sample size. The sample size for this study was initially calculated for the two eyes as being independent factors because the two eyes were thought to be able to have independent responses since the drops were being applied to the eyes nearly simultaneously. Nevertheless, this study was analysed in a paired eye fashion since the two eyes likely would have responded in a similar manner. Thus, the sample size of this study covered both situations. Nevertheless, the use of a paired analysis does result in an excess of sample size power (>95%), which may have resulted in an elevated type I error rate. Thus, differences were also evaluated for their clinical relevance, such as VAS score differences of 7 units, in addition to the statistical significance to help ensure that the conclusions of this study were clinically relevant.
Conclusions
Successful treatment of allergic conjunctivitis arises from both an effective and tolerable eye drop. Both Pataday and VISINE are topical treatments for allergic conjunctivitis. In this study, participants reported significantly better initial ocular comfort with Pataday compared VISINE. While both drops significantly reduced bulbar and limbal redness, the Likert questionnaires revealed participants had a “strong preference” or “preference” for Pataday over VISINE, and the VAS indicated that treatment with Pataday resulted in clinically meaningful better comfort at instillation than VISINE. This drop preference suggests that patients may have better treatment adherence with Pataday than VISINE. This trait may result in better drop acceptance, better treatment efficacy, and less costly medical care. However, further study of Pataday is needed to understand how patient reported outcomes are affected in patients with allergic conjunctivitis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abelson MB, Smith L, Chapin M. Ocular allergic disease: mechanisms, disease sub-types, treatment. Ocul Surf 2003; 1: 127–149.
- La Rosa M, Lionetti E, Reibaldi M, et al. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr 2013; 39: 18–25.
- Graziano FM, Stahl JL, Cook EB, et al. Conjunctival mast cells in ocular allergic disease. Allergy Asthma Proc 2001; 22: 121–126.
- Abelson MB, Mclaughlin JT, Gomes PJ. Antihistamines in ocular allergy: are they all created equal? Curr Allergy Asthma Rep 2011; 11: 205–211.
- Greiner J V, Udell IJ. A comparison of the clinical efficacy of pheniramine maleate/naphazoline hydrochloride ophthalmic solution and olopatadine hydrochloride ophthalmic solution in the conjunctival allergen challenge model. Clin Ther 2005; 27: 568–577.
- Kimchi N, Bielory L. The allergic eye: recommendations about pharmacotherapy and recent therapeutic agents. Curr Opin Allergy Clin Immunol 2020; 20: 414–420.
- Bielory L, Delgado L, Katelaris CH, et al. Special Series ICON Diagnosis and management of allergic conjunctivitis. 2020. doi:10.1016/j.anai.2019.11.014
- Asiedu K, Kyei S, Mensah SN, et al. Ocular Surface Disease Index (OSDI) versus the Standard Patient Evaluation of Eye Dryness (SPEED): a Study of a Nonclinical Sample. Cornea 2016; 35: 175–180.
- Pucker AD, Dougherty BE, Jones-Jordan LA, et al. Psychometric Analysis of the SPEED Questionnaire and CLDEQ-8. Invest Ophthalmol Visual Sci 2018; 59: 3307–3313.
- Papas EB, Keay L, Golebiowski B. Estimating a just-noticeable difference for ocular comfort in contact lens wearers. Invest Ophthalmol Vis Sci 2011; 52: 4390–4394.
- Sullivan BD, Crews LA, Sönmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea 2012;31:1000–1008.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381.
- Pucker AD, McGwin G, Franklin QX, et al. Application of systane complete for the treatment of contact lens discomfort. Cont Lens Anterior Eye 2020; 101399. DOI:10.1016/j.clae.2020.12.004
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 78: 64–65.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20: 1167–1173.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05 ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 78: 52–55.
- Pult H, Murphy PJ, Purslow C, et al. Limbal and bulbar hyperaemia in normal eyes. Ophthalmic Physiol Opt 2008; 28: 13–20.
- Greiner J V, Minno G. A placebo-controlled comparison of ketotifen fumarate and nedocromil sodium ophthalmic solutions for the prevention of ocular itching with the conjunctival allergen challenge model. Clin Ther 2003; 25: 1988–2005.