ABSTRACT
Purpose: To evaluate the relationship between clinical severity and tear levels of eosinophil cationic protein (ECP) and CC chemokine ligand 23 (CCL23) in patients with chronic allergic conjunctival disease (cACD). Subjects and methods: This clinical study included 21 patients with cACD, including atopic keratoconjunctivitis and vernal keratoconjunctivitis, and 14 subjects as controls. The clinical severity score was determined by objective findings. Tear ECP grade was evaluated using tear ECP levels determined by enzyme immunoassay. CCL23 expression in tears was also determined by enzyme immunoassay. Results: In the cACD group, tear ECP grade was significantly correlated with the clinical severity score. Clinical severity scores and tear ECP grades in the CCL23-positive subgroup were significantly higher than those in the CCL23-negative subgroup. Conclusion: We have confirmed the value of tear ECP grade and CCL23 expression in tears as a severity grading system in patients with cACD.
INTRODUCTION
Chronic allergic conjunctival disease (cACD), including vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC), is characterized by severe keratoconjunctivitis with giant papillae, gelatinous infiltration of the limbus, and shield ulcers, and its clinical course can include exacerbations and remissions over several years.Citation1,Citation2 The main medical treatments for cACD are instillation of ophthalmic solutions containing a steroid or an immunosuppressive agent. Precise assessment of disease severity by clinical severity score and tear biomarkers is critical for decision making regarding treatment and for follow-up in patients with cACD.
Objective assessment of the clinical severity of AKC/VKC requires a method to digitize the objective findings as a clinical score. We recently reported a 5-5-5 exacerbation grading scale for ACDCitation3 that can be used to determine the clinical severity of ACD.
Examination of tear biomarker levels is useful in the diagnosis of cACD, as well as determination of its severity and the efficacy of treatment for AKC/VKC. Various tear biomarkers for ACD have already been reported, including eosinophil cationic protein (ECP), a cytotoxic protein contained in specific granules of the eosinophil.Citation4 Tear ECP levels are thought to reflect the severity of eosinophilic inflammation in patients with ACD.Citation5–Citation7 Moreover, it has been reported that a steroid or immunosuppressant ophthalmic solution reduced tear ECP levels.Citation8 CCL23/myeloid progenitor inhibitory factor 1 (MPIF-1) is a member of the CC chemokine family and interacts with the CC chemokine receptor 1 (CCR1). CCL23 was originally isolated from a human aortic endothelial cell libraryCitation9 and from THP-1, a human monocytic cell line.Citation10 CCL23 expression has been observed with eosinophils in addition to monocytes and dendritic cells.Citation11 However, the relationship between clinical severity and tear levels of ECP and CCL23 is not fully understood.
In this study, we evaluated the relationship between clinical severity determined by the 5-5-5 exacerbation grading scale and tear levels of ECP and CCL23 in patients with cACD. We also investigated the usefulness of tear tests for ECP and CCL23 in patients with cACD.
SUBJECTS AND METHODS
This study was approved by the institutional review board of the Nihon University School of Medicine and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all study subjects.
Subjects
This study included 21 consecutive patients (21 eyes) with chronic ACD (AKC or VKC) treated at the Department of Ophthalmology, Nihon University Itabashi Hospital, Tokyo, Japan, from January 2013 to August 2013, and 11 healthy volunteers (controls). Demographic data for the subjects are shown in . Only non-treated patients or patients treated with anti-allergic ophthalmic solutions alone, such as mast cell stabilizers, histamine H1 receptor antagonists, corticosteroids, or immunosuppressive agents, were included in the study. Patients who used oral medicines or received injections to treat allergic disease and those who were receiving immunotherapy were excluded. Patients with ocular surface disease other than ACD, including lagophthalmos, blepharospasm, conjunctival chalasis, dry eye, infectious conjunctivitis, infectious keratitis, Stevens-Johnson syndrome, and ocular pemphigoid, and those who could not provide an adequate tear sample were also excluded.
TABLE 1. Demographic data of study patients and control subjects.
AKC and VKC were diagnosed by slit-lamp microscopy and a laboratory test for serum antigen-specific IgE antibody, according to the Japanese guidelines for ACD.Citation12
Clinical Severity Score
Scoring for clinical severity of AKC and VKC was performed using the 5-5-5 exacerbation grading scale for ACD.Citation3 This scale has been previously reported as a method for measuring the severity of ACD. Using this scoring method, a clinical score is determined by a total of points. Five severe clinical findings (active giant papillae, gelatinous infiltrates of the limbus, exfoliative epithelial keratopathy, shield ulcers, and papillary proliferation at the lower palpebral conjunctiva) are given 100 points each; 5 moderate clinical findings (blepharitis, papillary proliferation with a velvety appearance, Horner-Trantas spots, edema of the bulbal conjunctiva, and superficial punctate keratopathy) are given 10 points each; and 5 mild clinical findings (papillae at upper palpebral conjunctiva, follicular lesion at the lower palpebral conjunctiva, hyperemia of the palpebral conjunctiva, hyperemia of the bulbal conjunctiva, and lacrimal effusion) are given one point each. By using this method, the patients with AKC or VKC were divided into severe, moderate, and mild subgroups and given a clinical severity score, as shown in .
TABLE 2. Clinical severity score.
Determination of ECP and CCL23 in Tears
Tear sampling. Tears were sampled from the affected eye in unilateral cases or from the more severely affected eye in bilateral cases. Right eyes of 11 healthy volunteers who did not have allergic diathesis or a history of wearing contact lenses were used as controls.
Tears were sampled using the Schirmer I method with filter paper (Schirmer Tear Production Measuring Strips; Showa Yakuhin Kako, Tokyo, Japan),Citation6 and the Schirmer strips were stored at –20°C until further use. The Schirmer strips were thawed and eluted overnight at room temperature using 0.5 M NaCl and 0.5% Tween 20 containing 0.05 M phosphate-buffered solution (pH 7.2). The amount of tears obtained was calculated by considering 1 mm of a wet Schirmer strip to contain 1 μL of tears. Thus, the final concentration of the eluted solution corresponded to a 20-fold dilution of the original tear sample.
Enzyme immunoassay. Tear ECP levels were determined by chemiluminescent enzyme immunoassay (Immulyze®; LSI Medience Corporation, Tokyo, Japan). The lower limit of detection for ECP according to the manufacturer is 0.2 ng/mL. Tear CCL23 levels were determined using a magnetic bead assay (Bio-Plex Pro™ human chemokine assay; Bio-Rad Laboratories, Hercules, CA, USA). According to the manufacturer, the lower limit of detection for CCL23 is 0.5 pg/mL.
Tear ECP grading. We set up the tear ECP grading system to determine the severity of tear ECP levels. The tear ECP measurements were arranged from grade 1 to 5, as shown in .
TABLE 3. Tear ECP grade.
Determination of CCL23 expression in tears. CCL23 expression in tears was determined to be positive if it was above the cutoff value and negative if less than the cutoff value. The cutoff value was set to 0.5 pg/mL (10 pg/mL in a 20-fold tear dilution), which is the lower limit of the assay.
Statistical Analysis
Differences between groups were identified using the Mann-Whitney U test or chi-square test. Correlations were assessed using Spearman’s rank correlation. A P-value of less than 0.05 was considered to be statistically significant.
RESULTS
Relationship Between Clinical Severity Score and ECP Expression in Tears
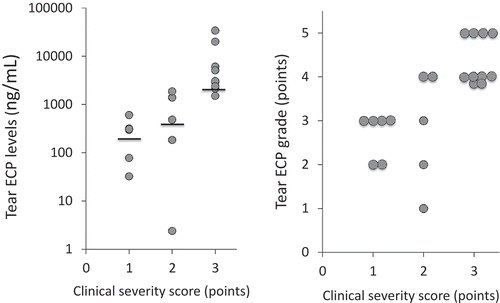
Median ECP levels in the subgroups with clinical severity scores of 1, 2, and 3 were 303 (range 32.4 to 604), 484 (2.40 to 1.87 × 103), and 3.00 × 103 (1.51 × 103 to 34.0 × 103) ng/mL, respectively (). In the AKC/VKC group, tear ECP grade was significantly correlated with the clinical severity score (r = 0.71, P<0.005, Spearman’s rank correlation; ).
Relationship Between Clinical Severity Score and CCL23 Expression in Tears
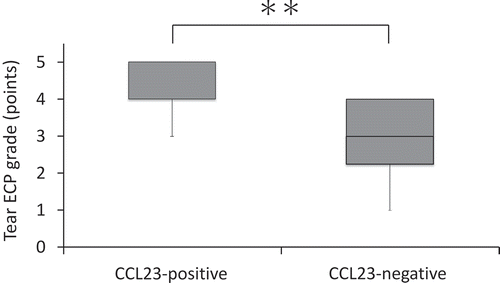
For CCL23 expression in tears, 7 of 21 patients in the AKC/VKC group were CCL23-positive, with mean (standard deviation) levels of 34.8 ± 18.4 pg/mL. Fourteen of 21 patients in the AKC/VKC group were CCL23-negative, with tear CCL23 levels in 14 patients being below the lower limit of detection.
Clinical severity scores in the CCL23-positive subgroup were significantly higher than those in the CCL23-negative subgroup (). Tear ECP grade in the CCL23-positive subgroup was significantly higher than in the CCL23-negative subgroup ().
TABLE 4. Relationship between CCL23 expression and clinical severity score.
DISCUSSION
Cycles of exacerbation and remission of cACD, including AKC and VK, continue for many years. In terms of medical treatment for these conditions, ophthalmic steroid, immunosuppressant, mast cell stabilizer, and histamine receptor antagonist solutions need to be given appropriately according to the severity of ACD. Another important issue in the treatment of cACD is adherence with administration of these solutions. To optimize adherence with medication, it is important that the patient understands his or her condition precisely, and participates in his or her own medical care in a positive way. Further, physicians must provide patients with precise practical information. The scoring methods used as part of the clinical examination for ACD help improve patient adherence with medication. In this clinical study, we investigated the validity of a scoring method for clinical severity and of the tear test in patients with ACD.
In this clinical study, we determined a clinical severity score based on objective findings using a 5-5-5 exacerbation grading scale. This scale includes clinical observations classified as severe, moderate, and mild to determine a clinical severity score, and is designed for patients with severe ACD and a high exacerbation grading score.Citation3 Therefore, we classified the severity of AKC and VKC as mild (1), moderate (2), or severe (3) and converted severity on the 5-5-5 exacerbation grading scale into clinical severity score.
The severity of ACD is also thought to depend on the degree of eosinophilic inflammation in the conjunctival tissue. Therefore, eosinophil-associated factors such as ECP and eotaxin were evaluated as biomarkers in the clinical tear test to evaluate the severity of ACDCitation5–Citation7,Citation13; ECP is a cytotoxic protein in eosinophil-specific granules. Histological investigation of conjunctival and corneal tissues in patients with AKC/VKC has shown deposition of eosinophilic-specific granule proteins such as ECP or major basic protein in shield ulcers, and eosinophils are thought to be associated with keratoconjunctival damage in patients with severe ACD.Citation14,Citation15 Further, tear ECP levels have been reported to increase in patients with ACD, particularly in those with VKC.Citation5–Citation7 However, the clinical significance of tear ECP levels in patients with ACD is not fully understood. In this study, we tried to diagnose the severity of AKC/VKC using tear ECP grade for the first time. In this clinical study, tear ECP grade correlated significantly with the clinical severity score, confirming that both the tear ECP grade and clinical severity score are useful clinical tools for assessing the severity of cACD.
This clinical study also evaluated the usefulness of CCL23 expression in tears as a biomarker for AKC/VKC. CCL23 interacts with CCR1 and recruits monocytes, macrophages, and dendritic cells that express CCR1 on their surface.Citation10 Further, CCL23 has been evaluated as a biomarker for certain inflammatory diseases, including rheumatoid arthritis,Citation16 systemic sclerosis,Citation17 and atopic dermatitis.Citation18 In this study, clinical severity scores and tear ECP grades in CCL23-positive patients with AKC/VKC showed high levels in comparison with those in CCL23-negative patients with AKC/VKC. Therefore, CCL23 is thought to be a useful biomarker expressed in tears in response to aggravation by AKC/VKC. In the eosinophilic inflammation associated with the pathophysiology of AKC/VKC, CCR3 expressed on eosinophils has attracted research attention, and CCR11/eotaxin-1, CCR24/eotaxin-2, CCR26/eotaxin-3, and RANTES, which are ligands for CCR3, were examined as eosinophilic chemokines associated with inflammation. In patients with VKC, it has been reported that levels of eotaxin-1 and eotaxin-2 in tears are significantly increased compared with those in controls, so these have also been identified as a biomarker for VKC.Citation13,Citation19 Further, there are reports that eosinophils are inflammatory cells expressing CCR1, and eosinophils express CCL23 in their cytoplasm.Citation20 In accordance with these previous reports, eosinophils are thought to be major inflammatory cells in the vicious cycle of allergic inflammation via CCR1. Therefore, in severe ACD, combined analysis of ECP and CCL23 in tears is thought to be a useful test for assessing allergic inflammation in the conjunctiva. Further investigation of the usefulness of combined analysis of ECP and CCL23 in tears will be necessary for various therapeutic drugs used in the medical treatment of cACD in the future.
In conclusion, we have confirmed the usefulness of tear ECP grade and expression of CCL23 in tears as a severity grading system in patients with cACD.
DECLARATION OF INTEREST
The authors declare that they have no conflicts of interest. The authors alone are responsible for the content and writing of this article.
ACKNOWLEDGMENTS
We wish to thank Mrs. Akiko Tomioka (Department of Visual Sciences, Division of Ophthalmology, Nihon University School of Medicine) for her expert technical assistance.
REFERENCES
- Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic sonjunctivitis: Clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15:482–488.
- Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36:777–784.
- Shoji J, Inada N, Sawa M. Evaluation of novel scoring system using 5-5-5 exacerbation grading scale for allergic conjunctivitis disease. Allergol Int. 2009;58:591–597.
- Venge P, Byström M, Carlson L, et al. Eosinophil cationic protein (ECP): Molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999;29:1172–1186.
- Montan PG, van Hage-Hamsten M. Eosinophil cationic protein in tears in allergic conjunctivitis. Br J Ophthalmol. 1996;80:556–560.
- Shoji J, Kitazawa M, Inada N, Sawa M, Ono T, Kawamura M, et al. Efficacy of tear eosinophil cationic protein level measurement using filter paper for diagnosing allergic conjunctival disorders. Jpn J Ophthalmol. 2003;47:64–68.
- Wakamatsu TH, Satake Y, Igarashi A, Dogru M, Ibrahim OM, Okada N, et al. IgE and eosinophil cationic protein (ECP) as markers of severity in the diagnosis of atopic keratoconjunctivitis. Br J Ophthalmol. 2012;96:581–586.
- Leonardi A, Borghesan F, Faggian D, Secchi A, Plebani M. Eosinophil cationic protein in tears of normal subjects and patients affected by vernal keratoconjunctivitis. Allergy. 1995;50:610–613.
- Patel VP, Kreider BL, Li Y, Li H, Leung K, Salcedo T, et al. Molecular and functional characterization of two novel human C-C chemokines as inhibitors of distinct classes of myeloid progenitors. J Exp Med. 1997;185:1163–1172.
- Youn BS, Zhang SM, Broxmeyer HE, Cooper S, Antol K, Fraser M Jr, et al. Characterization of CKbeta8 and CKbeta8-1: Two alternatively spliced forms of human beta-chemokine, chemoattractants for neutrophils, monocytes, and lymphocytes, and potent agonists at CC chemokine receptor 1. Blood. 1998;91:3118–3126.
- Matsumoto K, Fukuda S, Hashimoto N, Saito H. Human eosinophils produce and release a novel chemokine, CCL23, in vitro. Int Arch Allergy Immunol. 2011;155 Suppl 1:34–39.
- Takamura E, Uchio E, Ebihara N, Ohno S, Ohashi Y, Okamoto S, et al. Japanese guideline for allergic conjunctival diseases. Allergol Int. 2011;60:191–203.
- Shoji J, Inada N, Sawa M. Evaluation of eotaxin-1, -2, and -3 protein production and messenger RNA expression in patients with vernal keratoconjunctivitis. Jpn J Ophthalmol. 2009;53:92–99.
- Trocme SD, Kephart GM, Bourne WM, Buckley RJ, Gleich GJ. Eosinophil granule major basic protein deposition in corneal ulcers associated with vernal keratoconjunctivitis. Am J Ophthalmol. 1993;115:640–643.
- Messmer EM, May CA, Stefani FH, Welge-Luessen U, Kampik A. Toxic eosinophil granule protein deposition in corneal ulcerations and scars associated with atopic keratoconjunctivitis. Am J Ophthalmol. 2002;134:816–821.
- Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M, et al. Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor alpha, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor. Arthritis Rheum. 2008;58:2257–2267.
- Yanaba K, Yoshizaki A, Muroi E, Ogawa F, Asano Y, Kadono T, et al. Serum CCL23 levels are increased in patients with systemic sclerosis. Arch Dermatol Res. 2011;29–34.
- Novak H, Müller A, Harrer N, Günther C, Carballido JM, Woisetschläger M. CCL23 expression is induced by IL-4 in a STAT6-dependent fashion. J Immunol. 2007;178:4335–4341.
- Leonardi A, Jose PJ, Zhan H, Calder VL. Tear and mucus eotaxin-1 and eotaxin-2 in allergic keratoconjunctivitis. Ophthalmology 2003;110:487–492.
- Poposki JA, Uzzaman A, Nagarkar DR, Chustz RT, Peters AT, Suh LA, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:73–81.