ABSTRACT
Clinical relevance
Primary retinectomy in eyes not previously vitrectomized has been previously rarely performed in a minority of cases, unlike non-primary retinectomies in vitrectomized eyes.
Background
This paper aims to determine anatomical and functional outcomes of primary retinectomy, and to assess structural macular changes among successful cases.
Methods
In this retrospective multicentre cohort-study, 35 primary retinectomies in eyes undergoing initial vitrectomy for rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy C or D between 2014 and 2021 were included. The mean follow-up duration was 48 ± 59.24 months among successes and 46.54 ± 20.99 months among unsuccesses (p = 0.483).
Results
The anatomical success rate was 48.5% after one retinectomy and 60% after two retinectomies. Mean postoperative best corrected visual acuity (BCVA) was 1.85 ± 0.62 logMAR (6/425 Snellen equivalent). The difference from mean preoperative BCVA was not significant (p = 0.312). Final BCVA ≥ 6/60 was achieved in 17% of cases, and no cases gained ≥6/24. Final mean postoperative BCVA of successes was 1.69 ± 0.60 logMAR (6/294 Snellen equivalent) compared with 2.10 ± 0.57 logMAR (6/756 Snellen equivalent) of unsuccessful cases (p = 0.101). Post-operative macular optical coherence tomography was obtained from 95% of successes. Normal macular profile was found in 10% of cases, and the other cases demonstrated exudative maculopathy (60%), tractional maculopathy (20%) and macular atrophy (10%). Final BCVA was significantly higher in eyes with normal macular status compared to eyes with exudative maculopathy (p = 0.045) and macular atrophy (p = 0.025).
Conclusion
Primary retinectomy may be used for rhegmatogenous retinal detachment complicated with advanced proliferative vitreoretinopathy. Anatomical and functional outcome were inferior than non-primary retinectomies for grade C proliferative vitreoretinopathy. Functional outcome was influenced by macular status. Positive prognostic factors include final anatomical success and normal final macular anatomy.
Introduction
Retinectomy can be used in the management of rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy when other procedures were unsuccessful.Citation1 Retinectomy involves cutting and removing portions of retina to reduce persistent tractional forces that prevent the contact between neuroretina and retinal pigment epithelium.Citation2
Recently, pars plana vitrectomy has become the preferred choice for the management of rhegmatogenous retinal detachments and the use of peripheral retinectomies has gained favour in the management of severe grade C proliferative vitreoretinopathy with anterior retinal shortening.Citation3,Citation4 Primary retinectomy (PR) in eyes not previously vitrectomized has been previously performed in a minority of cases, and the anatomical and functional outcomes of PR have not been separately analysed.Citation3,Citation5,Citation6
Despite relatively high retinal reattachment rates following retinectomy, visual outcomes vary and are linked to the post-operative macular appearance. Macular optical coherence tomography (OCT) allows identification of subtle abnormalities, such as epiretinal membranes, cystoid macular oedema, macular atrophy and retinal pigment epithelium changes, which can explain the limited visual improvement in various cases.Citation7
The aim of this study is to report the anatomical and functional outcomes of a large series of PRs performed during the first pars plana vitrectomy for rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy at two tertiary referral vitreoretinal centres in the UK, and to evaluate structural macular changes in successful cases.
Methods
A retrospective observational multicentre chart-review study of consecutive patients who underwent PR for rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy C or D in eyes not previously vitrectomized was carried out in agreement with the tenets of the Declaration of Helsinki. The Institutional Review Boards of Royal Hallamshire Hospital, Sheffield, United Kingdom, ruled that approval was not required for this study given its retrospective nature. Electronic records of all patients who underwent PRs at the participating centres between 3 March 2014 and 12 November 2021 were retrospectively analysed. Cases were identified using the institutional ophthalmology electronic patient databases (Medisoft Ltd, Leeds, UK, OpenEyes™, Sunderland, UK).
All patients underwent a standard three-port 23-gauge or 25-gauge pars plana vitrectomy. Phacoemulsification or pars plana lensectomy were performed if the lens was found to obscure intraoperative fundus visualisation or impede adequate removal of the proliferative vitreoretinopathy membranes. A core vitrectomy was performed, followed by extensive trimming of the vitreous up to the vitreous base over 360°, generally using scleral indentation. Meticulous removal of all epiretinal and proliferative vitreoretinopathy membranes after staining them with membrane blue (DORC, Zuidland, the Netherlands) was performed with intraocular vitreoretinal forceps, starting from the posterior membranes and extending anteriorly up to the equator. Internal limiting membrane peeling at the posterior pole was performed at discretion of the surgeons.
Residual anterior traction and retinal detachment were evaluated after membranes peeling, and after membrane removal had been considered insufficient to relieve retinal tractions, a relaxing circumferential PR was performed. Endodiathermy was applied to the retina at the edge of the retinectomy and PR was performed with a vitreous cutter. The PR incision was placed peripherally along the posterior border of the vitreous base and was extended circumferentially as far as necessary to relieve all retinal tractions, care was taken to remove as much as possible of the peripheral non-functioning anterior retinal flap to minimise ischaemia, neovascularization and reproliferation. In some cases, intraoperative elevation of intraocular pressure was required to obtain haemostasis and minimise bleeding from the vessels at the edge of the PR. Subretinal membranes, if any, were peeled.
Upon completion of the PR, the retina was flattened with intravitreal injection of perfluoro-n-octane over the posterior pole until the level of the bubble extended up to the PR edge. Laser retinopexy was then applied in a confluent fashion in three to five rows along the posterior retinectomy edge, in some cases additional cryopexy was applied at the lateral edges of the PR. Perfluoro-n-octane was then exchanged for air, and air was exchanged for silicone oil 1300 or 5700 centistoke (RS-OIL 1300 centistoke, RS-OIL 5700 centistoke, Alchimia®, Padua, Italy) based on the preference of the surgeons.
Inclusion criteria were retinectomies performed in eyes with rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy C or D not previously vitrectomized (PRs) in which the retinectomy was performed as a primary adjunct procedure during the first vitrectomy. Eyes operated on with a retinectomy after a previous vitrectomy were excluded (non-primary retinectomies).
Characteristics recorded included patient demographics, laterality, pre- and post-operative best corrected visual acuity and intraocular pressure at last follow-up, pre-operative lens and macula status, grade and extent of proliferative vitreoretinopathy, surgical procedures performed intraoperatively including extent of the PR, intraocular tamponade used at the time of PR and the final intraocular tamponade status and whether anatomical reattachment was achieved. Primary anatomical success was defined as attached retina after PR in absence or presence of intraocular silicone oil tamponade, final anatomical success was defined as attached retina after the PR and a second retinectomy performed during a second procedure in absence or presence of intraocular silicone oil tamponade.
In patients who achieved anatomical success, macular spectral domain OCT (Spectralis HRA-OCT, Heidelberg Engineering GmbH, Heidelberg, Germany) was performed in order to evaluate the postoperative final macular appearance. Spectralis OCT scan patterns were used for all measurements and all eyes displayed a volume scan of 20 × 15 degrees in scan area with 19 B-scans spaced 242 μm apart. Quantitative assessment included the analysis of central foveal thickness and of ganglion cell-inner plexiform layer thickness.
Furthermore, analysis of all retinal layers of the macular OCT was performed to identify the location of cystoid macular oedema and to distinguish between exudative intraretinal cystoid spaces (exudative maculopathy – ), tractional intraretinal cystoid spaces (tractional maculopathy – ) and macular atrophy ().Citation8 Diagnostic characteristics of exudative maculopathy and tractional maculopathy have been previously described.Citation8
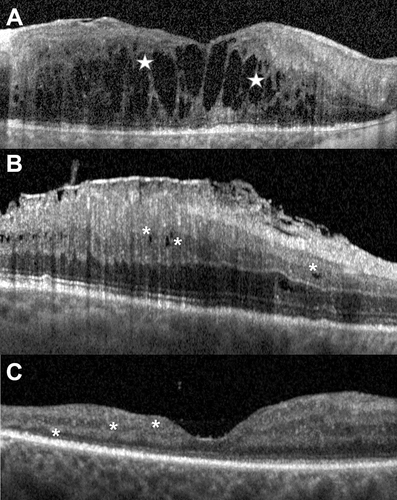
Figure 1. A: cross-sectional macular OCT horizontal scan of an eye with exudative maculopathy: the hyporeflective roundish cystoid spaces in the inner nuclear layer may merge (white stars) with those located in the Henle fibre layer-outer nuclear layer. B: cross-sectional macular OCT horizontal scan of an eye with tractional maculopathy: in this eye affected with a tractional epiretinal membrane, multiple hyporeflective round inner nuclear layer cystoid spaces (white asterisks) do not extend deeper in the retina or past the Henle fibre layer boundary. No outer retinal disruption is visible. C: cross-sectional macular OCT horizontal scan of an eye with macular atrophy. No intraretinal hyporeflective round cystoid spaces are found and all retinal layers of the macula are thinned (white asterisks).
Best corrected visual acuity was recorded at each visit and reported as Snellen Acuity, which was converted into logarithm of the minimal angle of resolution using conversion charts for statistical analysis.
Statistical analysis
Descriptive statistics were calculated for all variables of interest. Mean and standard deviation values were calculated for continuous variables, while frequency and percentage were calculated for categorical variables. Two-tailed Mann–Whitney U test, two-tailed Sign test and analysis of variance test (ANOVA) were used to compare the statistically significant difference in continuous variables among all subgroups. Pearson Correlation Coefficient was used to evaluate the correlation between final central foveal thickness and best corrected visual acuity. Outcome measures were anatomical success rate, final best corrected visual acuity and final macular status in patients with anatomical success at last follow-up exam.
Results
In total, 35 PRs in 35 eyes of 35 patients met the inclusion criteria and were analysed. Twenty-four were male and 11 were female, with a mean age of 52.74 ± 19.02 years (range 9–84 years). The 35 PRs were performed by 11 experienced vitreoretinal surgeons with standard three-port 23-gauge or 25-gauge pars plana vitrectomy in two tertiary referral vitreo-retinal units. All eyes had rhegmatogenous retinal detachment with established proliferative vitreoretinopathy (Grade C or D)Citation9 and had not undergone previous vitrectomy.
Postoperative follow-up ranged from 8 to 87 months after retinectomy (mean: 39.43 ± 22.19). The baseline characteristics of the patients before PR are summarised in . Intraoperative data is reported in . The distribution of retinectomy extension and pre-operative and post-operative intraocular pressures are summarised in .
Table 1. Pre-operative characteristics of 35 patients before primary retinectomy.
Table 2. Intraoperative data (35 procedures in 35 eyes).
Table 3. Distribution of retinectomy extension and preoperative and postoperative intraocular pressures.
Anatomical and functional outcomes
Post-operative data are synthetised in . Primary anatomical success was achieved in 17 cases after one PR (17/35, 48.5%). Of the 18 patients that re-detached, 10 underwent further retinectomy and secondary anatomical success was achieved in 4 of these giving a final anatomical success rate of 21/35 or 60%. At most recent follow-up of the 21 eyes that achieved final anatomical success, 6 (28%) patients had no intra-ocular tamponade while the majority (15, 72%) had a silicone oil in situ.
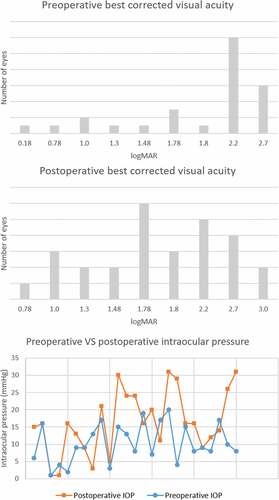
Figure 2. Distribution of pre-operative and post-operative best corrected visual acuity and intraocular pressure. Mean post-operative best corrected visual acuity was 1.85 ± 0.62 logarithm of the minimal angle of resolution (6/424 Snellen equivalent), the difference from mean pre-operative best corrected visual acuity (1.99 ± 0.61 logarithm of the minimal angle of resolution, 6/586 Snellen equivalent) was not significant (p = 0.312, two-tailed Mann–Whitney U test). Mean post-operative intraocular pressure was 14.94 ± 8.85 mmHg, the difference from mean pre-operative intraocular pressure (10.40 ± 5.57 mmHg) was significant P:= 0.016, two-tailed Mann–Whitney U test).
Silicone oil with 1300 centistoke viscosity was the most common tamponade of choice in these cases (53% versus 47% with 5700 centistoke). The duration of oil tamponade ranged between 4 and 87 months (mean 29.06 ± 21.62). The decision to keep oil in situ long-term (29 eyes) was made based on: (1) poor visual potential, 12 eyes (41%, best corrected visual acuities ranged between NPL and 6/362, mean 2.08 ± 0.42 logarithm of the minimal angle of resolution, 6/722 Snellen equivalent), (2) redetachment under oil tamponade, 5 eyes (17%), (3) anatomical success after a second retinectomy, 4 eyes (14%), (4) hypotony, 4 eyes (14%), and (5) relatively recent retinectomy (less than 12 months) in 4 eyes (14%).
Of the 14 patients which failed to achieve final anatomical success, 7 (50%) had 1300 centistoke silicone oil, 4 (29%) had 5700 centistoke silicone oil and 3 (21%) had no intraocular tamponade. Mean duration of follow-up was 48 ± 59.24 months among successful cases and 46.54 ± 20.99 months among unsuccessful cases; the difference was not significant (p = 0.483, two-tailed Mann–Whitney U test). Failure was due to retinal redetachment after a second retinectomy in 6 cases (43%), retinal redetachment under oil in 5 cases (36%) and redetachment after removal of silicone oil in 3 cases (21%).
The distribution of post-operative best corrected visual acuity and intraocular pressure are summarised in . The mean post-operative best corrected visual acuity was higher in cases that achieved anatomical success (1.69 ± 0.60, 6/294 Snellen equivalent) compared to those failing to achieve reattachment (2.10 ± 0.57, 6/756 Snellen equivalent); the difference was not significant (p = 0.101, two-tailed Mann–Whitney U test).
Reasons for worse final best corrected visual acuity compared to baseline were redetachment under oil (3 eyes, 43%), phthisis bulbi (2 eyes, 29%), exudative maculopathy (1 eye, 14%), macular atrophy (1 eyes, 14%). Eight cases out of 35 PRs underwent removal of silicone oil during the follow-up period, and 5/8 reached final anatomical success (62.5%). In the 5 cases where the retina remained attached after removal of silicone oil, final best corrected visual acuity remained stable in 1 eye (20%) and worsened in 4 cases (80%) compared to baseline.
Reasons for worse final best corrected visual acuity after successful removal of silicone oil were exudative maculopathy (1 case), tractional maculopathy (1 case), macular atrophy (1 case), phthisis bulbi (1 case). Mean post-operative intraocular pressure among successes was 15.8 ± 9.88 mmHg, and was 13.71 ± 6.95 mmHg among unsuccesses; the difference was not significant (p = 0.794, two-tailed Mann–Whitney U test).
No significant difference was recorded between final intraocular pressure among successes without silicone oil (11.8 ± 8.35 mmHg) and successes with silicone oil in situ (17.13 ± 9.99 mmHg, p = 0.528, two-tailed Mann–Whitney U test). After surgery, a higher proportion of eyes had raised intraocular pressure ≥22 mmHg (23%) compared to pre-operatively (0%).
Post-operative data and the distribution of post-operative best corrected visual acuity and intraocular pressure are summarised in .
Macular status
Post-operative macular OCT scans were available for 20 eyes of 20 patients out of the 21 successes (95%). Four (20%) had no intraocular tamponade and 16 (80%) had oil in situ at the time of the OCT. The post-operative macular findings after PRs are summarised in . Normal macular profile was found in 10% (2/20 – mean central foveal thickness 243.50 ± 5.50 μm, mean ganglion cell-inner plexiform layer thickness 81.65 ± 1.15 μm) of eyes, whereas an abnormal macular appearance was found in 90% (18/20) of eyes at last follow-up.
Table 4. Macular changes in 20 primary retinectomies and mean central foveal thickness.
Exudative maculopathy was diagnosed in 12 out of 20 eyes (60% – mean central foveal thickness 664.41 ± 234.39 μm, mean ganglion cell-inner plexiform layer thickness 192.90 ± 78.40 μm), whereas macular atrophy was observed in 2 out of 20 eyes (10% – mean central foveal thickness 129 ± 6 μm, mean ganglion cell-inner plexiform layer thickness 42.6 ± 0.9 μm). Tractional maculopathy was present in 4 out of 20 eyes (20% – mean central foveal thickness 376.75 ± 37.96 μm, mean ganglion cell-inner plexiform layer thickness 122.87 ± 5.42 μm).
Mean best corrected visual acuity was reduced in the three abnormal macular subgroups. Eyes with normal macular profile exhibited statistically significant better best corrected visual acuity (0.98 ± 0.66 logarithm of the minimal angle of resolution, 6/58 Snellen Equivalent) compared to the group with exudative maculopathy (1.69 ± 0.59 logarithm of the minimal angle of resolution, 6/294 Snellen Equivalent, p = 0.045, two-tailed Sign test), and with macular atrophy (mean best corrected visual acuity 2.16 ± 0.69 logarithm of the minimal angle of resolution, 6/868 Snellen Equivalent, p = 0.025, two-tailed Sign test); however, the difference was not statistically significant compared to the group with tractional maculopathy (mean best corrected visual acuity 1.60 ± 0.60 logarithm of the minimal angle of resolution, 6/240 Snellen Equivalent, p = 0.083, two-tailed Sign test). There was no statistically significant difference in final best corrected visual acuity in the eyes with exudative maculopathy, tractional maculopathy and macular atrophy (p = 0.46, analysis of variance test ANOVA).
Central foveal thickness was significantly lower in eyes with normal macular status when compared to exudative maculopathy (p = 0.008, two-tailed Sign test) and tractional maculopathy (p = 0.025, two-tailed Sign test), whereas central foveal thickness in the macular atrophy group was significantly lower when compared to eyes with normal macular profile (p = 0.024, two-tailed Sign test).
There was no statistically significant bivariate linear relationship between best corrected visual acuity and central foveal thickness (p = 0.211, r = −0.292, Pearson Correlation Coefficient) and ganglion cell-inner plexiform layer thickness (p = 0.772, r = −0.069, Pearson Correlation Coefficient) in all 20 eyes, or when considering the individual subgroups with exudative maculopathy (p = 0.256, r = −0.355 – p = 0.745, r = −0.105), macular atrophy and tractional maculopathy (p = 0.368, r = −0.452 – p = 0.259, r = 0.549).
Discussion
Pars plana vitrectomy is an established treatment for eyes with rhegmatogenous retinal detachment (including challenging cases complicated by proliferative vitreoretinopathy), with vitreoretinal interface syndrome and with aqueous misdirection,Citation4,Citation10–12 but less so for different conditions.Citation13–17 Recently pars plana vitrectomy has become the preferred technique for the management of complex
rhegmatogenous retinal detachments, and retinectomy is useful for complex retinal detachments with proliferative vitreoretinopathy, tractional retinal detachments or when different procedures such as scleral buckling and membrane peeling have failed.Citation18–21
Previous studies suggest retinectomies in cases of complex proliferative vitreoretinopathy-related rhegmatogenous retinal detachments, ocular traumas, tractional retinal detachments, where the risk of failure is higher.Citation4,Citation19,Citation22–24
This study shows that anterior proliferative vitreoretinopathy-C was the main indication for PRs (57% of cases) followed by proliferative vitreoretinopathy-D (23%). Positive prognostic factors for improved best corrected visual acuity in these eyes include anatomical success at last follow-up and normal macular status on OCT. Unlike previous reports,Citation4 a statistically significant bivariate linear relationship between final best corrected visual acuity and final central foveal thickness was not found.
An overall anatomical success rate of 48.5% after one PR and a final reattachment rate of 60% after two procedures is reported here. These results suggest that a final retinal reattachment via PR may be more difficult in eyes with rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy-C or D (for whom non-primary retinectomies have shown superior results).Citation4 As well, the present results are in line with previous series that reported final anatomical success rates of 47–85%.Citation19,Citation20,Citation23–25
The final best corrected visual acuity improved in 15 eyes (43%), was stable in 12 eyes (34%) and worsened in 8 eyes (23%). Best corrected visual acuity of 6/60 or better was achieved in 17% of cases, and no cases gained 6/24 or better. This concurs with previous literature showing that final vision was improved compared to baseline in 20–89%, with 6/60 or better vision achieved in 10–51%.Citation20,Citation21,Citation23–26
No cases in this study achieved a final best corrected visual acuity of 6/24 or better, unlike previous studies,Citation19 and final best corrected visual acuities were not significantly higher compared to baseline best corrected visual acuities. These results may be due to the original indication for PR (as eyes with advanced pathology), and to the high rate (86% – 18/21) of macular changes (exudative maculopathy, macular atrophy or tractional maculopathy) among successful cases. These data seem to suggest that surgery in advanced proliferative vitreoretinopathy remains worthwhile but case selection and improvements in surgical technique are required.
Various previous studies have reported that best corrected visual acuity increases after removal of silicone oil however not many have specifically investigated the effect of removal of silicone oil following a retinectomy.Citation2,Citation19,Citation26–28 These studies also either failed to separate patients with silicone oil at the final follow-up when reporting outcomes.Citation26,Citation27,Citation29 Furthermore, not all studies had follow-up durations as long as this study.Citation2,Citation30
The data reported here suggest that removal of silicone oil after a PR for advanced proliferative vitreoretinopathy in eyes which reached anatomical success may not improve final best corrected visual acuity, similarly from previous studies.Citation2 It is, however, known that removal of silicone oil carries a high risk of re-detachment (37.5% in this study).Citation4,Citation7,Citation19
Previous series have evaluated postoperative structural and functional macular integrity after surgery for retinal detachment or vitreoretinal interface syndromes,Citation10,Citation31,Citation32 but very few have looked at macular status after successful retinectomies.Citation4 Most of these studies had small sample sizes.Citation7,Citation33,Citation34 High reattachment rates, defining the anatomic success, are in contrast to the functional outcome, which relates to pre-operative and post-operative macular integrity.
In this series, a post-operative normal macular profile was found in 10% of successes. The remaining eyes showed exudative maculopathy (60%), tractional maculopathy (20%) and macular atrophy (10%), unlike previous reports.Citation7,Citation33,Citation34 Post-operative best corrected visual acuity was significantly higher when macular status was normal compared to eyes with exudative maculopathy (p = 0.045) or macular atrophy (p = 0.025). Statistical significance was not demonstrated when best corrected visual acuity was compared with eyes with tractional maculopathy (p = 0.083).
The higher best corrected visual acuity found in tractional maculopathy group despite anatomical changes is difficult to explain. An association between verticalised Henle fibres and lower best corrected visual acuity has been suggested.Citation35 The relatively lower central foveal thickness in the tractional maculopathy group may reflect reduced tractional damage of the Müller cell and of photoreceptor and therefore a better final best corrected visual acuity compared to the exudative maculopathy group.
Interestingly, two eyes of this series underwent internal limiting membrane peeling and silicone oil 5700 centistoke endotamponade during their PR surgery, with the first undergoing removal of silicone oil 14 months later and developing macular atrophy within one month, and the second developing exudative maculopathy under silicone oil tamponade. These results and the fact that 11 (92%) out of 12 eyes which developed exudative maculopathy and the other case which developed macular atrophy had silicone oil endotamponade at last follow up visit are in line with previous literature which showed two distinct patterns of macular thinning and thickening during silicone oil endotamponade.Citation36
Also, none of the four eyes which developed tractional maculopathy in this study underwent internal limiting membrane peeling during their PR surgery. Although the internal limiting membrane does not have natural contractile properties, it may serve as a scaffold for contractile tissue to exert tangential traction on the umbo.Citation37
Central foveal thickness was significantly lower in eyes with normal macular status when compared to eyes with exudative maculopathy and tractional maculopathy; however, central foveal thickness in the macular atrophy group was significantly lower when compared to eyes with normal macular status. A statistically significant correlation with final best corrected visual acuity was not found, unlike previous literature.Citation4 Also, ganglion cell-inner plexiform layer thickness was not significantly associated with final best corrected visual acuity. These results may be due to the advanced retinal pathology in these eyes.
Mean post-operative intraocular pressure at last follow-up was 14.94 ± 8.85 mmHg, the difference with mean pre-operative intraocular pressure (10.40 ± 5.57 mmHg) was statistically significant (p = 0.016). Interestingly, only 26% experienced a reduction in baseline intraocular pressure after PR, whereas intraocular pressure was stable or increased postoperatively in 54% of this series, which is different from previous series.Citation4,Citation38 The successful retinal reattachment and the silicone oil left in situ in the majority of the eyes (72%) may explain the postoperative increase of intraocular pressure from baseline in 46% of this series.
This study is thought to be the first to evaluate postoperative macular status after successful PRs in a large cohort of non-previously vitrectomized eyes and with a long follow-up. Only three studies included retinectomy cases to investigate macular profile in patients after vitrectomy for retinal detachment complicated by proliferative vitreoretinopathyCitation4,Citation7,Citation33; however, the series of patients undergoing retinectomy were smaller, follow-up duration was shorter,Citation7 retinectomy cases were not analysed separately,Citation33 or were performed in previously vitrectomized eyes.Citation4 Unlike previous reports,Citation7,Citation33 this study highlighted higher incidences of post-retinectomy exudative maculopathy (60%) and tractional maculopathy (20%), and a significant proportion of eyes with macular atrophy (10%).
This study has various limitations, including its retrospective design, which may cause ascertainment bias. Cases were operated on by different surgeons who may differ in clinical approach and surgical technique. The conversion of best corrected visual acuity from Snellen to logarithm of the minimal angle of resolution could cause inaccuracies as the relationships between the two measures are not directly proportional.Citation39,Citation40 When diagnosed, exudative maculopathy was treated with different combinations of intensive topical steroids and non-steroidal anti-inflammatory drugs according to the preferences of the surgeons, and intravitreal/periocular injections were not performed in this series, unlike previous literature.Citation41
The lack of statistically significant association between final best corrected visual acuity and final central foveal thickness may be related to the relatively small sample size of participating patients. The statistical power of the study which were not predetermined. Nevertheless, the strengths of this study include a relatively large study sample size for an uncommon and severe complication of retinal detachment and long follow-up duration with robust quantitative and statistical analysis.
In conclusion, anatomical and functional outcomes following PRs are lower compared to non-primary retinectomies for proliferative vitreoretinopathy-C but may be satisfactory. Retinal re-detachment after a second retinectomy or secondary to proliferative vitreoretinopathy development under silicone oil endotamponade or after planned removal of silicone oil were the main reasons for anatomical failure, and recognition of high-risk and early proliferative vitreoretinopathy cases, together with prompt and appropriate surgery can potentially improve outcomes.
Positive prognostic visual factors include anatomical success and normal final macular status, whereas no correlation was found between lower central foveal thickness and final best corrected visual acuity. The variety of macular OCT appearance following PR reflects functional outcomes. Further studies are required to explain the pathophysiology of exudative maculopathy and tractional maculopathy in these eyes.
Importantly, removal of silicone oil was not associated with improved final best corrected visual acuity and carries a high risk of re-detachment, with over a third of cases reported here redetaching.
Acknowledgements
The authors wish to thank Dr Vlassis Grigoropoulos MD for contributing in part of the raw data for the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Machemer, R. Retinotomy. Am J Ophthalmol 1981; 92: 768–774.
- Wong R, De Luca M, Shunmugam M, et al. Visual outcome after removal of silicone oil in patients undergoing retinectomy for complex retinal detachment. Int J Ophthalmol 2016; 9: 108–110.
- Tan HS, Mura M, Lesnik Oberstein SY, et al. Primary retinectomy in proliferative vitreoretinopathy. Am J Ophthalmol 2010; 149: 447–452.
- Grassi P, Melville S, Hariprashad A, et al. Structural and functional macular changes after retinectomy for retinal detachment complicated by proliferative vitreoretinopathy. Retina 2021; 41: 2531–2539.
- Quiram PA, Gonzales CR, Hu W, et al. Outcomes of vitrectomy with inferior retinectomy in patients with recurrent rhegmatogenous retinal detachments and proliferative vitreoretinopathy. Ophthalmol 2006; 113: 2041–2047.
- Hocaoglu M, Karacorlu M, Giray Ersoz M, et al. Retinotomy and retinectomy for anterior inferior proliferative vitreoretinopathy: can visual outcome be improved? Eur J Ophthalmol 2021: 11206721211012848. 10.1177/11206721211012848
- Stopa M, Kociecki J. Anatomy and function of the macula in patients after retinectomy for retinal detachment complicated by proliferative vitreoretinopathy. Eur J Ophthalmol 2011; 21: 468–472.
- Govetto A, Sarraf D, Hubschman JP, et al. Distinctive mechanisms and patterns of exudative versus tractional intraretinal cystoid spaces as seen with multimodal imaging. Am J Ophthalmol 2020; 212: 43–56.
- Machemer R, Aaberg TM, Freeman HM, et al. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 1991; 112: 159–165.
- Romano MR, Cennamo G, Grassi P, et al. Changes in macular pigment optical density after membrane peeling. PLoS One 2018; 13: e0197034. eCollection 2018.
- Foo E, Grassi P, Spiteri-Cornish K. Early vitrectomy in eyes with non-diabetic vitreous hemorrhage. Ther Adv Ophthalmol 2022; 14: 25158414221090099.
- Low S, Mohamed R, Davidson A, et al. A new paradigm for delivering personalised care: integrating genetics with surgical interventions in BEST1 mutations. Eye (Lond) 2020; 34: 577–583.
- Grassi P, Mudhar HS, Cornish KS. Traumatic suprachoroidal dislocation of crystalline lens. Ophthalmol Retina 2020; 4: 856–858.
- Grassi P, Chawla A, Rundle P. Vitrectomy for vitreous haemorrhage from vitreous operculum over retinocytoma. Ophthalmol Retina 2019; 3: 1055.
- Grassi P. photodynamic therapy for vasoproliferative tumour associated with retinitis pigmentosa and usher syndrome type 2. Clin Exp Optom 2022; 105: 91–93.
- Grassi P, Alexander P. Spontaneous resolution of serous macular detachment and outer retinal defect in optic disc pit maculopathy. Clin Exp Optom 2020; 103: 384–385.
- Grassi P, Wang H, Spiteri-Cornish K, et al. The effect of COVID-19 on the vitreoretinal service of a tertiary referral centre: real-world experience from the royal hallamshire hospital. Eur J Ophthalmol 2022; 32: NP335–NP338.
- Beuste T, Rebollo O, Parrat E, et al. Recurrences of retinal detachment after retinectomy: causes and outcomes. Retina 2020; 40: 1315–1324.
- Grigoropoulos VG, Benson S, Bunce C, et al. Functional outcome and prognostic factors in 304 eyes managed by retinectomy. Graefes Arch Clin Exp Ophthalmol 2007; 245: 641–649.
- Federman JL, Eagle RC Jr. Extensive peripheral retinectomy combined with posterior 360 degrees retinotomy for retinal reattachment in advanced proliferative vitreoretinopathy cases. Ophthalmol 1990; 97: 1305–1320.
- Iverson DA, Ward TG, Blumenkranz MS. Indications and results of relaxing retinotomy. Ophthalmol 1990; 97: 1298–1304.
- Lim AK, Alexander SM, Lim KS. Combined large radial retinotomy and circumferential retinectomy in the management of advanced proliferative vitreoretinopathy. Retina 2009; 29: 112–116.
- Faude F, Lambert A, Wiedemann P. 360 degrees retinectomy in severe anterior PVR and PDR. Int Ophthalmol 1998; 22: 119–123.
- Han DP, Rychwalski PJ, Mieler WF, et al. Management of complex retinal detachment with combined relaxing retinotomy and intravitreal perfluoro-n-octane injection. Am J Ophthalmol 1994; 118: 24–32.
- Morse LS, McCuen BW, Machemer R. Relaxing retinotomies. Analysis of anatomic and visual results. Ophthalmol 1990; 97: 642–647.
- Nagpal MP, Videkar RP, Nagpal KM. Factors having implications on re-retinal detachments after silicone oil removal. Indian J Ophthalmol 2013; 61: 534.
- Flaxel CJ, Mitchell SM, Aylward GW. Visual outcome after silicone oil removal and recurrent retinal detachment repair. Eye (Lond) 2000; 14: 834–838.
- Falkner CI, Binder S, Kruger A. Outcome after silicone oil removal. Br J Ophthalmol 2001; 85: 1324–1327.
- de Silva Dj, Kwan A, Bunce C, et al. Predicting visual outcome following retinectomy for retinal detachment. Br J Ophthalmol 2008; 92: 954–958.
- Han DP, Lewis MT, Kuhn EM, et al. Relaxing retinotomies and retinectomies. Surgical results and predictors of visual outcome. Arch Ophthalmol 1990; 108: 694–697.
- Abraham JR, Srivastava SK, Reese JL, et al. Intraoperative OCT features and postoperative ellipsoid mapping in primary macula-involving retinal detachments from the PIONEER study. Ophthalmol Retina 2019; 3: 252–257. Epub 2018 Oct 18.
- Poulsen CD, Petersen MP, Green A, et al. Fundus autofluorescence and spectral domain optical coherence tomography as predictors for long-term functional outcome in rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol 2019; 257: 715–723. doi: 10.1007/s00417-018-04222-w. Epub 2019 Jan 7.
- Kiss CG, Richter-Muksch S, Sacu S, et al. Anatomy and function of the macula after surgery for retinal detachment complicated by proliferative vitreoretinopathy. Am J Ophthalmol 2007; 144: 872–877.
- Benson SE, Grigoropoulos V, Schlottmann PG, et al. Analysis of the macula with optical coherence tomography after successful surgery for proliferative vitreoretinopathy. Arch Ophthalmol 2005; 123: 1651–1656.
- Govetto A, Hubschman JP, Sarraf D, et al. The role of Muller cells in tractional macular disorders: an optical coherence tomography study and physical model of mechanical force transmission. Br J Ophthalmol 2020; 104: 466–472.
- Lo DM, Flaxel CJ, Fawzi AA. Macular effects of silicone oil tamponade: optical coherence tomography findings during and after silicone oil removal. Curr Eye Res 2017; 42: 98–103.
- Ozturk Y, Ağın A, Gencoglu AY, et al. Comparison of intraocular tamponade in patients with peripheral tear-induced retinal detachment and coexisting macular hole without high myopia. Klin Monbl Augenheilkd 2023; 240: 897–902.
- Joussen AM, Walter P, Jonescu-Cuypers CP, et al. Retinectomy for treatment of intractable glaucoma: long term results. Br J Ophthalmol 2003; 87: 1094–1102.
- Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina 2010; 30: 1046–1050.
- Vesely P, Synek S. Repeatability and reliability of the visual acuity examination on logMAR ETDRS and Snellen chart. Cesk Slov Oftalmol 2012; 68: 71–75.
- Banerjee PJ, Quartilho A, Bunce C, et al. Slow-release dexamethasone in proliferative vitreoretinopathy: a prospective, randomized controlled clinical trial. Ophthalmol 2017; 124: 757–767. doi: 10.1016/j.ophtha.2017.01.021. Epub 2017 Feb 23.
