ABSTRACT
Clinical relevance
Traditionally, refraction is performed, and spectacles are manufactured in in 0.25D-steps. Trial and spectacle lenses manufactured in smaller increments may allow for a more accurate refraction and prescribed spectacles.
Background
To determine whether refraction in 0.05D-steps improves the proportion of eyes achieving achieve duochrome equality, and whether spectacles prescribed in 0.05D-steps offer any vision benefits, compared to 0.25D-steps.
Methods
Myopic young adults were enrolled into two prospective studies conducted at different sites. Study 1 comprised 66 participants (refracted under cycloplegia) while Study 2 comprised 51 participants (not cyclopleged). A standard refraction was performed in both studies and a trial frame and trial lenses were used to determine the spherical endpoint of duochrome equality (0.25D-steps first then 0.05D-steps). In Study 2, the cylindrical component was refined in 0.05D-steps before the spherical endpoint in 0.05D-steps. Monocular high-contrast-visual-acuity (HCVA) was measured while wearing the final refractions. Participants in Study 2 wore spectacles manufactured in 0.25D and 0.05D-steps for 7 days each in a randomized, double-masked study. Both spectacles appeared identical. Outcome measures assessed on dispensing and after 7 days of wear comprised monocular acuity-based measurements (HCVA, low-contrast-visual-acuity, vanishing-optotype-acuity, contrast-sensitivity) and subjective ratings. The Quality-of-Vision questionnaire and subjective preference were assessed after 7 days.
Results
Both studies showed a higher proportion of eyes achieved duochrome equality (P < 0.001) and better average monocular HCVA (P ≤ 0.006) in 0.05D-steps. Study 2 showed 0.05D-step spectacles provided better average results for all monocular acuity-based measurements (P < 0.006) and were preferred by 65% (P = 0.04) of participants after 7 days (P = 0.04). There were no differences between spectacles for any other measures (P > 0.1).
Conclusions
Refraction performed, and spectacles manufactured in 0.05D-steps for this study improved average acuity-based outcomes and were preferred by most participants to spectacles in traditional 0.25D-steps.
Introduction
Uncorrected refractive error is one of the most common causes of visual impairment worldwide.Citation1,Citation2 Spectacles are a simple and safe method of correcting refractive errors, and are traditionally manufactured in 0.25D-steps, where 0.25D of blur in the absence of accommodation approximates a one-line decrease on a standard visual acuity chart.Citation3 The most common reason reported for non-tolerance to spectacle corrections is related to the power of the lenses prescribed.Citation4
Tolerance on distance refractive power for single vision lenses is ±0.13D (ANSI Z80.1–2015), which is consistent with prescribing in 0.25D-steps. However, modern manufacturing methods permit much better precision to be achieved, e.g., BMF Precision Technology Inc. (Wuxi, China) manufacture trial and spectacle lenses to 0.05D-steps. Theoretically, refracting in 0.05D-steps should allow for a more accurate spectacle prescription. However, little information is available regarding the feasibility of refracting in less than 0.25D-steps.
The duochrome test has long been used for verification of the final refraction to enable precise determination of the spherical component by placing the least blurred image on the retina.Citation5 The test does not always result in equality between the red and green when traditional 0.25D increments are used,Citation6 suggesting some patients may not have a refraction and spectacles optimally correcting their refractive error. Further, smaller increments may allow for optimisation of the refraction and spectacles, but it is unknown whether this theoretical optimisation would result in any meaningful benefits compared to traditional refraction and spectacles.
The aims of this clinical investigation were twofold. First, to determine whether refraction in 0.05D-steps improves the proportion of those who achieve a subjective endpoint of red-green equality on the duochrome test compared to refraction in 0.25D-steps. Second, to assess whether spectacles manufactured in 0.05D-steps offer vision performance benefits compared to spectacles in 0.25D-steps.
Methods
All participants who enrolled into either study read and signed a written informed consent prior to the commencement of any study procedures, and both studies followed the tenets of the Declaration of Helsinki. The eligibility criteria for both studies are shown in . Both studies received approval from their respective university-based ethics committees and were registered on a clinical trial registry (Study 1: Chinese Clinical Trial Register [ChiCTR2100047074]; Study 2 Australian New Zealand Clinical Trials Registry [ACTRN12620000619943]).
Table 1. Study 1 and Study 2 eligibility criteria.
Trial lenses
A trial frame and trial lenses in 0.25D and 0.05D-steps were used to finalise monocular refraction in both studies. The same unmasked investigator performed refractions in 0.25D and 0.05D-steps (right eye first) on the same participant. Trial lenses in 0.25D and 0.05D-steps were both manufactured using the same crown glass material.
Study 1
There was only one visit in this prospective, single-centre study conducted at the Beijing Institute of Ophthalmology, Beijing Tongren Hospital (Beijing, China). Participants were cyclopleged with two drops of 1% cyclopentolate (Alcon, China) in each eye, instilled 10 min apart. A standard, monocular subjective refraction was performed using a trial frame about 30 min after the second drop. The spherical component of the subjective refraction was reduced (+0.75D added) monocularly, the cylindrical component was unchanged, and this was the starting point for determining the spherical component in both 0.25D and 0.05D-steps.
The spherical component was determined monocularly using a duochrome test, first in 0.25D-steps and then in 0.05D-steps. The same cylindrical component was used when determining both refractions. In each case, the final sphere was taken to be where equality was first achieved between the red and green letters of the duochrome test. If equality could not be achieved, the most negative power that did not cause a change from red to green was taken. High contrast visual acuity (HCVA) was measured monocularly in mesopic conditions using an MC-3 Mirror Chart Projector (Topcon, Japan) and recorded in logMAR, while wearing the final refractions.
Study 2
This was a prospective, single-centre, dispensing, randomised, double-masked, crossover study conducted at the School of Optometry and Vision Science, University of New South Wales (Sydney, Australia).
Study visits
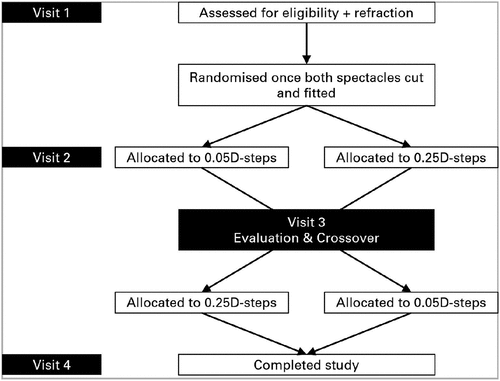
Participants attended a total of four study visits (). Refraction in 0.25D and 0.05D-steps were performed at Visit 1. Each participant chose one suitable spectacle frame and spectacles were manufactured in 0.25D-steps (control) and 0.05D-steps (test).
Participants returned for three more visits as shown in . The first pair of spectacles (Pair 1) were dispensed at Visit 2 and evaluated at Visit 3, and the second pair (Pair 2) were dispensed at Visit 3 and evaluated at Visit 4. The same assessments were performed with both spectacles at dispensing and evaluation visits, and both spectacles were worn for 7 days and a minimum of 8 h per day.
Spectacle lenses and frames
The control and test spectacle lenses were manufactured by BMF Precision Technology Inc. All lenses were custom-made in the same MR-8 material with an anti-reflective coating. The base curve and lens thickness for corresponding control and test lenses were also the same. Lens power was checked by the manufacturer using an LM-1800P digital lensmeter (Nidek, Japan), with increments set to 0.01D. The manufacturer set the tolerance for 0.05D-step lenses at ±0.02D.
Two identical frames (i.e., identical make, model, size, and colour) were ordered for each participant. Uncut control and test spectacle lenses were ordered for each participant, and the power was the same as the final refraction in 0.25D and 0.05D-steps, respectively. The same unmasked technician in Sydney cut and fitted control and test lenses into each of the frames and adjusted both pairs of spectacles for each participant. An LM-8 lensmeter (Topcon, Japan) was used to dot-up all lenses and the final horizontal and vertical centration distances were the same in both spectacles.
The same lensmeter was used to confirm the power of each control lens, and to measure the power of the corresponding test lens to make sure the difference compared to control was correct for both direction and approximate magnitude. Finished control and test spectacles were identical in appearance, and for all intents and purposes, the only difference between them was the difference in lens power obtained from the two refractions. The order of spectacles worn was randomly allocated according to a predetermined randomisation table.
Refraction
A phoropter was used to perform a standard, non-cyclopleged, monocular subjective refraction without binocular balancing at Visit 1. The cylindrical component used in 0.25D-steps refraction was the same as that obtained during subjective refraction. Prior to determining the spherical component in 0.05D-steps, the cylindrical component was further refined in 0.05D-steps using a 0.05D Jackson cross-cylinder. The cylindrical axes remained the same for both refractions. The procedures used to finalise the spherical component of both refractions (blurred starting point, order of refraction, and duochrome endpoint) were as described for Study 1. HCVA was measured monocularly in photopic conditions while wearing the final refractions.
Monocular visual acuity-based measurements
All acuity-based measurements were measured monocularly at dispensing and evaluation visits. HCVA and low contrast visual acuity (LCVA) were measured using a Thomson Test Chart Xpert 3Di (Thomson Software Solutions, UK), with 100% and 10% contrast levels, respectively, in photopic conditions and recorded in logMAR. The letter-by-letter termination rule, where each correctly identified letter scored 0.02 logMAR, was used to reduce test-retest variability values.Citation7,Citation8
Vanishing-optotype-acuity was measured using the Moorfields Acuity Chart (PA Vision, UK) at 4 metres, with a measurable acuity range of −0.30 to 1.00 logMAR. This chart incorporates a logMAR format, but a Sloan letter set is presented in a pseudo high-pass design.Citation9 Unlike conventional charts, the optotype “vanishes” after reaching the resolution threshold. This chart improves the test-retest variability of conventional acuity charts by filtering out low-frequency contents of letters and hence letters are much more equally discriminable.Citation9,Citation10
Contrast-sensitivity was measured using the Pelli-Robson chart (Precision Vision, USA) at 1 metre (spatial frequency~1 cycle per degree), which measures contrast-sensitivity ranging from 0 to 2.25 log units. Higher values represent better contrast-sensitivity.
Subjective measures
The frequency component of the Quality-of-Vision questionnaireCitation11 was administered at Visit 1 while wearing habitual spectacles, and at evaluation visits (Visits 3 and 4) after each lens type was worn for 7 days. Participants also reported average wearing time via recall at evaluation visits.
Vision-quality was rated for each lens type using a 100-millimetre visual analogue scale (0 to 100 in 1-point steps: 0=poor/worst imaginable and 100=excellent/perfect). At the dispensing visit, participants rated lens types for vision-quality (distance, near) and overall-vision-satisfaction. At the evaluation visit, participants rated lens types for vision-quality at daytime and night-time (distance, near), specific night-time tasks (driving, face recognition), and overall-vision-satisfaction.
Participants were asked to make a forced-choice preference between lens types at the crossover visit (Visit 3) and the final visit (Visit 4). At the crossover visit, participants were instructed to alternate wear between lens types, compare vision, and report their preference. At the final visit, participants reported their preference for either lens type after both had been worn for 7 days.
Sample size
Study 1
A minimum sample of 73 participants were required to detect a significant paired difference ± standard deviation (SD) in HCVA of 0.05 ± 0.15 logMAR between refractions at the 5% level of significance and 80% power. This study had no restrictions on best-corrected HCVA, but data were only included in the current analyses if HCVA was ≤0.00 logMAR for consistency with Study 2 (). This added restriction reduced the sample to 66, but also reduced the variability in HCVA measurements, and thus a lower SD of 0.10 logMAR was assumed to calculate the available power. The final sample of 66 provided >90% power to detect a paired difference in HCVA of 0.05 ± 0.10 logMAR.
Study 2
A minimum sample of 42 participants were required to complete the study to detect a significant paired difference of 8 ± 18 unitsCitation12 between control and test spectacles on a rating scale of 0–100 for subjective vision quality at the 5% level of significance and 80% power. A sample size of 42 also provided >85% power to detect a paired difference in visual acuity of 0.05 ± 0.10 logMAR. The study aimed to enrol at least 49 eligible participants to account for a 15% drop-out rate.
Statistical analysis
Data analyses were performed with SPSS version 28 (IBM, USA), and significance was set at 5%. Variables measured on a categorical scale were reported as percentages, and differences between 0.25D And 0.05D-steps were assessed with the χ2 test.
Quality-of-Vision scores were calculated in a denovo Rasch analysis and scaled in accordance with the original designCitation11 prior to analysis. Variables measured on an interval scale were summarised as means ± SD. Raw data measured on an interval scale were assessed by observing frequency histograms and Q-Q plots. If observed values deviated from expected normal values, an appropriate transformation was applied prior to statistical analysis.
A linear mixed model with subject random intercepts was used to compare variables measured on an interval scale. Each model included lens type (0.05D and 0.25D-steps) as a fixed factor, while the model assessing Quality-of-Vision scores also included habitual spectacles. The models assessing monocular acuity-based measurements while wearing spectacles included visit (dispensing and evaluation) as a fixed factor, and the interaction between lens type and visit. Significant interactions were assessed at each level of the interacting term and a Bonferroni correction was applied where applicable. Each model accounted for within-participant correlation from two-eyed data and repeated measurements.
Further analyses were performed to assess associations between preference and demographic factors, and to determine whether larger differences between refractions resulted in better outcomes. Participants from Study 2 were categorised based on spectacle preference at Visit 4 (0.05D-steps vs. 0.25D-steps) and the demographic factors assessed were age (years), myopia (M based on refraction in 0.05D-steps), and sex (male/female).
Participants from Study 2 were divided into one of three groups (category: untreated, treated, mixed) based on the absolute difference in the spherical-equivalent (M) between refractions in each eye as shown below:
Untreated: <0.10D in both eyes.
Treated: ≥0.10D in both eyes.
Mixed: <0.10D in one eye and ≥0.10D in the other eye.
Analyses were restricted to those variables which showed a significant difference between lens types in the main analyses. The final sample of 51 provided >85% power at the 5% level of significance to detect a difference in visual acuity of 0.05 ± 0.10 logMAR between groups.
Results
Demographics
Study 1
Ninety-eight participants were screened, 14 did not meet the eligibility criteria due to the refractive error being outside the allowable range, and 18 were excluded due to the HCVA criterion of Study 2 not being met. Sixty-six participants comprising 23 males and 43 females, with an average age of 24.1 ± 2.3 years (20–27 years), were included in these analyses.
Study 2
Seventy-two participants were screened and 19 did not meet the eligibility criteria, primarily due to refractive error being outside the allowable range. Spectacle lenses were ordered for 53 participants, but one voluntarily discontinued and data from a second was excluded from the analysis due to a lens fitting error. Fifty-one participants comprising 34 males and 17 females, with average age 26.8 ± 5.9 years (19–42 years), were included in these analyses.
One participant reported a mild headache while wearing 0.05D-steps that resolved within three days, which is less than the 7day average adaptation time reported for spectacles.Citation13 No adaptation problems were reported with 0.25D-steps.
Six participants reported an average wearing time of less than 8 h per day while wearing at least one pair of study spectacles. This was reported by three participants while wearing both pairs, one wearing 0.25D-steps, and two wearing 0.05D-steps.
Refraction and duochrome equality
Results are shown in . Refractions were decomposed into M and astigmatic vector components J0 and J45Citation14 prior to analysis.
Table 2. Study 1 and 2 results for M, J0, J45, high contrast visual acuity, and duochrome equality, when refracted in 0.25D-steps and 0.05D-steps. Data are presented as mean ± SD and percentages. A lower mean score in logMAR indicates a better result. Mean differences are also shown to three decimal points when means in 0.25D-steps and 0.05D-steps are the same to two decimal points. Analyses have not been performed on J0 and J45 in Study 1, as the cylindrical component was the same for both refractions. Significant P-values (P < 0.05) are shown in bold, italicised font.
For both studies, there was no difference between refractions for M (P > 0.1), the maximum absolute difference between refractions for the spherical component was 0.20D, and a significantly higher proportion of participant eyes achieved duochrome equality (P < 0.001) and better HCVA (P ≤ 0.006) with 0.05D-steps. For Study 2, there was no difference between refractions for J0 and J45 (P > 0.8).
Monocular visual acuity-based measurements
All results are shown in . For all acuity-based measurements, significantly better results were achieved in 0.05D-steps (P ≤ 0.006), there was no significant effect for visit (P > 0.1), nor a significant interaction between lens type and visit (P > 0.2).
Table 3. Monocular visual acuity-based measurements while wearing spectacles manufactured in 0.25D and 0.05D steps. Data are presented as mean ± SD. A lower mean score in mean logMAR and a higher mean score in log units indicate better results. Significant P-values (P < 0.05) are shown in bold, italicised font.
Subjective measures
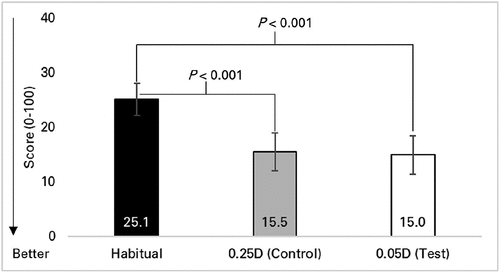
Quality-of-Vision scores are shown in . Frequency of symptoms were worse with habitual spectacles compared to both study lens types (P < 0.001) while there was no difference between study lens types (P > 0.99).
Figure 2. Quality-of-Vision scores while wearing habitual spectacles and spectacles manufactured in 0.25D and 0.05D steps. Mean scores are shown. A lower mean score indicates a better result (reduced symptom frequency). Only comparisons with significant differences (p < 0.05 on Bonferroni correction) are shown. Error bars = 95% confidence intervals.
Vision-quality ratings are shown in . All ratings were negatively skewed, and thus a logarithmic transformation was applied to each rating (ln[101-rating]) prior to statistical analysis. There were no significant differences in vision-quality ratings between lens types at dispensing (P > 0.41). Vision-quality (near) at daytime was significantly better after 7 days of wear while wearing 0.05D-steps (1.2 vs. 1.6 log units, P < 0.001), but the difference was negligible (mean difference = 0.5 units). There were no other differences after 7 days of wear (P > 0.1).
Table 4. Vision quality ratings on Day 1 (dispensing) and Day 7 (evaluation) with spectacles manufactured in 0.25D and 0.05D steps. Data are presented as mean ± SD units. A higher mean rating indicates a better result. F and p-values are calculated using log transformed data (ln[101 – rating]). Significant P-values (P < 0.05) are shown in bold, italicised font.
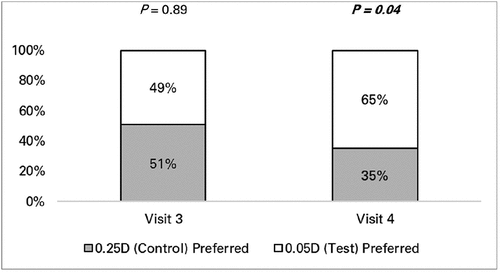
Subjective preference results are shown in . There was no difference in preference between lens types at Visit 3 crossover (P = 0.89). A significantly higher proportion of participants preferred 0.05D-step lenses at Visit 4 after completing 7 days of wear with both lens types (P = 0.04).
Further analyses
Participants who preferred 0.05D-steps were less myopic (M: −2.6 ± 1.1D vs. −3.6 ± 1.5D, P = 0.008). There were no differences for age (P = 0.60) or sex (P > 0.99).
Out of the 51 participants, 9 were categorised as untreated, 16 as treated, and 26 as mixed. Refraction in 0.05D-steps yielded 6 untreated participants with more plus in both eyes (maximum plus difference = 0.08D), 10 treated with more minus in both eyes, and 17 mixed with more minus in the eye with the larger refraction difference.
There was no difference in the distribution of preference between groups at Visit 4 (P = 0.66). Results for acuity-based measurements are shown in .
Figure 4. Differences in monocular visual acuity-based measurements between spectacles manufactured in 0.25D and 0.05D steps for the three groups untreated, treated, and mixed. Mean results are shown for A) high contrast visual acuity, B) low contrast visual acuity, C) vanishing-optotype-acuity, and D) contrast-sensitivity. Only comparisons with significant differences (p < 0.05 on Bonferroni correction) are shown. P-values above columns refer to between group differences and P-values below columns refer to within group differences. Error bars = 95% confidence intervals.
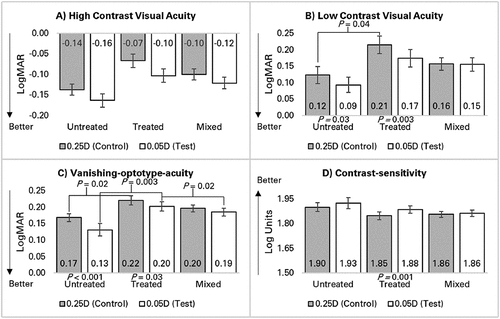
Differences in HCVA between lens types were independent of category (P = 0.29). LCVA with 0.25D-steps was significantly better in the untreated group compared to treated (P = 0.04) while there were no differences compared to mixed (P > 0.1). There was no difference in LCVA between groups with 0.05D-steps (P = 0.06).
The untreated and treated groups achieved better LCVA with 0.05D-steps (P ≤ 0.03) while there was no difference in mixed (P = 0.88). Vanishing-optotype-acuity with 0.25D-steps was significantly better in the untreated group compared to treated (P = 0.02) while there were no differences compared to mixed (P > 0.2). Vanishing-optotype-acuity with 0.05D-steps was significantly better in both the untreated and mixed groups compared to treated (P ≤ 0.02).
The untreated and treated groups achieved better vanishing-optotype-acuity with 0.05D-steps (P ≤ 0.03) while there was no difference in mixed (P = 0.053). There was no difference in contrast-sensitivity between groups for either 0.25D or 0.05D-steps (P > 0.1). The treated group achieved better contrast-sensitivity with 0.05D-steps (P = 0.001), while there was no difference in untreated or mixed (P > 0.07).
Discussion
The current studies are the first to provide evidence that the red-green duochrome test is sensitive for use as an endpoint when refracting in 0.05D-steps. The finer increments afforded by 0.05D-steps appear to have contributed to the higher proportion of eyes that achieved red-green equality compared to traditional refraction in 0.25D-steps, with or without cycloplegia (). Accommodation was also a factor, as demonstrated by the significantly higher proportion of non-cyclopleged eyes that achieved equality with either refraction in Study 2 compared to cyclopleged eyes in Study 1 (P ≤ 0.02 with Bonferroni correction).
This higher proportion of eyes achieving equality with accommodation suggests over-minusing may have occurred in Study 2. However, given there was no difference between refractions (), the extent of any over-minusing appears to be the same in both refractions, and thus unlikely to have influenced the final outcomes. The finding from Study 2 that not all eyes achieved equality when refracted in 0.05D-steps agrees with reports that duochrome may not work on everyone,Citation6 possibly due to some participants preferring one colour despite changes in refraction.Citation6
Both studies also showed better HCVA when refracted in 0.05D-steps (). However, the observed mean differences in HCVA (0.03 and 0.01 logMAR for Studies 1 and 2, respectively) may be dismissed as being clinically irrelevant. One of the premises of the current analyses is smaller increment steps may allow fine-tuning of the refraction endpoint. If true, then the difference in refractions may represent the difference in blur.
After extrapolating the relationship between blur and acuity (0.25D of blur approximates one line or 0.10 logMARCitation3 to smaller increments, the mean difference in M of 0.09D (Study 1) and 0.03D (Study 2) are expected to yield better HCVA in 0.05D-steps by 0.03 and 0.01 logMAR, respectively, which is the same as the observed differences. Thus, rather than being clinically irrelevant, the observed differences in HCVA between refractions are expected for the small difference in blur.
A small focal error of 0.25D has been reported to potentially affect acuity and comfortCitation15–17 The results from Study 2 suggest that even smaller differences in blur may affect acuity, with all four monocular acuity-based measurements significantly better when wearing spectacles in 0.05D-steps (). The small differences noted with refraction were also present in the spectacle prescriptions, and thus, mean differences in acuity-based measurements were also small.
Compared to habitual spectacles, study spectacles were based on the most current refraction and contained unblemished lenses with an anti-reflective coating. Thus, it is not surprising that the frequency of symptoms was worse with habitual spectacles compared to either study lens type (). It is also probable that habitual spectacles were used as a point of comparison for lens types, which may have contributed to low Quality-of-Vision scores and high vision-quality ratings with both lens types (), resulting in small differences that were either not significantCitation18 or not meaningful. By contrast, acuity-based measures do not appear to have been influenced by previous wearing experience.
Subjective preference was also not subject to previous wearing experience because it was forced choice for either lens type. Preference was no better than chance when spectacles were directly compared at Visit 3 (), which is not surprising given that participants had limited time for comparison, and both spectacles were identical in appearance, differing only slightly in prescription. Regardless, 0.05D-steps were preferred after participants were given the opportunity of wearing both spectacles for 7 days, presumably due to the slightly reduced blur with 0.05D-steps and better acuity-based results.
Aberrometers are currently used to provide a spectacle prescription to 0.01D-steps and manufacturers provide spectacle lenses to 0.01D-steps, but at least one retailer supplying this service and product provides a disclaimer regarding both the clinical utility and accuracy of lenses to 0.01D-steps.Citation19 Study 2 shows 0.05D-steps offer mild improvements in acuity-based measures and are preferred to traditional spectacles in 0.25D-steps.
Finalising the spherical component in 0.05D-steps using a trial frame and duochrome was also straightforward, with or without cycloplegia, and results in a higher proportion of eyes achieving duochrome equality compared to 0.25D-steps, albeit the procedure was more time consuming.
In terms of cost, single-vision lenses in 0.05D-steps are anticipated to be comparable to custom-made single-vision lenses. The further analyses showed some expected findings where a few acuity-based measurements were worse in the treated group compared to untreated when wearing spectacles in 0.25D-steps, and better in the treated group when wearing 0.05D-steps (). There were also some unexpected findings, comprising the differences in HCVA which were independent of category, spectacles in 0.05D-steps were better for a few measurements in the untreated group, and the untreated and mixed groups achieved better vanishing optotype-acuity while wearing 0.05D-steps than the treated group. Some of these unexpected findings may be explained by refraction in 0.05D-steps minimising blur even in the untreated group. However, the reasons for worse results in the treated group with 0.05D-steps compared to the other groups steps are unclear but are unlikely to be attributable to a statistical quirk as they occurred in three out of four acuity-based measurements.
Regardless, improvements for some acuity-based measurement in the untreated group with 0.05D-steps, in conjunction with the non-significant difference in distribution preference between groups suggest that any potential vision benefits with 0.05D-steps may not be limited to only those who demonstrate the greatest difference between refractions.
There are a few potential limitations of these analyses. The results for duochrome equality may have been biased because an unmasked investigator performed both refractions. Using two investigators, with one investigator masked to the previous refraction result, would yield a more robust methodology but the required resources were not available. The duochrome endpoint, however, was determined by the participant, who was masked to the type of refraction performed. Any potential bias was further minimised by using the same starting point of +0.75D blur for both refractions, and thus accommodation was controlled before finalising the spherical component with duochrome. Therefore, using unmasked investigators is unlikely to have affected the overall findings.
Refraction in 0.25D-steps was always performed first, which may have increased the proportion of eyes achieving equality in 0.05D-steps due to a learning effect. If a learning effect occurred, there should be a difference between eyes for duochrome equality because the right eye was always refracted first in both studies. There were no differences for duochrome equality between eyes in either study for refraction in 0.25D (P > 0.1) or 0.05D-steps (P > 0.09), and thus performing refraction in 0.25D-steps first is also unlikely to have affected the overall findings.
Binocular balancing was not performed, suggesting the final refraction may not have been controlled for accommodation.Citation6 Binocular balancing, however, is not necessary when there is no accommodation,Citation6 as occurred in Study 1 because participants were cyclopleged, nor when peripheral fusion is maintained,Citation6 as occurred in both studies because a trial frame was used to finalise refractions.
The confidence limit for the repeatability of conventional subjective refraction procedures has been reported at ±0.27D,Citation20 which is much higher than refraction in 0.05D-steps. However, repeatability has only been assessed in 0.25D-steps, not 0.05D-steps. The current analyses do not suggest any difficulty in refracting in 0.05D-steps, with similar refraction results recorded at two different sites, but repeatability in 0.05D-steps was not specifically assessed. The repeatability of refraction in 0.05D-steps may be assessed in a future study.
Other limitations include the restricted range of refractive errors evaluated and the inclusion of only healthy young adults achieving a minimum HCVA of 6/6 or better. It should be noted that spectacles in 0.05D-steps are not intended in those with a high refractive error because of the increased effect of vertex distance negating any potential benefit. Indeed, those who preferred 0.05D-steps after 7 days were, on average, less myopic than those who preferred 0.25D-steps. Spectacles in 0.05D-steps are also not intended for those with reduced acuities because finer increments in refractive correction would be of little benefit in these patients.
Conclusion
A higher proportion of participant eyes achieved duochrome equality and better average HCVA with refraction in 0.05D-steps, with or without cycloplegia. Spectacles in 0.05D-steps worn during this study offered better average HCVA, LCVA, vanishing-optotype-acuity, and contrast-sensitivity, and were preferred by most participants over spectacles in 0.25D-steps after 7 days of wear.
Acknowledgments
The authors thank AccelTech Technology Inc. (Singapore) for supplying lenses and frames used in this study. The authors also wish to acknowledge Ms Dubravka Huber as the unmasked technician, Dr Rajini Peguda, Dr Revathy Mani and Prof Konrad Pesudovs for their contributions to data collection (RP) and support with statistical analyses (RM and KP).
Disclosure statement
Two of the authors (DT and FC) are employed by nthalmic Pty Ltd, who supported this research. None of the authors or nthalmic Pty Ltd have a financial or proprietary interest in either trial lenses or spectacle lenses manufactured in 0.05D-steps.
Data availability statement
These data that support the findings of these analyses are available from the second author (DT: [email protected]) upon reasonable request.
Additional information
Funding
References
- Naidoo KS, Leasher J, Bourne RR et al. Global vision impairment and blindness due to uncorrected refractive error, 1990–2010. Optom Vis Sci 2016; 93: 227–234. doi:10.1097/OPX.0000000000000796.
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012; 96: 614–618. doi:10.1136/bjophthalmol-2011-300539.
- Smith G. Relation between spherical refractive error and visual acuity. Optom Vis Sci 1991; 68: 591–598. doi:10.1097/00006324-199108000-00004
- Freeman CE, Evans BJ. Investigation of the causes of non-tolerance to optometric prescriptions for spectacles. Ophthalmic Physiol Opt 2010; 30: 1–11. doi:10.1111/j.1475-1313.2009.00682.x.
- Gantz L, Schrader S, Ruben R et al. Can the red-green duochrome test be used prior to correcting the refractive cylinder component? Plos One 2015; 10: e0118874. doi:10.1371/journal.pone.0118874.
- Momeni-Moghaddam H, Goss DA. Comparison of four different binocular balancing techniques. Clin Exp Optom 2014; 97: 422–425. doi:10.1111/cxo.12198.
- Mimouni M, Shamir RR, Cohen AD et al. A comparison of different scoring terminations rules for visual acuity testing: from a computer simulation to a clinical study. Curr Eye Res 2019; 44: 790–795. doi:10.1080/02713683.2019.1589524.
- Arditi A, Cagenello R. On the statistical reliability of letter-chart visual acuity measurements. Invest Ophthalmol Visual Sci 1993; 34: 120–129.
- Shah N, Dakin SC, Redmond T et al. Vanishing optotype acuity: repeatability and effect of the number of alternatives. Ophthalmic Physiol Opt 2011; 31: 17–22. doi:10.1111/j.1475-1313.2010.00806.x.
- Shah N, Dakin SC, Whitaker HL et al. Effect of scoring and termination rules on test–retest variability of a novel high-pass letter acuity chart. Invest Ophthalmol Visual Sci 2014; 55: 1386–1392. doi:10.1167/iovs.13-13340.
- McAlinden C, Pesudovs K, Moore JE. The development of an instrument to measure quality of vision: the quality of vision (QoV) questionnaire. Invest Ophthalmol Visual Sci 2010; 51: 5537–5545. doi:10.1167/iovs.10-5341.
- Papas EB, Keay L, Golebiowski B. Estimating a just-noticeable difference for ocular comfort in contact lens wearers. Invest Ophthalmol Visual Sci 2011; 52: 4390–4394. doi:10.1167/iovs.10-7051.
- Strang NC, Gray LS, Winn B et al. Clinical evaluation of patient tolerance to autorefractor prescriptions. Clin Exp Optom 1998; 81: 112–118. doi:10.1111/j.1444-0938.1998.tb06729.x.
- Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci 1997; 74: 367–375. doi:10.1097/00006324-199706000-00019.
- Atchison DA, Schmid KL, Edwards KP et al. The effect of under and over refractive correction on visual performance and spectacle lens acceptance. Ophthalmic Physiol Opt 2001; 21: 255–261. doi:10.1046/j.1475-1313.2001.00588.x.
- Miller AD, Kris MJ, Griffiths AC. Effect of small focal errors on vision. Optom Vis Sci 1997; 74: 521–526. doi:10.1097/00006324-199707000-00020.
- Rosser DA, Murdoch IE, Cousens SN. The effect of optical defocus on the test–retest variability of visual acuity measurements. Invest Ophthalmol Visual Sci 2004; 45: 1076–1079. doi:10.1167/iovs.03-1320.
- Walline JJ, Bailey MD, Zadnik K. Vision-specific quality of life and modes of refractive error correction. Optom Vis Sci 2000; 77: 648–652. doi:10.1097/00006324-200012000-00011.
- OPSM Australia [Internet]. Clarifye. 2020 [accessed 2023 Jan 20]. https://www.opsm.com.au/clarifye-digital-eye-exam.
- Rosenfield M, Chiu NN. Repeatability of subjective and objective refraction. Optom Vis Sci 1995; 72: 577–579. doi:10.1097/00006324-199508000-00007.