ABSTRACT
Obstetric antiphospholipid syndrome (OAPS) is an autoimmune disorder with severe life-threatening complications shown during pregnancy. It has been reported that the increase in CD16+CD56dim natural killer (NK) cells in peripheral blood are risk factors for recurrent miscarriages, but this expression of CD16+CD56dim NK cells in OAPS patients has not been reported, and the mechanism is not clearly illustrated. In this study, we compared the distributional profiles of different NK cell subsets and the expressions of NK cell-activating receptors in peripheral blood of patients with OAPS and healthy women. Our results showed significantly increased NKG2A−NKG2D+ subset and decreased NKG2A+NKG2D− subset in CD3− CD16+CD56dim NK cells, CD3−CD16−CD56bright NK cells and CD56+T cells in OAPS patients compared with those in healthy control women. The CD27−CD11b+ subset significantly increased in CD3−CD16+CD56dim NK cells in OAPS patients compared with those in healthy control women. In addition, the NKG2A−NKG2D+ subset in CD3−CD16+CD56dim NK subset in triple positivity was higher than single positivity OAPS patients. At the optimal diagnostic threshold established by ROC analysis, using the cut-off of NKG2A−NKG2D+ and CD27−CD11b+ subset in CD3−CD16+CD56dim NK cells is 10.10% and 92.75%, the sensitivity of NKG2A−NKG2D+ and CD27−CD11b+ to detect patients with OAPS compared with healthy control results was 94.1% and 60.8%, and specificity was 84.2% and 89.5%, respectively, with an area under the curve (AUC) of 0.903 and 0.829, respectively. The NKG2A−NKG2D+ subset in CD3−CD16+CD56dim NK cells was positively correlated with the antiphospholipid antibodies lg anti-aCL IgG, lg anti-aCL IgM, lg anti-aCL IgA, lg anti-β2GP1 IgM and Complement 4(C4), while the CD27+CD11b+ subset in CD3−CD16+CD56dim NK cells was correlated with lg anti-β2GP1 IgG and lg anti-β2GP1 IgA. These results suggested that the NK cytotoxic function enhanced in OAPS patients and unbalanced of NK activating receptors and inhibiting receptors may contribute to the immune pathogenesis of OAPS.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disease characterised by vascular thrombosis and recurrent fetal loss with the persistent presence of circulating antiphospholipid antibodies (aPLs) (Chaturvedi and McCrae Citation2017; Cochery-Nouvellon et al. Citation2017). In 2013, a new clinical criteria were proposed to separate APS into thrombotic APS (TAPS) characterized by thrombosis and obstetric APS (OAPS) characterized by adverse pregnancy events. (Antovic et al. Citation2018; Cochery-Nouvellon et al. Citation2017). OAPS has been referred to as a severe acquired risk factor for recurrent abortions, fetal losses, pre-eclampsia, placental insufficiency and represents an important health threat for women of childbearing age (Antovic et al. Citation2018; Carolis et al. Citation2018; Marchetti et al. Citation2013). Natural killer (NK) cells have been implicated to play a critical role in the processes of reproduction (Abel et al. Citation2018; Van den Hoogen et al. Citation2016), which are associated with implantation failures, recurrent miscarriage and infertility due to either cytotoxicity or receptor expressions (Lee et al. Citation2019; Ryan et al. Citation2001; Tabiasco et al. Citation2006). NK cells can be subdivided into two main cell populations, CD16+CD56dim and CD16−CD56bright NK cells. It has been reported that the increase of CD16+CD56dim NK cells in the endometrium and peripheral blood are risk factors for recurrent miscarriages (Tabiasco et al. Citation2006). CD16−CD56bright NK cells in peripheral blood are recruited into the decidua during pregnancy and differentiate into the decidual NK cells upon exposure to the local microenvironment and associated with the pathogenesis of foetal loss, intrauterine growth restriction and preterm birth (Kitaya Citation2008; Lee et al. Citation2019). However, the distribution of CD16+CD56dim and CD16− CD56bright NK cells has seldom been reported in OAPS patients, and the mechanism is not clearly illustrated as well.
NK cells express a series of receptors to identify relevant ligands on target cells, which can activate their cytotoxic and secretory functions (Tabiasco et al. Citation2006). The NKG2 family belongs to the c-type lectin family, which include inhibitory receptors NKG2A, NKG2B, and activating receptors NKG2C, NKG2D and NKG2E (Ryan et al. Citation2001). NKG2 family receptors can directly activate or inhibit peripheral NK cells, and are considered as the main activated receptors mediating NK cell cytotoxic function (Kumar Citation2018). Natural cytotoxicity receptors (NCR) belong to another important NK receptor families, which consist of three activating receptors NKp46, NKp44 and NKp30. Therefore, whether activating and inhibiting receptor phenotypes change in OAPS patients need to be further explored (Tabiasco et al. Citation2006).
It has been reported that the level of maturation and function of human NK cells can be detected through the classification of CD11b/CD27 phenotype, so as to establish a new model of the development and function of human NK cells, and to link the mouse model with human NK cells. Resembling the subsets in mice, human NK cells have been further divided into four functionally distinct subsets based on the surface density of CD27 and CD11b: CD27− CD11b −, CD27+ CD11b−, CD27+ CD11b+ and CD27− CD11b+. The CD27− CD11b−NK cells display an immature phenotype and potential for differentiation, the CD27+ CD11b− and CD27+ CD11b+ NK cells show strong ability to secrete cytokines, and the CD27−CD11b+ NK cells show high cytolytic function. The specific microenvironment and complex cellular interactions provide crucial signals for modifying the features of NK cells (Fu et al. Citation2011; Zhang et al. Citation2017).
At present, there are limited reports on the expression of NK cell receptors and the distributional profiles of NK cell subsets according to CD11b/CD27 expressions in OAPS patients, and the precise process of autoimmune responses in the pathogenesis of OAPS remains unknown. Therefore, the aim of this study was to compare the subsets frequencies and the phenotypic characterizations of circulating NK cells in patients with OAPS and healthy women, and to detect the correlations between these parameters and antiphospholipid antibodies. Our results suggest that the imbalance of circulating NK cell receptors involves in the pathogenesis of OAPS especially for increased NKG2A−NKG2D+CD3−CD16+CD56dim NK cell subset was observed in OAPS patients which may offer a new biomarker for estimating immunological states and potential targets for the development of novel therapies for OAPS patients.
Materials and methods
Study populations
A total of 43 OAPS patients were recruited in this study. All the OAPS patients were newly diagnosed by 2006 Sydney APS criteria from December 2018 to July 2020 in Pecking University Third Hospital. Meanwhile, 30 age-matched healthy women were selected as controls. EDTA anticoagulated venous blood samples were collected from each participant. The study was performed in accordance with the Helsinki Declaration and was approved by the Peking University Third Hospital Medical Science Research Ethics Committee. Informed consent was obtained from all patients. The clinical characteristics of OAPS patients and healthy control women are summarized in .
Table 1. Characteristics of OAPS and healthy control
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation with Ficoll–Hypaque (Sigma Chemical Co, St Louis, MO, USA). The concentration of samples was adjusted to 1*106/mL. PBMCs were resuspended in PBS containing 10% fetal bovine serum and stained with anti-CD3 (PerCP-Cy, cloneSP34-2), anti-CD56 (Alexa Fluor®647, cloneB519),anti-CD16 (APC-H7, clone3G8), anti-CD27 (PE, cloneM-T271), anti-NKG2D (PE-Cy™7, clone1D11), anti-CD11b (BB515, cloneICRF44), anti-NKG2A (BV421, clone20d5) and anti-NKp46 (BV510, clone9E2). All antibodies were purchased from BD Biosciences (San Diego, CA, USA). The concentration of antibodies was used according to the instructions of the recommended adding volume of BD company. PBMCs were incubated with antibodies for 30 min, then washed with PBS twice and analyzed by flow cytometry using a FACS Canto II (BD Biosciences San Jose, CA, USA). Isotype antibodies were used as controls.
The detection of aPLs and complement components
Serum aCL (IgG, IgM and IgA) and anti-β2GPI (IgG, IgM and IgA) were detected by a chemiluminescence immunoassay on BIO-FLASH Chemiluminescent Analyser (Biokit S. A., Barcelona, Spain). The cut-off values for the positivity of antibodies above were 20.0 CU according to the recommendations. The positivity of aCL or anti-β2GPI was defined as at least one subtype (IgG, IgM and IgA) that was positive. Serum C3 and C4 were detected by kinetics nephelometry following the manufacturer’s package inserts for Beckman Coulter reagents on the IMMAGE 800 analyzer (Beckman Coulter Inc, Brea, CA)
Statistical analysis
Data are reported as mean±SEM and were analyzed using GraphPad Prism V5.0 software (GraphPad Software Inc., San Diego, CA). Comparisons between groups were performed using Mann–Whitney U test and One-way ANOVA. The Spearman’s correlation test was used to evaluate correlations between groups. Graphic analyses were performed using GraphPad Prism V5.0. Data of Flow cytometry were analyzed using Flowjo software (Treestar, Ashland, OR, USA). A P value <.05 was considered to indicate a statistically significant difference.
Results
The percentages of NK cells and CD3+CD56+ T cells in OAPS patients and healthy controls
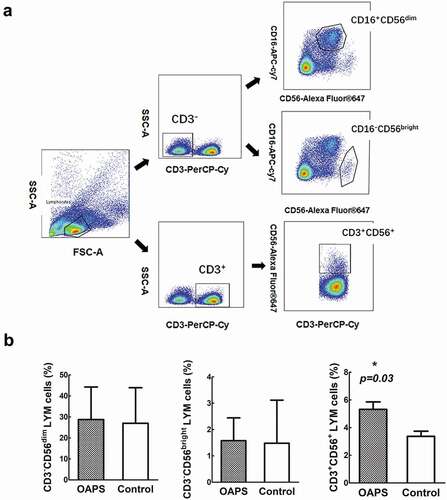
Human NK can be subdivided into two main cell populations. About 90% are cytotoxic CD3−CD16+CD56dim NK cells, while the remaining 10% are non-cytotoxic CD3−CD16−CD56bright NK cells (Domaica et al. Citation2012). The CD56 + T cells (define as CD3+CD56+) comprise 5–15% of peripheral circulating T cells in human and express the typical NK cell surface marker CD56. CD56+T cells are also characterized by some NK cell-like properties, such as the large granular lymphocyte morphology and the capacity to destroy NK-sensitive target cells. The gating strategies of CD3−CD16+CD56dim NK cells, CD3−CD16−CD56bright NK cells and CD56+T cells are shown in . We first compared the expression of these subsets in OAPS patients and healthy controls. The percentages of CD3−CD16+CD56dim and CD3−CD16−CD56bright NK cells in OAPS patients in higher than healthy control but not there was no statistical significance (p = .934, p = .651, respectively), CD56+T cells in OAPS patients in significantly higher than healthy control group (p = .03) ()
Figure 1. Comparisons of frequencies of frequencies of CD3-CD16+ CD56dim, CD3- CD16-CD56 bright NK cells and CD3+ CD56 + T cells in patients with OAPS and healthy control women. (a) Flow cytometry plots showing gating strategies of CD3-CD16+ CD56dim, CD3-CD16-CD56 bright NK cells and CD3+ CD56 + T cells from lymphocytes in a representative subject. (b) Frequencies of different CD3-CD16+ CD56dim, CD3-CD16-CD56 bright NK and CD3+ CD56 + T lymphocyte subsets in OAPS patients and healthy control subjects.
Increased peripheral NKG2A−NKG2D+CD3−CD16+CD56dim NK cell subset was positively correlated with aPLs in OAPS patients
To further investigate the functional characteristics of NK cells in OAPS patients, we analyzed the expressions of NK receptors NKG2A and NKG2D. NK cytotoxic activity results from a balance between inhibitory and activating cell surface receptors with their specific ligands expressed at the cell surface of target cells. NKG2D is an activating receptor while NKG2A is an inhibiting one, so the increased NKG2A−NKG2D+ subset represents the enhancement of cytotoxic. The results showed that the NKG2A−NKG2D+ subset was significantly increased in CD3−CD16+CD56dim NK cells, CD3−CD16−CD56bright NK cells and CD56+T cells (p < .001, p < .001, p < .001, respectively) and the NKG2A+NKG2D− subset was significantly decreased in CD3−CD16+CD56dim NK cells, CD3−CD56bright NK cells and CD56+T cells (p < .001, p = .006, p < .001, respectively) compared with those in healthy control women (). We further evaluated the sensitivity and specificity of NKG2A/NKG2D in CD3−CD56dim NK cells in OAPS and healthy control women to explore their diagnosis efficacy (). At the optimal diagnostic threshold established by ROC (receiver-operator characteristic) analysis, using 10.10% as the cutoff point of NKG2A−NKG2D+ subset in CD3−CD16+CD56dim NK cells, the sensitivity to detect patients with OAPS compared with healthy control results was 94.1% and specificity was 84.2%, respectively, with an area under the curve (AUC) of 0.903 (0.831–0.976) ().
Table 2. The diagnostic efficacy of NKG2A/NKG2D and CD27/CD11b in CD3−CD56dim NK cells in OAPS
Figure 2. Comparisons of frequencies of NKG2A and NKG2D expression in in OAPS patients and healthy control women. (a)Representative flow plots showed the portions of NKG2A and NKG2D expression from CD3- CD16+ CD56dim, CD3- CD16-CD56 bright NK cells and CD3+ CD56 + T cells. Bar charts show the frequencies of different NKG2D and NKG2A subsets in (b) CD3- CD16+ CD56dim NK cells, (c) CD3-CD16-CD56bright NK cells and (d) CD56 + T cells in OAPS patients and healthy control women. (e) ROC curve showing diagnostic accuracy of NKG2A-NKG2D+ subset in CD3-CD16+ CD56dim NK cells to distinguish patients with OAPS. (f) The correlations between NKG2A-NKG2D+ expression and the common logarithms of the concentration of antiphospholipid antibodies. (g) The correlations between NKG2A-NKG2D+ expression and the concentration of the complement C3 and C4. The red underline indicates p < .05. Data were showing as mean ± SD. All P values were two-tailed and considered significant when less than 0.05. *P < .05, **P < .01, ***P < .001.
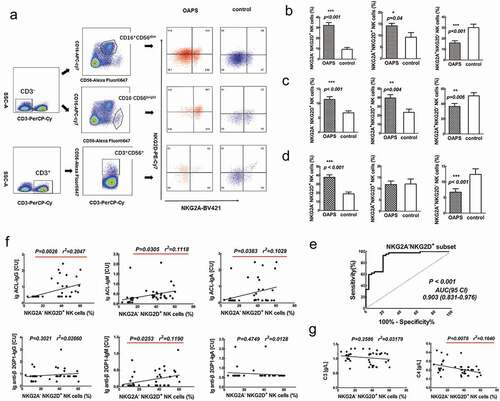
Correlation between NKG2A−NKG2D+ subset in CD3−CD16+CD56dim NK and the antiphospholipid antibodies are summarized in The NKG2A−NKG2D+ subset in CD3−CD16+CD56dim NK cells was positively correlated with the common logarithms of antiphospholipid antibodies lg ACL-IgG (p = .0026, r2 = 0.2047), lg ACL-IgM (p = .0305, r2 = 0.1118), lg ACL-IgA (p = .0383, r2 = 0.1029) Ig β2GP1-IgM (p = .0253, r2 = 0.1190). Apart from that, we also tested the correlation between this subset and Compliment 3 and Compliment 4 (). The NKG2A−NKG2D+ subset in CD3−CD56dim NK cells was negatively correlated with Complement 4 (p = .0078, r2 = 0.1640) () in OAPS patients. Collectively, the increase of peripheral NKG2A−NKG2D+ subset in CD3−CD16+CD56dim NK cells was correlated with aPL antibodies formation; thus, influence the disease severity of OAPS.
Increased peripheral CD27−CD11b+CD3−CD16+CD56dim NK cell subset was positively correlated with aPLs in OAPS patients
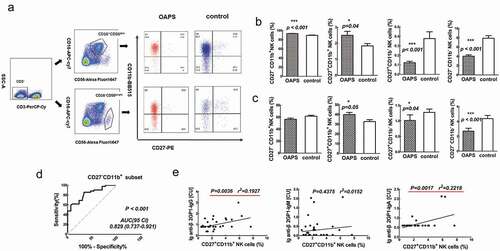
We next compared the phenotypic characteristics of CD27/CD11b in CD3−CD16+CD56dim and CD3−CD16−CD56bright NK cells between OAPS and healthy controls. Each CD27/CD11b population could be characterized by unique functional and phenotypic attributes. It has been found that large CD27 −CD11b− populations of NK cells from decidua, which display an immature phenotype and potential for differentiation. CD27+ CD11b− and CD27+ CD11b+ NK cells show the ability to secrete cytokines, while CD27−CD11b+ NK cells exhibit high cytolytic function. In this study, the CD27−CD11b+ subset was significantly increased in CD3−CD56dim NK cells (p < .001), the CD27+CD11b+ subset was significantly increased in CD3−CD56dim NK cells and CD3−CD56bright NK cells (p = .04, p = .05), the CD27+CD11b− subset was significantly increased in CD3−CD56dim NK cells and CD3−CD56bright NK cells (p < .001, p = .04), and the CD27−CD11b− subset was significantly decreased in CD3−CD56dim NK cells and CD3−CD56bright NK cells (p < .001, p < .001) in OAPS patients compared with those in healthy control women (). Research shows that CD27−CD11b− exhibits an inactive phenotype, suggest that the inactive subset of NK cells was reduced in the peripheral blood of OAPS patients.
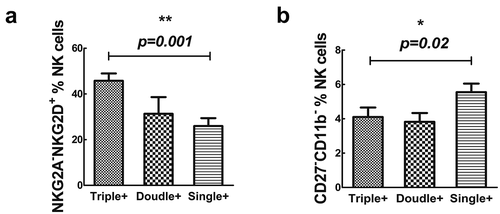
Figure 3. The NKG2A-NKG2D+subset (A) and CD27-CD11b-(B) in CD3-CD16+ CD56dim subset in triple positivity, double positivity and single positivity in OAPS patients. Results are expressed as mean ± SD. *P < .05, **P < .01, ***P < .001.
To investigate the sensitivity and specificity of CD27/CD11b in CD3−CD56dim NK cells in OAPS and healthy control women, we evaluate the optimal diagnostic threshold established by ROC analysis, while using 92.75% as the cutoff point of CD27−CD11b+ subset in CD3−CD56dim NK cells, the sensitivity to detect patients with OAPS compared with healthy control results was 60.8% and specificity was 89.5%, with an AUC of 0.829 (0.737–0.921) ().
Correlation between CD27+CD11b+ subset in CD3−CD16+CD56dim NK and the antiphospholipid antibodies are summarized in . The CD27+CD11b+ subset in CD3−CD56dim NK cells was positively correlated with the common logarithms of antiphospholipid antibodies lg anti-β2GP1-IgG (p = .0036, r2 = 0.1927) and lg anti-β2GP1-IgA (p = .0017, r2 = 0.2218) (). However, there were no significant associations between other cell subsets and the values of other clinical indicators.
The percentage of NKG2A/NKG2D and CD27/CD11b subsets in CD3−CD56dim NK cells in triple, double and single positivity OAPS patients
Antiphospholipid antibodies profiles in OAPS patients including 31 patients expressed positivity only one kind of antiphospholipid antibodies (LA+ or anti-ACL+ or anti-β2GP1+), 6 patients exhibited double positivity (LA+, aCL+, anti-β2GP1- or LA+, aCL-, anti-β2GP1+ or LA-, aCL+, anti-β2GP1+) and 6 patients showed triple positivity (LA+, aCL+, anti-β2GP1+).
The percentage of NKG2A−NKG2D+ subset in triple positivity was higher than single positivity (P = .001) and the percentage of CD27−CD11b− subset in triple positivity was lower than single positivity in CD3−CD56dim subset NK cells in OAPS patients (P = .02) (). But there was no significant difference between triple positivity and double positivity, or double positivity and single positivity. Moreover, there is no significant difference of NKG2A/NKG2D and CD27/CD11b subsets in CD3−CD56bright subsets.
Figure 4. Comparisons of frequencies of CD27 and CD11b expression in in OAPS patients and healthy control women. (a) Representative flow plots showed the portions of CD27and CD11b expression from CD3- CD16+ CD56dim and CD3-CD16-CD56 bright NK cells. Bar charts show the frequencies of different CD27and CD11b subsets in (b) CD3-CD16+ CD56dim NK cells, (c) CD3- CD16-CD56 bright NK cells and in OAPS patients and healthy control women. (d) ROC curve showing diagnostic accuracy of CD27-CD11b+ subset in CD3-CD16+ CD56dim NK cells to distinguish patients with OAPS. (e)The correlations between the change in the percentage of CD27+ CD11b+ in CD3- CD16+ CD56dim NK cells and the common logarithms of antiphospholipid antibodies. The red underline indicates p < .05.Data were showing as mean ± SD. All P values were two-tailed and considered significant when less than 0.05. *P < .05, **P < .01, ***P < .001.
Discussion
OAPS patients were associated with significantly higher risks of pregnancy-induced hypertension, fetal loss, abortion, thrombosis, and preterm delivery (Liu and Sun Citation2019). Therefore, the researches on diagnosis and mechanism of OAPS are of great importance. NK cells make up over 60% in the uterus of human in the early pregnancy. As an allogeneic graft, the fetus is able to establish and maintain pregnancy. Thus, the balance of functions of NK cells is very important for the promotion of fetal growth and maintenance of immune tolerance (Fu et al. Citation2017). Mounting evidence has linked NK cells to reproductive failure particularly when they become adversely activated and mediate fetal demise by releasing perforin (Yougbare et al. Citation2017). It is reported that the decidua NK comes from peripheral blood and is recruited into the uterus and decidua under the action of chemokines, and subsequently differentiated into uterine NK. (Chrysoula and Linda Citation2005). The phenotype of uNK is similar to a small subgroup of peripheral blood NK cells; however, in peripheral blood CD16−CD56bright NK cells are generally agranular (Lash et al. Citation2010). Despite some controversy, there are studies show that peripheral NK (pNK) cells are closely related to uterine NK cells, in addition to the directly cytotoxic function, pNK also act as regulators of adaptive immunity, for example, by interacting and providing stimulatory signals for the components of the adaptive immune system, including T cells and dendritic cells (De Carolis et al. Citation2009). NK cells dysfunction can lead to spontaneous abortion and other adverse consequences (Perricone et al. Citation2007). So, the detection of peripheral NK cells can indirectly help to understand the local immune status of the uterus, so as to explore the mechanisms of repeated abortion in patients with OAPS (Esteve-Valverde et al. Citation2016). NK cells are divided into CD16−CD56bright and CD16+CD56dim NK subsets. CD16−CD56bright NK cells are mainly responsible for cytokine secretion, whereas CD16+CD56dim NK cells are primarily responsible for cytotoxicity (Fan et al. Citation2008). CD3+CD56+T cells are characterized by some NK cell-like properties, such as the large granular lymphocyte morphology and the capacity to destroy NK-sensitive target cells, which were presumed to display properties of both NK cells and T cells and exhibited capacities of cytotoxicity and cytokine production (Fan et al. Citation2016). Previous research showed that the proportion of NK cells in circulating lymphocytes in humans increased in peripheral blood of the recurrent spontaneous abortion (RSA) patients (Perricone et al. Citation2007), and little is known about the potentials of CD56+T cells in OAPS or other autoimmune diseases. In this study, the CD56+T cells in OAPS patients in significantly higher than healthy control group. The increased CD56+T cells indicate the enhancement of cytotoxicity in OAPS patients. However, the results still need further investigation with larger sample size.
NK cells express a series of immune receptors to identify relevant ligands on target cells, which maintain the immune balance between activation and tolerance of NK cells. The previous research in unexplained spontaneous abortion indicated that the patients have higher expression of activation receptors NKp46 and NKp44 in decidual NK (dNK) cells but have no change in peripheral blood (Zhang et al. Citation2008). In this study, we verified the abnormal expressions of NK activating receptor NKG2D and inhibitory receptor NKG2A. The results showed NKG2A−NKG2D+ subset was increased and the NKG2A+NKG2D− subset was decreased significantly in OAPS patients in both CD3−CD16+CD56dimNK cells, CD3−CD16−CD56brightNK cells and CD3+CD56+T cells compared with those in healthy control women. CD3−CD16−CD56bright NK cells are the main component of NK cells in deciduous in the early pregnancy, and were considered mainly responsible for cytokine secretion, but not responsible for cytotoxicity; however, this conclusion is controversial (Zhang et al. Citation2008). In this study, the NKG2A−NKG2D+ subset in CD3−CD16−CD56brightNK cells also increased significantly, indicating that CD56brightNK cells may also play a cytotoxic function in the pathological process of OAPS patients. Moreover, our results showed the percentage of NKG2A−NKG2D+ subset in CD3−CD16+CD56dim NK cells in triple aPLs positivity was higher than single aPL positivity and the percentage of CD27−CD11b− subset in CD3−CD16+CD56dim NK cells in triple aPL positivity was lower than single aPL positivity OAPS patients. In the earlier criteria for the classification of APS, a single positivity of any of the three tests mentioned before fulfill the serologic criteria. However, there is growing evidence strengthen the concept that the more positive tests of aPLs, the higher risk for thromboembolic events and pregnancy morbidity, triple aPLs (LA/aCL/aβ2GPI) positive population has a more severe course of the disease (Hernández-Molina et al. Citation2013; Pengo et al. Citation2010). Triple aPLs positivity was considered as a high-risk type in the risk stratification in OAPS patients (Liu and Sun Citation2019), our study suggests that the increased NK cells cytotoxic effect may be associated with adverse pregnancy events. In addition, we also detected the expressions of NK cells receptors NKp46 and NKp30, the NKG2A+NKp46− subset increased and the NKG2A−NKp46+ subset decreased significantly in CD56bright NK cells (supplement f 1), but there were no significant differences in NKp30 subsets. The differences between NK activation status in peripheral blood and in decidua and their interaction effects need to be further studied.
It has been reported that the level of maturation and function of human NK cells can be detected through the classification of CD11b/CD27 phenotype. The CD27+CD11b− and CD27+CD11b+NK cells show a strong ability to secrete cytokines, while CD27− CD11b+NK cells exhibit high cytolytic function, and CD11b−CD27−NK cells display an immature phenotype (Fu et al. Citation2014). In this study, the CD27−CD11b+ subset in OAPS patients was significantly increased in CD3−CD16+CD56dim NK cells compared with those in healthy control women, suggest that the cytotoxicity of NK cells was enhanced in the peripheral blood of OAPS patients. Taken together, these results may indicate that the activation of NK enhanced in the peripheral blood of OAPS patients.
We further investigated the diagnosis value of NKG2A/NKG2D and CD27/CD11b subsets in CD3−CD16+CD56dim NK cells. At the optimal diagnostic threshold established by ROC analysis, using the cutoff of NKG2A−NKG2D+ subset in CD3− CD16+CD56dim NK cells is 10.10%, the sensitivity and specificity to detect patients with OAPS compared with healthy control results was 94.1% and 84.2%, with an area under the curve (AUC) of 0.903 (0.831–0.976); using the cutoff of CD27−CD11b+ subset in CD3− CD16+CD56dim NK cells is 92.75%, the sensitivity to detect patients with OAPS compared with healthy control results was 60.8% and specificity was 89.5% with an AUC of 0.829 (0.737–0.921). These AUC proves that NKG2A−NKG2D+ and CD27−CD11b+subset in CD3−CD16+CD56dim NK cells are valuable for evaluating OAPS.
In human peripheral blood lymphocytes, CDl6+CD56dim NK cells are key effectors of antibody-dependent cell-mediated cytotoxicity (ADCC). CD3−CD16+CD56dim NK cells expressing FcγRIII (CD16), which can recognize the target cells covered by antibodies and lead to the ADCC effect of NK cells and the production of cytokines, resulting in NK cell-specific cytotoxic effect. The OAPS patients contain anti-phospholipid antibodies in their circulation; therefore, we come up with a hypothesis whether CD16+CD56dim NK cells mediated ADCC in the APS patients. In this study, the NKG2A−NKG2D+ portion in CD3−CD16+CD56dim subset was correlated with antiphospholipid antibodies lg ACL-IgG, lg ACL-IgM, lg anti-β2GP1-IgM, and C4 in OAPS patients. So we speculate the aPLs form immune complexes on trophoblast cells to create targeted binding sites for NK cell Fcγ receptors. There are researches indicate that NK cells mediated cytotoxicity of invasive trophoblasts as a pathological mechanism in fetal alloimmune thrombocytopenia, and suggest that anti-NK cell therapies may prevent immune-mediated pregnancy loss.
However, there are still some limitations in this study at present. Firstly, the sample size is relatively small, apart from that, we didn’t follow the relationship of the pregnancy outcomes or therapeutic effects of the OAPS patients with high NKG2A−NKG2D+ or CD27−CD11b+ NK cells in CD3−CD16+CD56dim subset. Functional studies of whether NK cells play a role through ADCC response need to be further verified by detecting CD107a, interferon-γ or perforin to uncover the precise molecular mechanisms during the pathogenic process of OAPS (Rubin et al. Citation2017).
However, there are still some limitations in this study at present. Firstly, the sample size is relatively small, besides, we didn’t follow the relationship of the pregnancy outcomes or therapeutic effects of the OAPS patients with high NKG2A−NKG2D+ or CD27−CD11b+ NK cells in CD3−CD16+CD56dim subset. Functional studies of whether NK cells play a role through ADCC response need to be further verified by detecting CD107a, interferon-γ or perforin to uncover the precise molecular mechanisms during the pathogenic process of OAPS (Rubin et al. Citation2017). Apart from that, we will collect some decidua samples and establish animal models for further research in the next step.
In conclusion, our study investigated the subsets distribution and phenotypes of peripheral NK cells and receptors in OAPS patients and healthy women. Our study indicates that unbalanced NK activating receptors and inhibiting receptors may contribute to the immune pathogenesis of OAPS. The percentage of NKG2A−NKG2D+CD3−CD56dim NK cells may serve as a good biomarker for patients with OAPS, leading to new strategies for the disease diagnosis.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abel AM, Yang C, Thakar MS, Malarkannan S. 2018. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 9:1869.
- Antovic A, Sennstrom M, Bremme K, Svenungsson E. 2018. Obstetric antiphospholipid syndrome. Lupus Sci Med. 5(1):e000197.
- Carolis SD, Tabacco S, Rizzo F, Giannini A, Botta A, Salvi S, Garufi C, Panici PB, Lanzone A. 2018. Antiphospholipid syndrome: an update on risk factors for pregnancy outcome. Autoimmun Rev. 17(10):956–66.
- Chaturvedi S, McCrae RK. 2017. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 31:406–17.
- Chrysoula D, Linda CG. 2005. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 26:44–62.
- Cochery-Nouvellon E, Mercier E, Bouvier S, Balducchi JP, Quere I, Perez-Martin A, Mousty E, Letouzey V, Gris JC. 2017. Obstetric antiphospholipid syndrome: early variations of angiogenic factors are associated with adverse outcomes. Haematologica. 102:835–42.
- De Carolis C, Perricone C, Perricone R. 2009. NK cells, autoantibodies, and immunologic infertility: a complex interplay. Clin Rev Allergy Immunol. 39:166–75.
- Domaica CI, Fuertes MB, Uriarte I, Girart MV, Sardanons J, Comas DI, Di Giovanni D, Gaillard MI, Bezrodnik L, Zwirner NW. 2012. Human natural killer cell maturation defect supports in vivo CD56(bright) to CD56(dim) lineage development. PloS One. 7:e51677.
- Esteve-Valverde E, Ferrer-Oliveras R, Alijotas-Reig J. 2016. Obstetric antiphospholipid syndrome. Rev Clín Esp (English Edition). 216:135–45.
- Fan X, Zhu L, Liang H, Xie Z, Huang X, Wang S, Shen T. 2016. Antibody-dependent CD56+ T cell responses are functionally impaired in long-term HIV-1 infection. Retrovirology. 13:76.
- Fan YY, Yang BY, Wu CY. 2008. Phenotypic and functional heterogeneity of natural killer cells from umbilical cord blood mononuclear cells. Immunol Invest. 37:79–96.
- Fu B, Tian Z, Wei H. 2014. Subsets of human natural killer cells and their regulatory effects. Immunology. 141:483–89.
- Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. 2011. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 133:350–59.
- Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z, Sun R, Tian Z, Wei H. 2017. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. 47:1100–13.
- Hernández-Molina G, Espericueta-Arriola G, Cabral AR. 2013. The role of lupus anticoagulant and triple marker positivity as risk factors for rethrombosis in patients with primary antiphospholipid syndrome. Clin Exp Rheumatol. 31:382–88.
- Kitaya K. 2008. Accumulation of uterine CD16(-) natural killer (NK) cells: friends, foes, or Jekyll-and-Hyde relationship for the conceptus? Immunol Invest. 37:467–81.
- Kumar S. 2018. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology. 154:383–93.
- Lash GE, Robson SC, Bulmer JN. 2010. Review: functio nal role of uterine natural killer (uNK) cells in human early pregnancy decidua. Trophoblast Res. 24:S87–S92.
- Lee CL, Vijayan M, Wang X, Lam KKW, Koistinen H, Seppala M, Li RHW, Ng EHY, Yeung WSB, Chiu PCN. 2019. Glycodelin-A stimulates the conversion of human peripheral blood CD16-CD56bright NK cell to a decidual NK cell-like phenotype. Hum Reprod. 34:689–701.
- Liu L, Sun D. 2019. Pregnancy outcomes in patients with primary antiphospholipid syndrome: A systematic review and meta-analysis. Medicine. 98:e15733.
- Marchetti T, Cohen M, de Moerloose P. 2013. Obstetrical antiphospholipid syndrome: from the pathogenesis to the clinical and therapeutic implications. Clin Dev Immunol. 2013:159124.
- Pengo V, Banzato A, Denas E, Bison G, Padayattil Jose S, Ruffatti A. 2010. Antiphospholipid syndrome: critical analysis of the diagnostic path. Lupus. 19:428–31.
- Perricone C, De Carolis C, Giacomelli R, Zaccari G, Cipriani P, Bizzi E, Perricone R. 2007. High levels of NK cells in the peripheral blood of patients affected with anti-phospholipid syndrome and recurrent spontaneous abortion: a potential new hypothesis. Rheumatology. 46:1574–78.
- Rubin TS, Zhang KJ, Gifford C, Lane A, Choo S, Bleesing JJ, Marsh RA. 2017. Perforin and CD107a testing is superior to NK cell function testing for screening patients for genetic HLH. BLOOD. 129:2993–99.
- Ryan JC, Naper C, Hayashi S, Daws MR. 2001. Physiologic functions of activating natural killer (NK) complex-encoded receptors on NK cells. Immunol Rev. 181:126–37.
- Tabiasco J, Rabot M, Aguerre-Girr M, El Costa H, Berrebi A, Parant O, Laskarin G, Juretic K, Bensussan A, Rukavina D, et al. 2006. Human decidual NK cells: unique phenotype and functional properties – a review. Placenta. 27(Suppl A):S34–9.
- Van den Hoogen LL, van Roon JAG, Radstake TRDJ, Fritsch-Stork RDE, Derksen RHWM. 2016. Delineating the deranged immune system in the antiphospholipid syndrome. Autoimmun Rev. 15:50–60.
- Yougbare I, Tai WS, Zdravic D, Oswald BE, Lang S, Zhu G, Leong-Poi H, Qu D, Yu L, Dunk C, et al. 2017. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat Commun. 8:224.
- Zhang QF, Yin WW, Xia Y, Yi YY, He QF, Wang X, Ren H, Zhang DZ. 2017. Liver-infiltrating CD11b(-)CD27(-) NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell Mol Immunol. 14:819–29.
- Zhang Y, Zhao A, Wang X, Shi G, Jin H, Lin Q. 2008. Expressions of natural cytotoxicity receptors and NKG2D on decidual natural killer cells in patients having spontaneous abortions. Fertil Steril. 90:1931–37.
