ABSTRACT
The extraction of nickel (Ni) from sulfide resources commences with flotation to produce a concentrate which is then smelted to produce a nickel-enriched phase called matte, and further refined to produce pure Ni products as well as by-products, such as cobalt (Co), copper (Cu) and precious metals. However, the traditional concentrate smelting-matte refining process, whilst technologically robust, is capital intensive and suffers from several environmental and technical issues such as sulfur dioxide emissions, poor recovery of cobalt and difficulty processing concentrates high in magnesia and arsenic without appropriate blending with high grade concentrates to dilute the concentration of these species. The direct hydrometallurgical processing of nickel sulfide concentrates and whole ores may be a remedy to these issues and hydrometallurgy offers several advantages over pyrometallurgy such as potentially lower capital costs, the ability to process lower grade materials and produce marketable metals or compounds directly from ore/concentrate. Despite the advantages of hydrometallurgy over traditional base metal sulfide smelting, the hydrometallurgical processing of nickel concentrates has been limited to a small handful of commercial operations, some of which only produce an upgraded intermediate for pyrometallurgical processing. In Part I of this three-part series, a comprehensive review of piloted processes for direct hydrometallurgical processing of nickel sulfide concentrates is presented, followed by a survey of industrial operations which have carried out direct leaching of nickel sulfide concentrates. A review of research activities and challenges/opportunities in the direct hydrometallurgical processing of nickel sulfide concentrates are presented in Part II and Part III of this series.
1. Introduction
Nickel (Ni) is an essential metal for society with stainless steels being its major end use, though high purity nickel sulfate, a key raw material for production of lithium-ion battery (LIB) cathodes essential for electric vehicles (EVs) is a rapidly growing market (McRae Citation2020a). Nickel demand has traditionally been driven by stainless steel production, either in the form of class 2 (<99.8% Ni) nickel products such as nickel oxide, ferronickel and nickel pig iron (NPI), or higher purity class 1 (>99.8% Ni) nickel such as nickel powder, briquettes or electrowon cathode (Campagnol et al. Citation2017). Campagnol et al. (Citation2017) have indicated that demand for class 1 nickel in the form of high purity nickel sulfate for lithium-ion batteries is expected to reach 570,000 tonnes (Ni metal basis) in 2025.
Terrestrial nickel resources are contained in sulfide and lateritic deposits, with 30–40% of the land-based nickel resources being contained in sulfide deposits with the balance in laterite deposits (Dalvi, Bacon, and Osborne Citation2004; Hoatson, Jaireth, and Jaques Citation2006; Mudd Citation2010). Major nickel sulfide deposits are in Australia, Canada, Russia and Southern Africa whilst most nickel laterite deposits are located in Indonesia, New Caledonia, Brazil and the Philippines (Hoatson, Jaireth, and Jaques Citation2006). Despite laterites being more abundant than sulfide deposits, Ni production has historically been derived from sulfides due to these resources being more amenable to beneficiation and less challenging to process, resulting in lower costs relative to Ni production from laterites (Elias Citation2002; Mudd Citation2010). However, recent data from the United States Geological Survey (USGS) show that Ni production from laterite ores has overtaken sulfide resources over the 2011–2016 period (McRae Citation2018, Citation2020b). The key driver appears to be Chinese stainless-steel producers using NPI rather than class 1 nickel to reduce manufacturing costs (Campagnol et al. Citation2017); Ni production data from China show that approximately 60% of Ni production in 2016 was in the form of ferronickel and NPI (McRae Citation2020b). Since 2014, NPI production has also expanded in Indonesia, coinciding with the ban by the Indonesian government on the export of direct shipping nickel laterite ores (McRae Citation2020b).
Lateritic ores are not typically amenable to beneficiation and therefore direct processing of whole ore using hydrometallurgical or pyrometallurgical techniques is necessary with the choice of processing method being dependent on the type of ore (Dalvi, Bacon, and Osborne Citation2004; Elias Citation2002; Marsh, Anderson, and Gray Citation2013). High pressure acid leaching (HPAL) is the preferred method for the extraction of Ni and Co from oxide (limonite) and clay silicate type ores to produce a diverse range of products (i.e. mixed sulfide precipitate, mixed hydroxide precipitate, metal powder, cathode) whilst saprolitic ores are generally processed via pyrometallurgy to produce either ferronickel or sulfide matte (Dalvi, Bacon, and Osborne Citation2004; Elias Citation2002; Marsh, Anderson, and Gray Citation2013). Nickel laterite projects employing hydrometallurgical or pyrometallurgical processing have often suffered from high capital expenditure and cost blowouts, have required long ramp up times, suffered from technical issues relating to commissioning and ramp up, and in the case of HPAL, have at times failed to achieve nameplate capacity (Jones et al. Citation2010; McDonald and Li Citation2020; Oxley, Smith, and Caceres Citation2016). The extraction of Ni from sulfide ores is much simpler relative to laterites and is dominated by pyrometallurgical processing. This typically involves comminution and flotation to produce a nickel concentrate which is subsequently smelted and converted to produce a sulfur-deficient, metal-enriched phase termed matte, which is then refined to produce pure Ni, Cu, Co metal products and precious and platinum group metals (PGMs) where applicable (Crundwell et al. Citation2011b). A simplified flow chart of the flotation-smelting-matte refining process for treatment of nickel sulfide ores is presented in . There is great deal of variation in the standard flow sheet with respect to flotation practice (i.e. mineralogy, liberation size, reagents, etc.) and converter matte refining, however concentrate smelting is dominated by either flash smelting or roasting-electric furnace smelting technology whilst matte converting is almost exclusively done using Peirce-Smith converters (Moskalyk and Alfantazi Citation2002; Warner et al. Citation2007).
Figure 1. Simplified traditional process flowsheet for the extraction of Ni, Cu and Co from nickel sulfide resources.
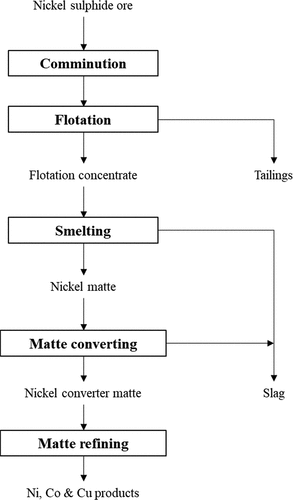
The objective of concentrate smelting is to remove part of the sulfur and iron present in the concentrate by oxidation; sulfur is oxidized to sulfur dioxide (SO2) whilst iron forms iron oxide which is fluxed by reaction with silica to form an iron silicate slag (Crundwell et al. Citation2011f). This iron silicate slag also removes any non-sulfide gangue present in the concentrate which dissolves into the molten slag (Crundwell et al. Citation2011f). Typical base metals content of concentrates utilized for smelting contain a combined Ni, Cu and Co grade of 8% to above 20%, though lower grade concentrates having a combined base metal concentration of 3–4% are smelted in Southern Africa due to the high PGM content of these materials (Warner et al. Citation2007). Concentrate smelting is carried out either by roasting-electric furnace smelting or via flash smelting and the key process steps in both processes are presented in ; the key principles of both methods are covered in detail elsewhere (Crundwell et al. Citation2011c, Citation2011f). The production details of both pyrometallurgical processing routes are presented in including typical Ni and Cu grades in the concentrates, mattes and slags. It can be seen from that either pyrometallurgical route is very efficient at recovering Ni, Cu and precious metals to the matte. Base metal contents in slags produced from flash smelting are generally higher and recoveries are lower relative to the electric furnace route due to the more oxidizing conditions employed, however slags are processed to recover base metals through slag cleaning operations. Cobalt recoveries are poor by either route (Matousek Citation1982; Warner et al. Citation2007).
Figure 2. Process steps during the pyrometallurgical processing of nickel sulfide concentrates to produce nickel converter matte via flash smelting or the roasting-electric furnace melting route. Adapted from Crundwell et al. (Citation2011f).
Table 1. Production details of nickel concentrate smelting via the roasting-electric furnace smelting route and the flash smelting route. Data for roasting-electric furnace smelting and flash smelting adapted from Crundwell et al. (Citation2011c, Citation2011f).
The final product from nickel concentrate smelting by either route is a molten nickel sulfide enriched phase (matte) which typically contains 15–40% Ni, 20–40% Fe, and 20–25% S as well as Cu, Co and any PGMs (Crundwell et al. Citation2011a). The molten matte is transferred to a converter where iron is oxidized using air or oxygen and removed from the matte as an iron silicate slag; the final product, termed converter matte, typically contains 40–70% Ni, 0.5–4% Fe, 21–24% S as well as Cu and Co (Crundwell et al. Citation2011a; Warner et al. Citation2007). There is a great deal of variation with regards to the processing of nickel converter matte to recover base and precious metals, with direct hydrometallurgical processing of granulated converter matte being practiced at some refineries (Warner et al. Citation2007). In other instances, such as mattes arising from smelting of nickel-copper concentrates and concentrates high in PGMs, slow cooling of the converter matte is carried out to enable copper and nickel phase separation and the formation of coarse sized crystals of chalcocite (Cu2S), heazlewoodite (Ni3S2) and a metallic alloy phase enriched in precious and platinum group metals; the cooled matte is beneficiated to produce separate Cu and Ni-sulfide concentrates and a PGM-enriched concentrate for further processing (Bulatovic Citation2007; Crundwell et al. Citation2011e; Warner et al. Citation2007). Magnetic separation is carried out at Anglo American Platinum’s refinery in Rustenburg, South Africa, on slow-cooled mattes to recover the PGMs, with the non-magnetic fraction (nickel-copper matte) being processed via hydrometallurgy in a dedicated base metals refinery producing copper cathode and metallic nickel and cobalt powders (Crundwell et al. Citation2011d). Processes for the smelting of nickel flotation concentrates to produce matte followed by matte refining to produce Ni, Co and Cu products are well established. The primary advantages of nickel sulfide concentrate smelting are the following (Crundwell et al. Citation2011b, Citation2011c, Citation2011d, Citation2011f):
Combustion of part of the sulfur and iron in the concentrate can supply most of the energy requirements for smelting (flash smelting only) and converting, with the added advantage of removing a large part of the iron and sulfur from the concentrate as slag and sulfur dioxide (SO2), respectively, simplifying downstream matte refining,
Starting from a nickel flotation concentrate of grade 5–20% Ni, smelting and converting produces a nickel matte containing 40–70% Ni, resulting in a considerable reduction in size of downstream leaching and refining equipment relative to direct leaching of flotation concentrates,
Precious metals report to the matte and are more concentrated relative to the original flotation concentrates
Against these advantages are several drawbacks such as:
Difficulty processing nickel concentrates high in arsenic (As) and magnesia (MgO), which can render many concentrates, particularly those produced from disseminated sulfide ores and talc-carbonate ores, unsuitable if they cannot be blended with higher quality concentrates to reduce the concentration of the deleterious species,
Poor recovery of cobalt to the matte phase during smelting and converting, where typically 30–50% of cobalt is lost to the metallurgical slag (Matousek Citation1982; Warner et al. Citation2007),
High capital cost associated with concentrate smelting and matte converting, including the capture of SO2 and its conversion to sulfuric acid. The latter can only be justified where there is a market for the sulfuric acid that is produced (Lu et al. Citation2000),
Plant hygiene issues relating to the use of ladles and launders during pyrometallurgical processing (Dutrizac Citation1992).
Elevated MgO grade (as talc, orthopyroxene etc.) in nickel smelter feeds cause operational problems for the smelter due to MgO raising the slag liquidus temperature and viscosity, leading to poor slag-matte separation and higher metal losses due to entrainment (Eksteen, Oraby, and Nguyen Citation2020; Lotter et al. Citation2008). This necessitates furnace operation at higher temperature, which shortens the life of the furnace refractories. The MgO content in the flotation concentrate(s) must be limited to below 7–10% to prevent operational problems during smelting (Feng et al. Citation2012; Senior and Thomas Citation2005). Arsenic in the smelter feed is also problematic both from an environmental and operational perspective. Toguri, Babaie, and Sridhar (Citation1995) has shown that the presence of As complicates the refining of Cu-Ni mattes, leading to a decrease in grain size of chalcocite and alloy phases during cooling and altering the morphology of chalcocite (from dendritic to spherical in the presence of As). Whilst As can sometimes be rejected at the concentrator, this can be an issue when the As is present as niccolite (NiAs) and gersdorffite (NiAsS), which will result in nickel losses to the mill tailings.
Other situations where smelting may be unjustified include if the size of the resource to be processed is too small to justify the high capital cost associated with a dedicated smelter and matte refining facility, or when the transport cost to ship nickel concentrate(s) to a toll smelter is prohibitively high (Fleming et al. Citation2000). Under these circumstances, direct hydrometallurgical processing of nickel sulfide concentrates can be a viable alternative, and many of the technical, economic and environmental challenges imposed on smelting have spurred research and development activities around hydrometallurgical processes for the extraction of Ni, Co and other base metals (Cu, Zn) from sulfide concentrates. Direct hydrometallurgical processing of whole nickel sulfide ores has also received considerable attention, particularly bioleaching, which was the subject of a review by Watling (Citation2008). Despite the level of research and development over the years on nickel concentrate hydrometallurgy, the adoption of hydrometallurgical processes for the extraction of Ni, Co and Cu from nickel flotation concentrates remains low, particularly given several operations utilize some form of hydrometallurgical processing to produce an upgraded sulfide product for smelting, or the operation originally built for processing sulfide concentrates has switched over to other nickel sulfide feedstocks.
The purpose of Part I of this three-part series is to review and summarize piloted leaching processes as applied to the direct hydrometallurgical processing of nickel sulfide flotation concentrates and secondly, to survey commercial nickel operations which have implemented hydrometallurgical processing. An overview of the geology and mineralogy of nickel sulfide deposits, as well as current beneficiation practice is also provided. Following an examination of pilot plant developments and commercial operations concerning the hydrometallurgy of nickel sulfide concentrates, laboratory research activities concerning direct leaching of nickel sulfide concentrates are reviewed in Part II and Part III of this series and the challenges and research opportunities relating to nickel sulfide concentrate hydrometallurgy are discussed.
2. Nickel sulfide deposits
Nickel sulfide deposits are found on most continents, however major deposits are found in Russia, Australia, Canada, and Southern Africa (Hoatson, Jaireth, and Jaques Citation2006). Canada and Russia possess the largest resources of nickel sulfides, followed by Australia and South Africa; however, Australia has the largest nickel resources when laterites are also considered (Hoatson, Jaireth, and Jaques Citation2006). All nickel sulfide deposits are magmatic in origin and are broadly characterized as either sulfide rich (>5%) or sulfide poor (<5%); for sulfide rich deposits, Ni and Cu are of primary economic interest whilst for sulfide poor deposits, generally PGMs are of economic importance (Naldrett Citation1999; Song, Wang, and Chen Citation2011). The major nickel sulfide deposits including the nickel grade, contained nickel metal and year of discovery are presented in ; typical Ni and Cu concentrations in magmatic Ni-Cu sulfide deposits are 0.7–3% Ni and 0.2–2% Cu (Eckstrand and Hulbert Citation2007); the deposits of the Bushveld Igneous Complex (South Africa) are lower grade but possess high concentrations of PGMs for which they are primarily mined. The variation in Ni grade among some of the major deposits is indicative of the proportion of high-grade massive sulfide ore to low grade disseminated sulfide ore (Barnes, Barnes, and Perring Citation2007). It can be seen from that the Sudbury complex (Canada) and the Noril’sk-Talnakh deposits (Russia) are the largest sulfidic nickel deposits, being far larger than the other listed deposits based on contained Ni metal alone. Whilst Australia possesses significant reserves of sulfidic nickel and is ranked after Canada and Russia, the deposits are much smaller.
Table 2. Nickel grade, contained nickel metal (in tonnes) and year of discovery of major nickel sulfide deposits across the globe; data adapted from Hoatson, Jaireth, and Jaques (Citation2006) unless stated otherwise.
2.1. Sulfide mineralogy
A list of economically important nickel sulfide and arsenide minerals, as well as common sulfidic gangue in nickel sulfide deposits is presented in . Pentlandite is the most important nickel sulfide mineral and commonly occurs with pyrrhotite and chalcopyrite. Other nickel sulfides listed in can be important where alteration has taken place. Violarite is a supergene alteration product of pentlandite and typically occurs in pyrite-violarite and transition zones above the primary zone (massive pentlandite-pyrrhotite ore) (Marston et al. Citation1981; Nickel, Ross, and Thornber Citation1974) and can be economically important during the mining of some massive nickel sulfide ores. Millerite forms as an alteration product of pentlandite (Bide, Hetherington, and Gunn Citation2008; Holwell et al. Citation2017) and is an important nickel sulfide mineral in some deposits such as Mt Keith, Black Swan and the Otter Shoot, Kambalda (Dowling et al. Citation2004; Grguric et al. Citation2007; Keele and Nickel Citation1974). Niccolite and gersdorffite can occur in nickel sulfide deposits as a result of hydrothermal alteration; their presence in nickel concentrates is undesirable due to the deleterious effects of As during smelting and typically requires dilution to an acceptable level by blending with low As concentrates (Grguric et al. Citation2007). Pyrrhotite, an iron sulfide, is the primary gangue sulfide that occurs in nickel sulfide deposits though it typically contains Ni, either in solid solution or as fine pentlandite intergrowths (Rezaei et al. Citation2017; Toguri Citation1975). Typically, the Ni concentration in solid solution in pyrrhotite is 0.4–0.6% and the presence of micron-size inclusions of pentlandite can raise the nickel content even higher (Rezaei et al. Citation2017; Toguri Citation1975). Hence during beneficiation of nickel sulfide deposits, nickel loss due to pyrrhotite rejection is inevitable.
Table 3. Economically important nickel sulfide and arsenide minerals and their mode of occurrence in nickel sulfide deposits listed in terms of increasing Ni content (wt%) and sulfidic gangue typically present in nickel sulfide deposits.
2.2. Ore textures
Nickel sulfide ores are broadly classified into the following categories: massive sulfide ore, matrix ore (also termed net-textured ore) and disseminated sulfide ore, the distinction being based on the sulfide content and silicate texture (Barnes and Lightfoot Citation2005). Massive sulfide ores consist of 75–100% sulfides as pyrrhotite, pentlandite and chalcopyrite, with or without minor pyrite (Barnes et al. Citation2018; Barnes, Barnes, and Perring Citation2007). Where silicate inclusions are present in massive ores, they are generally dispersed and isolated (Barnes et al. Citation2017; Jones Citation1996). Matrix sulfide ores comprise silicate inclusions in a matrix of sulfide, the sulfide making up 30–70% of the ore (Barnes et al. Citation2017). Disseminated sulfide ores contain between 1% and 33% sulfides dispersed in a silicate matrix, the sulfides are not interconnected and are present as patches in the silicate interstices or as globules (Barnes and Lightfoot Citation2005). The presence of multiple ore textures/types in a single deposit is not uncommon; for example, the Jinchuan deposit (Gansu province, China) contains both disseminated and matrix ore (Naldrett Citation2004) whilst all three ore textures are found at the Noril’sk-Talnakh camp (Barnes et al. Citation2018; Eckstrand and Hulbert Citation2007). The massive sulfide ores at Noril’sk-Talnakh are high grade, containing 3.42% Ni and 5.38% Cu, but are of lower tonnage (88.7 Mt) relative to the lower grade disseminated ore (1706.3 Mt) which grades 0.51% Ni and 1.02% Cu (Eckstrand and Hulbert Citation2007). Consequently, different flotation regimes have been employed for processing the various types of Ni-Cu sulfide ores present at the Noril’sk-Talnakh camp (Kozyrev et al. Citation2002).
2.3. Beneficiation practice
Nickel sulfide ores are subjected to mineral beneficiation to reject sulfidic and non-sulfidic gangue and produce a nickel concentrate grading from 5% to 25% Ni suitable for smelting (MäKinen and Taskinen Citation2008). Beneficiation is accomplished through comminution and froth flotation (refer to ); in some instances, magnetic separation is employed before or after flotation to reject monoclinic pyrrhotite which is ferromagnetic (Agar Citation1991; Bulatovic Citation2007). The choice of unit operations and operating parameters is largely dictated by the type of ore being processed i.e. massive sulfide ore vs disseminated sulfide ore which subsequently influences the liberation size and choice of flotation collectors and depressants for separation of the values from the gangue. Other factors to consider include if the ore has been altered, which can bring about mineralogical and textural changes; the effects of supergene alteration and serpentinization on nickel sulfide flotation have been well documented (Eltham and Tilyard Citation1973; Kerr Citation2002; Mani et al. Citation1997; Mishra, Viljoen, and Mouri Citation2013; Sizgoric Citation1981). Flotation practice differs by geographical location. Bulk sulfide flotation is generally carried out on Western Australian nickel ores which tend to be low in copper and contain substantial concentrations of magnesium bearing minerals and hydrophobic gangue such as talc (Bulatovic Citation2007; Eltham and Tilyard Citation1973; Pietrobon et al. Citation1997). Bulk sulfide flotation is also carried out on ores from the Merensky Reef and UG-2 chromitite in South Africa due to the association of PGMs with base metal sulfides in these ores (Xiao and Laplante Citation2004); the concentrates are lower grade (3–4% combined base metals) but possess high concentrations of PGMs (100–400 g/t) (Warner et al. Citation2007). Flotation practice in Russia and Canada differs from Western Australia where the deposits tend to be massive copper-nickel sulfide ores. In these ores, pyrrhotite is the major gangue mineral and flotation is carried out to reject pyrrhotite and produce separate copper and nickel concentrates. Magnetic separation is generally incorporated in the flowsheets to remove monoclinic pyrrhotite (Agar Citation1991; Bulatovic Citation2007). Pyrrhotite rejection is necessary as the smelting of pyrrhotite generates large quantities of SO2 but yields very little nickel (Senior, Shannon, and Trahar Citation1994). Pyrrhotite rejection however must be balanced against Ni losses during beneficiation since pyrrhotite contains Ni in solid solution or as exsolved pentlandite that is too fine to liberate (Bulatovic Citation2007; Kerr Citation2002; Senior, Shannon, and Trahar Citation1994). Sequential flotation is employed to process massive sulfide ore from the Noril’sk-Talnakh deposit in Russia and produce separate copper, nickel and pyrrhotite concentrates (Bulatovic Citation2007; Kozyrev et al. Citation2002); the pyrrhotite concentrate has a high concentration of Ni, Cu and PGMs and is processed further in a dedicated hydrometallurgical facility to recover the contained metals (refer to Section 3.2.2).
3. Piloted processes and commercial operations
In response to the technical and environmental hurdles presented by the traditional concentrate smelting-matte refining route, the direct leaching of nickel sulfide flotation concentrates has been intensely researched and a number of these processes have been piloted and, in some cases, have seen commercial implementation. Several nickel processing operations have practiced hydrometallurgical processing of nickel sulfide flotation concentrates or whole ore at commercial scale and these are summarized in . It can be seen in that there is wide variability in terms of the feedstocks processed, finished nickel products and leaching technology employed. A number of these operations are now closed or no longer process flotation concentrates, having undergone modifications to the original refineries to accommodate changes in feedstock (i.e. Kwinana, WA and Fort Saskatchewan, Canada). Other operations such as the Nadezhdin mill (Russia) and the Cosmic Boy nickel concentrator (West Australia) carry out hydrometallurgical processing to produce upgraded intermediates which are blended with flotation concentrates destined for smelting. The processing of nickeliferous pyrrhotite concentrates is practiced at the Nadezhdin mill (Russia) whilst historic production took place in Canada (refer to ). The Canadian operations are unique in that roast-leach type processes were utilized to extract base metals from the pyrrhotite and produce an iron oxide residue suitable for production of iron ore pellets destined for steel making. The purpose of this section is to summarize piloted processes that have been developed to extract Ni directly from flotation concentrates and review several commercial operations that have practised direct hydrometallurgical processing. While a number of these operations are now closed or no longer process flotation concentrates, a description has been provided for historical purposes.
Table 4. Commercial nickel operations that have processed nickel-bearing sulfide concentrates via hydrometallurgical methods.
3.1. Ammoniacal leaching processes
The ability of ammonia to selectively dissolve Cu, Ni, Co and Zn from their ores and concentrates has formed the basis for several commercially developed leaching processes. The first commercial operations utilizing ammonia-ammonium carbonate solution as a lixiviant were deployed to extract Cu from mill tailings by Kennecott in Alaska, and Calumet and Heca in Michigan, USA (Forward and Mackiw Citation1955; Meng and Han Citation1996). Anaconda’s Arbiter process and BHP’s Escondida process were developed to extract copper from sulfide flotation concentrates utilizing ammonia-ammonium sulfate as a lixiviant (Duyvesteyn and Sabacky Citation1993; Kuhn, Arbiter, and Kling Citation1974). Ammonia-ammonium lixiviants have been utilized for the extraction of Ni and Co from lateritic ores via the Caron process, which involves reduction roasting followed by ammonia-ammonium carbonate leaching (Caron Citation1950) and was in use at the Yabulu refinery in Queensland for processing laterite ores (now in care and maintenance) and by Inco (now Vale) until 1982 for treating pyrrhotite concentrates via a modified process (see Section 3.6.2). The only commercialized ammonia leaching process to date for the direct treatment of nickel sulfide concentrates is the Sherritt-Gordon ammonia pressure leaching process which is currently in use at Fort Saskatchewan, Canada, and Kwinana, Western Australia. The Sherritt-Gordon process is essentially a pressure oxidation process carried out in aqueous ammonia-ammonium sulfate at elevated temperature using air as an oxidant; the key operating characteristics and features of the process are listed in and compared against other piloted and commercialized processes. The leaching technology was developed to process nickel concentrate from Lynn Lake (Canada) grading 10–16% Ni, 1–2% Cu and 0.3–0.4% Co, where a hydrometallurgical process was deemed to be more cost effective than concentrate smelting and matte refining (Forward Citation1953; Forward and Mackiw Citation1955; Kerfoot Citation1989).
Table 5. Summary of hydrometallurgical processes developed for the direct leaching of nickel sulfide flotation concentrates and status of the technology.
In the Sherritt-Gordon Process, base metals are dissolved from the sulfide mineral particles by reaction with oxygen, water and ammonia; ferrous iron is oxidized and precipitated as hydrous ferric oxide (Forward Citation1953; Forward and Mackiw Citation1955). Sulfidic sulfur (S2-) is oxidized to thiosulfate (S2O32-) which then undergoes further oxidation by dissolved oxygen in solution to ultimately form sulfate (SO42-) and sulfamate (NH2SO32-) via a series of sequential reactions involving intermediates such as trithionate (S3O62-) (Forward Citation1953; Forward and Mackiw Citation1955). Dissolved copper in solution is believed to play a catalytic role in oxidation of thiosulfate to trithionate, sulfate and sulfamate (Forward Citation1953). Batch leaching tests by Forward (Citation1953) showed that the nickel dissolution rate was influenced by factors such as temperature, oxygen partial pressure and free ammonia concentration; 95% of Ni could be extracted in under an hour by leaching at 220°F (104.4°C). Higher leaching temperatures and oxygen partial pressures, and longer retention times favor the oxidation of thiosulfate and trithionate to sulfate and sulfamate, however, a key aspect of the Sherritt-Gordon process is the retention of a portion of the unsaturated sulfur containing ions in solution for subsequent copper removal (Forward Citation1953; Forward and Mackiw Citation1955). Copper removal is achieved by boiling the solution (termed “copper boil”), where Cu(II) ions react with thiosulfate and trithionate in solution, precipitating copper as covellite (CuS) and chalcocite (Cu2S) (Forward Citation1953; Forward and Mackiw Citation1955). Precious and platinum group metals present in nickel matte are reported to be partially extracted during ammoniacal pressure leaching and are subsequently precipitated during the “copper boil” stage, reporting to the copper sulfide precipitate (Wishaw Citation1993). The copper-free solution produced from the ‘copper boil’ is treated in an autoclave at 245°C and air pressure of 4100 kPa to destroy residual unsaturated sulfur compounds and ammonium sulfamate present in solution (termed oxydrolysis) prior to metallic nickel recovery by pressure hydrogen reduction (Forward Citation1953; Wishaw Citation1993). The oxydrolysis step is necessary to prevent sulfur contamination in the final nickel product and to remove sulfamate, which is a herbicide and would not be permissible in the final ammonium sulfate by-product if used as a fertilizer (Wishaw Citation1993). The final products from the Sherritt-Gordon process are metallic nickel powder or briquettes, cobalt metal powder, briquette or sulfide precipitate, and ammonium sulfate (Forward Citation1953; Wishaw Citation1993).
The operations at Fort Saskatchewan and Kwinana have demonstrated the adaptability of the Sherritt-Gordon ammonia pressure leach technology to processing different nickel sulfide feedstocks (flotation concentrates, matte or mixed sulfide precipitate). A significant advantage of the leach process for concentrates is its selectivity toward Ni, Cu and Co, with iron present in pentlandite, chalcopyrite and pyrrhotite being converted to a hydrous ferric oxide residue (Forward and Mackiw Citation1955). Pyrite present in the concentrate does not react in the ammoniacal solution and reports to the residue; this does however mean that any Ni and Co present in pyrite are not dissolved during leaching (Forward and Mackiw Citation1955). Other advantages of the Sherritt-Gordon ammonia leach process are that metallic nickel can be recovered directly from solution by hydrogen reduction, and part of the ammonia can be recovered for re-use in the leaching stage. The main disadvantages of the Sherritt-Gordon ammonia leach process are the high cost of ammonia relative to sulfuric acid, though this is partially offset by recovery and recycling, the generation of ammonium sulfate [(NH4)2SO4] as a by-product, which requires disposal if there is no market for it, and cobalt recovery and refining is considerably more complex relative to acid-based leaching processes. Cobalt refining at Fort Saskatchewan involves complex multi-stage processing of cobalt intermediates (MSP or Co(III) hexamine sulfate) via leaching, recrystallization and hydrogen reduction; by contrast cobalt recovery is simpler in acid sulfate systems where cobalt separation from nickel is well established and is achieved by conventional solvent extraction and electrowinning (SX-EW).
3.1.1. Fort Saskatchewan, Canada
The ammonia pressure leach process was originally developed to treat Ni-Cu-Co flotation concentrate from Sherritt-Gordon Mines’ Lynn Lake concentrator in Canada (Deng Citation1994; Forward Citation1953; Forward and Mackiw Citation1955; Kerfoot Citation1989). Once the Lynn Lake Mine became exhausted (mid 1970s) the refinery continued to operate, processing pentlandite concentrates from across Canada and nickel mattes from overseas (Kerfoot and Cordingley Citation1997). The Fort Saskatchewan nickel refinery continues to operate to this day, still employing ammonia pressure leaching; however, since 1990 mixed Ni-Co sulfide precipitate produced as an intermediate from nickel laterite processing at Moa Bay (Cuba) is the primary feed to this refinery (Kerfoot and Cordingley Citation1997; Kofluk and Freeman Citation2006). The flowsheet for the original refinery operation at Fort Saskatchewan for concentrate processing is presented in and involved ammonia pressure leaching in a train of autoclaves operating at a temperature range of 80–95°C and used compressed air as the oxidant at a pressure of 800 kPa (Kerfoot and Cordingley Citation1997). Nickel, cobalt and copper are leached into solution as their ammine complexes whilst sulfur is oxidized to sulfate, sulfamate and unsaturated sulfur compounds such as trithionate, thiosulfate etc (Forward Citation1953; Forward and Mackiw Citation1955). Iron present in the concentrate is oxidized and precipitates as a hydrous ferric oxide residue (Forward and Mackiw Citation1955). Copper is removed from the leach liquor by precipitation as its sulfide by reaction with unsaturated sulfur compounds present in the liquor (Forward Citation1953; Forward and Mackiw Citation1955). An interesting feature of the Sherritt-Gordon ammonia leach process is control of the degree of sulfur oxidation in the autoclaves to ensure there is sufficient unsaturated sulfur compounds present in the liquor for subsequent copper sulfide precipitation. Following on from copper precipitation, further removal of residual unsaturated sulfur compounds and sulfamate in the Ni-Co leach liquor is carried out by boiling in an autoclave (Forward Citation1953; Forward and Mackiw Citation1955). The process chemistry of the copper removal and oxydrolysis stages are discussed in detail by Forward (Citation1953) and Forward and Mackiw (Citation1955).
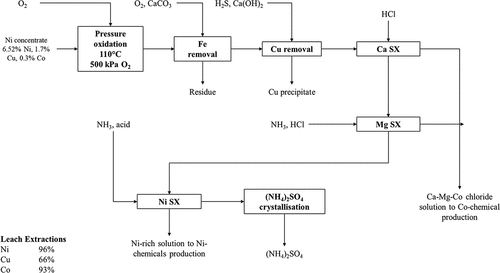
Figure 3. Simplified process flowsheet of the Sherritt-Gordon ammonia pressure leach process for leaching of Ni sulfide concentrates and recovery of Ni via pressure hydrogen reduction; refining of the mixed sulfide precipitate has been omitted. Typical operating conditions of some unit operations in the Sherritt-Gordon process are presented along with overall metal recoveries during processing of a nickel concentrate grading 10–14% Ni. Adapted from Forward (Citation1953), Forward and Mackiw (Citation1955), Kerfoot and Cordingley (Citation1997) and Wishaw (Citation1993).
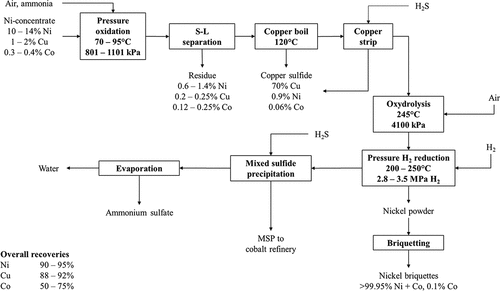
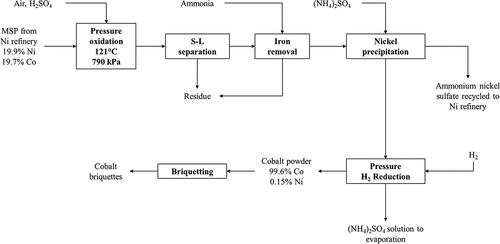
Metallic Ni powder is produced via hydrogen reduction of the copper-free solution in an autoclave at elevated temperature and pressure and the resultant powder is briquetted (Forward Citation1953; Forward and Mackiw Citation1955). The solution from Ni reduction is treated with H2S to precipitate Ni and Co as a mixed sulfide precipitate (MSP) and the barren solution is then processed further to recover ammonium sulfate as a by-product (Forward Citation1953; Forward and Mackiw Citation1955; Mackiw et al. Citation1958). The MSP product is further processed in a cobalt refinery, the main process steps of which are depicted in ; leaching is carried out via sulfuric acid pressure oxidation in an autoclave at 121°C and pressure of 790 kPa (absolute) using air as an oxidant (Mackiw et al. Citation1958). The leach solution is purified to remove dissolved iron by adjusting the pH to 4.9–5.1 using ammonia and sparging air to oxidize any ferrous iron to the ferric state (Mackiw et al. Citation1958). After iron removal, solid ammonium sulfate is added to increase the concentration of (NH4)2SO4 in solution to the level required for cobalt reduction, and to remove Ni from solution as nickel ammonium sulfate which is recycled to the nickel reduction circuit (Forward Citation1953; Mackiw et al. Citation1958). Cobalt is recovered from solution by hydrogen reduction at elevated temperature and pressure in an autoclave to form cobalt powder (Mackiw et al. Citation1958).
Figure 4. Cobalt recovery from mixed sulfide precipitate (MSP) as originally practiced at Fort Saskatchewan (Canada) prior to 1990. Adapted from Forward (Citation1953) and Mackiw et al. (Citation1958).
The refinery at Fort Saskatchewan has undergone significant modifications to the leaching and cobalt refining circuit due to changes in the feed, with the refinery switching over to the processing of MSP from Moa Bay, Cuba. The MSP grades 55 wt% Ni and contains high concentrations of cobalt (5 wt%) and could not be processed satisfactorily in the original refinery (Kerfoot and Cordingley Citation1997). Mixed sulfide precipitate from Moa Bay comprises 80% of the feed to the refinery, the balance being nickel matte and nickel sulfate by-product from copper refineries (Kerfoot and Cordingley Citation1997). Nickel and cobalt are dissolved from the MSP by pressure leaching in aqueous ammonia using air as an oxidant; nickel matte (70 wt% Ni) is dissolved in a separate autoclave train also via ammonia pressure leaching (Kerfoot and Cordingley Citation1997). The combined Ni-Co leach liquor is subjected to Ni-Co separation by precipitating cobalt as a Co(III) hexamine salt, which has a low solubility in saturated ammoniacal solution, through the addition of anhydrous ammonia (Kerfoot and Cordingley Citation1997). The Ni-rich solution is processed in the exact same sequence of operations previously described i.e. copper sulfide precipitation, oxydrolysis and hydrogen reduction (Kerfoot and Cordingley Citation1997). Copper sulfide precipitation is however achieved through the addition of elemental sulfur and SO2, rather than relying on unsaturated sulfur compounds present in the leach liquor (Kerfoot and Cordingley Citation1997). The cobalt(III) hexamine sulfate is processed in a separate circuit which involves repulping in water to dissolve any precipitated nickel hexamine sulfate; the purified cobalt(III) hexamine sulfate is re-dissolved in ammonium sulfate solution followed by Co(III) reduction to Co(II) using metallic cobalt powder and finally hydrogen reduction in an autoclave at high temperature and pressure to produce high-grade metallic cobalt powder (Kerfoot and Cordingley Citation1997). A portion of this metallic cobalt powder is recycled to the Co(III) reduction stage with the balance being briquetted (Kerfoot and Cordingley Citation1997).
3.1.2. Kwinana, Western Australia
The Sherritt-Gordon ammonia pressure leach process is employed at BHP Nickel West’s refinery in Kwinana, WA (formerly owned by WMC Resources) and was originally built to process nickel flotation concentrates from Kambalda. The plant was commissioned in 1970, however, with the commissioning of the Kalgoorlie nickel smelter in 1973, co-processing of matte and concentrate commenced, with the refinery switching to an all-matte feed in 1985 (Deng Citation1994; Wishaw Citation1993). Some advantages of switching to all matte feed were stated to be higher Ni tenors in the leach liquor, an increase in Ni throughput, reduced cooling requirements in the autoclaves due to the lower sulfur content of the matte feed, and generation of a smaller volume of leach residue (Deng Citation1993, Citation1994; Wishaw Citation1993). The operating conditions of the autoclave train at the Kwinana refinery processing flash furnace smelter matte grading 66 wt% Ni (ground to a P90 of 106 µm), are a temperature range of 85–95°C and a pressure of 750–1000 kPa using compressed air as the oxidant (Wishaw Citation1993; Woodward Citation2014; Woodward and Bahri Citation2007). The Ni-Cu-Corich ammoniacal solution is processed in a similar manner to the original Sherritt-Gordon process at Fort Saskatchewan which was previously described (refer to ). The primary difference is that further processing of the MSP product for recovery of cobalt values does not take place (Wishaw Citation1993). The refinery at Kwinana has undergone an expansion with the addition of a plant to produce 100,000 tonnes per annum of nickel sulfate (Anon, Citation2021) , a key ingredient in LIB cathodes used in electric vehicles.
3.2. Sulfuric acid pressure oxidation processes
Sulfuric acid pressure oxidation processes have been commercially employed since the 1980s for processing of Ni-Cu mattes, zinc concentrates and refractory gold concentrates (Berezowsky et al. Citation1991) and have seen limited use in the processing of nickel sulfide concentrates. The sulfuric acid required for oxygen pressure leaching can be introduced through recycling of raffinates from solvent extraction-electrowinning circuits and/or can be generated in-situ during leaching from hydrolysis of ferric iron and oxidation of part or all the elemental sulfur. Oxygen pressure leaching processes are characterized according to the operating temperature at which leaching is performed which has an effect on sulfur speciation and oxygen consumption during leaching; low-temperature oxidation (100–110°C) takes place below the melting point of elemental sulfur (113–119°C), intermediate temperature oxidation (120–180°C) takes place above the melting point of elemental sulfur but below a temperature at which majority of the sulfur is further oxidized to sulfate whilst high-temperature pressure oxidation is typically carried out at 180–220°C where sulfur is fully oxidized to sulfate (Berezowsky Citation2000; Honey, Muir, and Hunt Citation1997). Further details of the process chemistry during oxygen pressure leaching of nickel sulfide concentrates are discussed by Berezowsky (Citation2000). Sulfuric acid pressure oxidation processes for the treatment of nickel flotation concentrates are by far the most numerous and advanced with respect to pilot plant development and commercial deployment. The key features of the piloted processes are presented in . Several nickel processing operations have practiced pressure oxidation which are presented in . Two of these operations have been closed (Fredericktown and Garfield, both in the USA) but have been briefly described for historical purposes.
3.2.1. Piloted pressure leach processes
3.2.1.2 Activox®
The Activox® process was originally developed by Western Minerals Technology (WMT) for oxidative leaching of base metal sulfide concentrates (Johnson, Evans, and Turner Citation2000) and has also been applied to treatment of refractory gold ores (Corrans, Johnson, and Angove Citation1993). The Activox® process is essentially a low temperature (80–110°C), pressure oxidation process utilizing oxygen gas as the oxidant, whereby base metals are leached from the concentrate whilst sulfide is oxidized to elemental sulfur, thus reducing the oxygen requirement for leaching versus high temperature, high pressure oxidation processes (Corrans, Johnson, and Angove Citation1993; Johnson, Evans, and Turner Citation2000). Elemental sulfur reports to the leach residue and since the leaching process operates below the melting point of sulfur (approximately 120°C), there is no need for the addition of a sulfur dispersing agent during leaching. The leaching media is an acid-sulfate system, with the sulfuric acid being generated in situ by oxidation of sulfide minerals in the feed (Johnson, Evans, and Turner Citation2000). The concentrate to be leached is subjected to ultra-fine milling down to a P80 of 10 µm, thereby improving leaching kinetics in subsequent pressure oxidation (Corrans, Johnson, and Angove Citation1993; Johnson, Evans, and Turner Citation2000; Palmer and Johnson Citation2005). Chloride addition during Activox® leaching has been stated to be beneficial for copper extraction (Adams and Johnson Citation2001) which is consistent with the findings by Subramanian and Ferrajuolo Citation1976) and McDonald and Muir (Citation2007). Typical residence times for leaching nickel sulfide concentrates are reported to be 1–2 hours (Johnson, Evans, and Turner Citation2000), chalcopyrite being refractory requires longer times (up to 5 hours) (Corrans, Johnson, and Angove Citation1993). Precious metals are not leached in the process and report to the leach residue from which they are recovered by conventional means (Johnson, Evans, and Turner Citation2000; Palmer and Johnson Citation2005). Iron is initially leached from the concentrate but undergoes hydrolytic precipitation, forming goethite and hematite (Corrans, Johnson, and Angove Citation1993; Palmer and Johnson Citation2005). There is no guarantee of goethite formation and the study by McDonald and Muir (Citation2007) on chalcopyrite leaching under Activox® conditions (108°C 700 kPa O2 pressure) found that goethite formation only occurred during leaching with 10 g/L Cl−. Leaching with sulfuric acid and chloride resulted in formation of natrojarosite and poorly crystalline iron oxyhydroxides such as 2-line ferrihydrite (McDonald and Muir Citation2007). Metal recoveries during the leaching of a range of sulfide concentrates using the Activox® process have been reported to be greater than 95% for Ni and Co, and 75–90% for Cu, with 40–80% conversion of sulfidic sulfur to elemental sulfur (Palmer and Johnson Citation2005). Activox® leaching technology has been applied to the treatment of a variety of nickel sulfide concentrates from Western Australia and Africa (Corrans, Johnson, and Angove Citation1993; Johnson, Evans, and Turner Citation2000), and was the chosen leaching technology for the Yakabindie Nickel Project, Western Australia, and the Tati nickel project, Botswana (Johnson, Evans, and Turner Citation2000; Palmer and Johnson Citation2005; Robles, Cronje, and Nel Citation2009). The technology has not been applied commercially for the processing of nickel flotation concentrates.
3.2.1.2. CESL process
The CESL process was originally developed by Cominco (now Teck Resources Limited) for the hydrometallurgical processing of copper sulfide concentrates, as an alternative to smelting, but was also adapted for the processing of nickel sulfide concentrates (Jones, Hestrin, and Moore Citation1998). The CESL process is a medium temperature pressure oxidation process taking place under an oxygen pressure of 1379 kPa (gauge) and temperature above the melting point of sulfur (120°C) but below 160°C to limit oxidation of elemental sulfur to sulfate. The first step in the CESL process is re-grinding of flotation concentrate(s) in a ball mill to a P95 of 45 µm to liberate the sulfide minerals and improve leaching kinetics (Jones, Hestrin, and Moore Citation1998; Mayhew et al. Citation2013). Oxidative dissolution of concentrates takes place in an acidic sulfate solution containing up to 60 g/L H2SO4, 12 g/L chloride and 5–10 g/L Cu(II), the role of chloride being to limit oxidation of elemental sulfur to sulfate (Jones, Citation1996; Jones, Hestrin, and Moore Citation1998). Brown and Papangelakis (Citation2005) have also shown that chloride ions have a dispersing effect on elemental sulfur and it is possible that chloride in the CESL process serves an additional function as a sulfur dispersing agent during leaching. In the absence of any suitable sulfur dispersing agent, sulfide mineral dissolution kinetics would likely be inhibited due to encapsulation of unreacted sulfides by molten elemental sulfur (Brown and Papangelakis Citation2005; Tong and Dreisinger Citation2009). The specific role of copper ions in the CESL process is not stated, however WMC Resources previously recognized the benefits of copper ions in combination with chloride during pressure oxidation of nickel sulfide concentrates which formed the basis for a patent (Crossley et al. Citation1998). It may be that in the presence of sufficient chloride ions in solution, the Cu(II)/Cu(I) couple functions as a redox mediator during oxygen pressure leaching. Iron sulfides in the concentrate(s) are oxidized and precipitated as hematite; in the case of pyrrhotite, oxidation also produces elemental sulfur whilst sulfide in pyrite is oxidized to sulfuric acid (Defreyne et al. Citation2006). Whilst only 50% to 75% of the pyrite is reported to undergo oxidation in the CESL process, its presence in concentrates is stated to be undesirable as it increases oxygen consumption and heat generation during pressure oxidation and generates sulfuric acid which must be neutralized downstream (Defreyne et al. Citation2006). The heat generation due to pyrite oxidation may not be an issue if the process can be operated autogenously whilst the acid generation from pyrite might also not be a problem if a use can be found for the acid such as in a heap leach operation.
Once the base metals have been leached into solution, the autoclave leach slurry is subjected to solid–liquid separation and the leach liquor is neutralized with limestone to remove iron and aluminum from solution (Jones et al. Citation2010; Mayhew et al. Citation2013). Copper can be recovered as cathode via solvent extraction and electrowinning (SX-EW) whilst Ni and Co can be recovered as a mixed hydroxide precipitate (MHP), mixed sulfide precipitate (MSP), or separated via SX to produce individual products (Jones et al. Citation2010; Jones, Mayhew, and O’Conner Citation2009; Mayhew et al. Citation2013). The CESL process has been tested on a range of nickel concentrates ranging in Ni grade from 2.1 to 20.4 wt% at bench and pilot plant scale, including concentrates high in MgO arising from the flotation of disseminated Ni sulfide ores which are not suitable for smelting (Jones et al. Citation2010; Jones, Hestrin, and Moore Citation1998; Jones, Mayhew, and O’Conner Citation2009; Mayhew et al. Citation2013). High Ni and Co recoveries have been reported for a range of concentrates leached using the CESL process, being above 95% for both elements (Jones, Hestrin, and Moore Citation1998; Jones, Mayhew, and O’Conner Citation2009). Despite being tested at bench and pilot plant scale, the CESL process has not been deployed commercially for the processing of nickel sulfide concentrates.
3.2.1.3. PlatsolTM
PLATSOLTM leaching technology was originally developed by International PGM Technologies Limited at SGS Lakefield Research (Canada) for processing bulk polymetallic sulfide flotation concentrates containing Cu, Ni, Co, gold (Au) and PGMs from the NorthMet deposit (Duluth Gabbro, Minnesota), owned by Polymet Mining Corporation (Dreisinger et al. Citation2005; Ferron et al. Citation2002; Fleming et al. Citation2000). The bulk sulfide concentrates were considered uneconomical for smelting and hence a hydrometallurgical process was an attractive alternative to development of a dedicated smelting facility or shipping flotation concentrate to toll smelters (Fleming et al. Citation2000). The leaching technology was also tested on a range of different base metal sulfide concentrates containing a range of Ni grades from 3 to 15 wt% as well as Co, Cu and PGMs (Ferron et al. Citation2000, Citation2002; Fleming et al. Citation2000). The leaching process is essentially a high-temperature pressure oxidation process carried out at 225°C utilizing oxygen gas as the oxidant in sulfuric acid media containing dissolved chloride (Dreisinger et al. Citation2005; Ferron and Fleming Citation2001); base and precious metals are dissolved from concentrates under the conditions employed and total oxidation of sulfidic sulfur to sulfate takes place (Fleming et al. Citation2000). Most of the iron (>90%) in the concentrate reports to the leach residue as hematite (Ferron et al. Citation2000; Fleming et al. Citation2000). Key features of the process are the addition of an ultra-fine milling stage (P80 15–20 µm) and chloride addition (5–20 g/L NaCl) which facilitates dissolution of Au and PGMs during leaching via formation of soluble chloro complexes (Ferron et al. Citation2000; Fleming et al. Citation2000). Base metals recovery during leaching was reported to be unaffected by fine grinding or chloride addition, however recoveries of Au, Pt and Pd were improved both when ultra-fine milling and chloride addition were employed (Fleming et al. Citation2000). Gold recoveries during leaching were also reportedly affected by the grinding media, with ceramic media giving the best results during leaching versus steel grinding media, which was believed to be due to cementation of dissolved gold onto iron powder produced during attrition grinding using steel media (Fleming et al. Citation2000). At the high temperatures employed, leaching kinetics were very rapid with more than 95% of the Ni, Cu and Co being leached from concentrates in the first 20 minutes with longer retention times (up to 180 min) being necessary to dissolve the precious metals (Dreisinger et al. Citation2005; Ferron and Fleming Citation2002; Ferron et al. Citation2000). A demonstration plant utilizing PLATSOLTM leaching technology was built at SGS Lakefield for processing bulk sulfide flotation concentrate produced from the NorthMet deposit (Ferron and Fleming Citation2001, Citation2002); however, the leaching technology does not appear to have been industrially implemented.
3.2.1.4. Kell process
The Kell process is a multi-step hydrometallurgical process that has been developed for the extraction of base metals, gold, silver and PGMs from flotation concentrates as an alternative to smelting. The first stage in the Kell Process is high-temperature pressure oxidation (170–230°C) in a sulfate medium using oxygen gas to dissolve base metals and oxidize sulfidic sulfur to sulfate; precious and platinum group metals are retained in the leach residue (Liddell and Adams Citation2012; Liddell et al. Citation2019). By comparison, PGMs are intentionally dissolved in the PLATSOLTM process, also utilizing high-temperature pressure oxidation, via the addition of 5–20 g/L NaCl. The leach liquor arising from the pressure leaching stage (Kell process) is processed to recover base metals whilst the leach residue is further processed to recover Au, Ag and PGMs (Liddell and Adams Citation2012; Liddell et al. Citation2019). The leach residue may be subjected to a HCl leach at ambient pressure to dissolve silver and residual base metals retained in the residue followed by roasting of the residue at 500–1000°C to convert PGM minerals to a readily leachable form (Liddell et al. Citation2019). Test work performed by Adams, Liddell, and Holohan (Citation2011) found that reducing conditions during roasting are ideal for subsequent PGM recovery. Recovery of precious and platinum group metals from the roasted leach residue takes place via an ambient pressure oxidative leach in a chloride medium using chlorine gas as an oxidant (Liddell et al. Citation2019). The process has been tested on a range of PGM bearing sulfide concentrates, primarily from Southern Africa and average base metals (Ni, Cu, Co) recoveries are 96–98% whilst Pt and Pd recoveries are 98% (Liddell et al. Citation2019).
3.2.1.5. IGO processTM
IGO Limited, an ASX listed nickel miner, has recently patented a process for production of high purity/battery grade nickel sulfate directly from nickel sulfide concentrates via oxygen pressure leaching, solution neutralization and purification, and solvent extraction to produce nickel sulfate hydrate crystals (Grassi et al. Citation2019). The main feature of the invention is the stripping of nickel from the loaded organic phase using a sulfuric acid solution saturated in Ni such that nickel sulfate hexahydrate crystals are immediately precipitated from solution (Grassi et al. Citation2019). Nickel dissolution from the concentrate is proposed to take place either via low temperature (100 – 120°C) or high temperature (>200°C) pressure oxygen leaching. For the former case, ultra-fine grinding (P80 6.6 µm) improved nickel leaching from the concentrate (Grassi et al. Citation2019) just like in Activox®. Grassi et al. (Citation2019) reported near complete extraction of base metals within 60 min during high-temperature pressure leaching; however, the resultant leach liquors contained high concentrations of dissolved iron (up to 22 g/L total Fe, mostly as Fe(III)) and free acid (up to 78 g/L H2SO4) which would increase limestone consumption in downstream neutralization. The high iron and acid concentrations in the leach liquor is not necessarily an issue if a use can be found for them, such as leaching nickel sulfide flotation tailings or nickel oxide ores, though this is not mentioned in the invention. The IGO ProcessTM has not been commercially deployed; however, IGO Limited has indicated that they are assessing opportunities for partnership/collaboration to leverage the technology (IGO Limited Citation2020).
3.2.2. Commercial operations
3.2.2.1. Garfield Cobalt Refinery, USA
The Garfield cobalt refinery in Utah, USA, operated by Calera Mining Company was the first application of sulfuric acid pressure oxidation to the processing of nickel-bearing sulfides (Berezowsky Citation2000). The plant was operational from 1953 to 1959 and shut down due to a shortage of ore (Meddings and Evans Citation1971). The plant processed a sulfide concentrate from the Blackbird Mine in Idaho (USA) grading 17.5% Co, 1.0% Ni and 0.5% Cu; major sulfide minerals in the concentrate were cobaltite (CoAsS) and pyrite with minor gersdorffite, chalcopyrite and arsenopyrite (Berezowsky Citation2000). The pressure leaching process in use at the refinery involved total pressure oxidation at 375°F (190.6°C) and a total pressure of 3600 kPa using air as the oxidant in a lead and brick lined autoclave (Berezowsky Citation2000). Under these conditions, 95–97% of the Co was leached (Berezowsky Citation2000); the extraction efficiencies for Ni and Cu have not been stated. Majority of the iron and arsenic in the concentrate was precipitated as basic iron sulfate and ferric arsenate (Meddings and Evans Citation1971). The leach slurry from the autoclave was neutralized to remove residual arsenic and iron, and copper was recovered by cementation with cobalt powder (Meddings and Evans Citation1971). Cobalt and Ni recovery was via pressure hydrogen reduction to produce an impure product after adjustment of the solution with ammonia to produce the desired ammonia to metal ratio in solution (molar ratio of 2) for reduction (Meddings and Evans Citation1971). Construction and operation of the plant was rushed, and no piloting of the process occurred, as such the plant was plagued with numerous issues due to the abrasive and corrosive nature of the slurry (Berezowsky Citation2000).
3.2.2.2. Fredericktown metals refinery, USA
The Fredericktown refinery in Missouri, USA, operated by National Lead Company was the second commercial plant to utilize sulfuric acid pressure oxidation in the processing of nickel-bearing sulfide concentrates (Berezowsky Citation2000). The plant was operational between 1954 and 1960 and like the Garfield refinery was closed due to lack of feed (Meddings and Evans Citation1971). The feed to the leach plant was a flotation concentrate grading 5.0% Ni, 4.2% Co and 4.8% Cu, as siegenite and chalcopyrite, produced as a by-product of copper-lead mining (Berezowsky Citation2000; Meddings and Evans Citation1971). The pressure leaching process in use at the refinery involved total pressure oxidation at 232°C and a total pressure of 5300 kPa using air as the oxidant (Berezowsky Citation2000). Most of the iron was precipitated in the autoclave as basic iron sulfate and further removal of iron post-pressure leaching took place through pH adjustment and aeration to oxidize residual ferrous iron (Meddings and Evans Citation1971). Metallic Ni, Cu and Co products were recovered by pressure hydrogen reduction (Meddings and Evans Citation1971).
3.2.2.3. Kokkola refinery, Finland
There has been a long history of Ni/Co refining at Kokkola by Outokumpu Oy, starting with Ni and Co extraction from pyrite-pyrrhotite concentrate from the Outokumpu copper mine via sulfation roasting between 1966 and 1987 (refer to Section 3.6.3). From December 1991, the Kokkola refinery began processing a low grade, high magnesia (10% MgO) nickel sulfide flotation concentrate from the Hitura mine (Finland) grading 5–7% Ni. The plant used a pressure oxidation process (HIKO process – refer to ) in autoclaves at temperatures below the melting point of sulfur (<120°C) with oxygen gas at 500 kPa pressure (Berezowsky Citation2000; Nyman et al. Citation1992). Additional details of the HIKO process such as acid addition to the leach charge, concentrate feed particle size, leaching time and degree of sulfide conversion to elemental sulfur are unknown (Berezowsky Citation2000). The leach solution was subjected to iron precipitation using limestone followed by copper precipitation as a sulfide using H2S (Nyman et al. Citation1992). Following on from iron and copper removal, the solution was subjected to SX using di-2-ethylhexyl phosphoric acid (D2EHPA) to remove calcium followed by separation of cobalt (and magnesium) from nickel using Ionquest 801, an organophosphorus-based extractant (Nyman et al. Citation1992). The raffinate from Co/Mg SX was then sent to Ni SX; the final products were purified Ni and Co chemicals (Nyman et al. Citation1992); no details however are given of the Ni SX circuit i.e. type of extractant used, nor the Co refining circuit after Co/Mg SX. The status of the pressure leaching plant is unknown though the refinery has changed ownership a number of times and is currently a cobalt refinery operated by Umicore (Cooke Citation2021; Törmänen and Tuomela Citation2021) and the nickel mine that supplied concentrate to the leaching plant has been closed since 2015 (Björkman Citation2019). A simplified process flow diagram of the HIKO process used at the Kokkola refinery is presented in .
Figure 5. Simplified process flow diagram of the Outokumpu HIKO process used at the Kokkola refinery (Finland) for processing of nickel sulfide concentrates via low temperature pressure oxidation and recovery of nickel and cobalt chemicals. Operating conditions of the leaching process and metal extractions during leaching for a given nickel concentrate are presented. Adapted from Nyman et al. (Citation1992) Honey, Muir, and Hunt (Citation1997) and Berezowsky (Citation2000).
3.2.2.4. Long Harbour, Canada
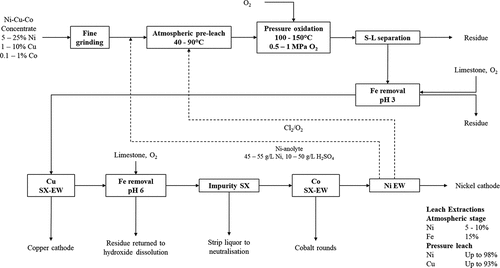
Hydrometallurgical processing of nickel flotation concentrate is carried out by Vale Limited at Long Harbor using nickel concentrate produced from the Voisey’s Bay nickel deposit; the first shipment of metallic nickel produced from the plant occurring in 2015 (Anon Citation2015). A simplified flowsheet of the Voisey’s Bay Process is presented in and the key features of the leaching stages are summarized in . The process commences with regrinding of the flotation concentrate to below 20 µm followed by a first stage leach in sulfuric acid (return electrolyte from Ni-electrowinning) at ambient pressure using a mixture of chlorine gas and oxygen as the oxidant (produced from the Ni-electrowinning circuit). This is followed by a second stage leach at elevated pressure using oxygen as the oxidant in an autoclave (Crundwell et al. Citation2011b; Kerfoot et al. Citation2001; Stevens et al. Citation2009). The clarified leach solution produced from the two-stage leach process undergoes a first stage of iron removal where 95% of the iron is removed by raising the pH to 3 using limestone and aerating with oxygen to oxidize any ferrous iron in the solution to the ferric state (Stevens et al. Citation2009). Following on from the first iron removal stage, copper is recovered from the solution via solvent extraction-electrowinning (SX-EW) using LIX 84, a commercially available hydroxyoxime extractant (Stevens et al. Citation2009). The raffinate from Cu SX undergoes a second stage of iron removal, followed by cadmium removal (by sulfide precipitation) and finally an impurity SX stage using D2EHPA to remove calcium, magnesium and other impurities (Fe, Pb, Zn, Mn) prior to cobalt solvent extraction (Crundwell et al. Citation2011b; Stevens et al. Citation2009). The purified raffinate is then subjected to Co SX-EW using Cyanex 272, an organophosphinic acid extractant. The raffinate from Co SX is sent to the Ni electrowinning circuit; the final products produced at Long Harbor are nickel cathode, copper cathode and cobalt rounds (Crundwell et al. Citation2011b; Stevens et al. Citation2009).
Figure 6. Simplified process flow diagram for the hydrometallurgical processing of nickel concentrate from Voisey’s Bay (Canada) at Vale’s Long Harbour operations to produce Ni, Cu and Co cathode via a combination of pressure oxidation, solvent extraction (SX) and electrowinning (EW). Some unit operations such as hydroxide re-leaching after solid/liquid separation and cadmium removal prior to impurity SX have been omitted for simplicity. Adapted from Crundwell et al. (Citation2011b), Kerfoot et al. (Citation2001) and Stevens et al. (Citation2009). Conditions utilized in atmospheric pre-leach and pressure oxidation and extraction efficiencies are based on the original patent filed by Kerfoot et al. (Citation2001) and may not reflect actual conditions in current use.
3.2.2.5. Nadezhdin mill, Russia
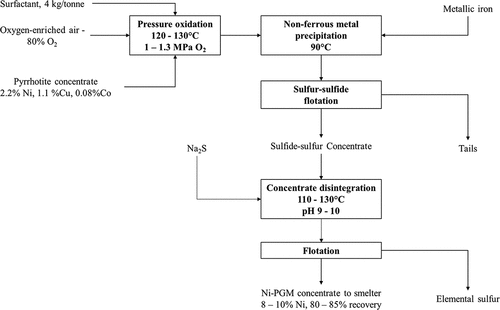
Flotation of massive sulfide ores from the Noril’sk-Talnakh ore field (Siberia) produces three products, copper concentrate, nickel concentrate and pyrrhotite concentrate (Bulatovic Citation2007; Kozyrev et al. Citation2002). The pyrrhotite concentrates produced typically contain 2–5% Ni, 1–4% Cu and up to 15 g/t of PGMs (Berezowsky Citation2000; Deng Citation1992, Citation1993; Klyushnikov et al. Citation2021). Nickeliferous pyrrhotite concentrates are processed to recover the base and platinum group metals content via a combination of autoclave pressure leaching and residue flotation at the Nadezhdin mill, operated by Noril’sk Mining and Metallurgy Corporation (now Nornickel) (Deng Citation1992, Citation1993). The autoclaves were commissioned in 1979 and involved leaching pyrrhotite concentrates at 125–135°C and pressure of 1.5 MPa, using 80% O2 as the oxidant with addition of 4 kg/t of lignin sulfonic acid salts as a sulfur dispersing agent (Berezowsky Citation2000; Deng Citation1992, Citation1993). The leaching process results in oxidation of 96% of the pyrrhotite in the concentrate and conversion of 65% of sulfidic sulfur in the feed to elemental sulfur; 67% of the Ni is initially leached into solution but is subsequently precipitated from the leached slurry as a sulfide (Deng Citation1992, Citation1993). Precipitation of dissolved base metals is carried out by treating the leach pulp with metallic iron at 90°C which together with elemental sulfur present in the leach slurry precipitates the dissolved metals as their sulfides (Berezowsky Citation2000).
The precipitated base metal sulfides along with unreacted sulfides and PGMs are separated from elemental sulfur and iron oxides via sequential flotation (Deng Citation1992, Citation1993). Firstly, flotation is carried out to reject iron oxides formed during pressure oxidation and recover an elemental sulfur-metal sulfide flotation concentrate (Berezowsky Citation2000). The concentrate is then treated in an autoclave at 110–130°C and pH 9–10 with addition of 5–7 g/L Na2S to disintegrate the concentrate into a mixture of elemental sulfur and metal sulfides; the pulp is then subjected to flotation to produce a sulfide concentrate and an elemental sulfur product (Berezowsky Citation2000). The final nickel grade in the concentrate is 8–10% with an overall recovery of base and platinum group metals to the final concentrate of 80–85% (Berezowsky Citation2000). The base and platinum group metal enriched product is then combined with the nickel concentrate produced from the beneficiation circuit and processed further via flash smelting (Berezowsky Citation2000; Klyushnikov et al. Citation2021). A simplified process flow diagram is presented in . The pressure oxidation autoclaves at the Nadezhdin mill do not have sufficient capacity to process all the nickeliferous pyrrhotite concentrate produced and as such, a large proportion of the concentrate is diverted to stockpile (Klyushnikov et al. Citation2021; Moskalyk and Alfantazi Citation2002).
Figure 7. Simplified process flow diagram for the hydrometallurgical processing of nickeliferous pyrrhotite concentrates from Talnakh (Russia) at the Nadezhdin mill via a combination of pressure oxidation, non-ferrous metal sulfide precipitation and flotation to recover a sulfide concentrate for flash smelting and elemental sulfur. Operating conditions of major unit operations and overall nickel recovery and final nickel product grade during processing of a pyrrhotite concentrate grading 2.2% Ni are presented (from Berezowsky Citation2000). Adapted from Kitay, Mechev, and Volkov (Citation1991) and Berezowsky (Citation2000).
3.3. Atmospheric sulfuric acid leaching
The only processes developed to date employing chemical leaching of nickel concentrates in a sulfate medium at atmospheric pressure are the Intec Nickel Process developed by Intec Limited, and the Albion ProcessTM developed by Glencore (formerly Xstrata). Neither of these processes have been deployed commercially for the processing of nickel sulfide concentrates though the Albion ProcessTM has been commercially deployed for processing gold, copper and zinc concentrates and may therefore be suitable for leaching of nickel flotation concentrates. The key features of both processes are presented in and are briefly described below.
3.3.1. Intec Nickel Process
The Intec Nickel Process is a two-stage atmospheric leaching process developed for processing base metal sulfide concentrates containing precious metals and PGMs. Much of the development work for the Intec Process focused on copper extraction from copper sulfide concentrates (Moyes and Houlis Citation2003; Palmer and Johnson Citation2005). The first stage leach involves carrying out oxidative dissolution of reground flotation concentrate (ground to P80 25 µm) at 80°C in a sulfuric acid solution containing halides (Cl-, Br-) using air (21% O2) as an oxidant (Moyes and Houlis Citation2003). Nickel, copper and cobalt dissolution efficiencies in the first leaching stage were reported to be greater than 95%, with approximately 50% of the Au and PGMs dissolving in this leaching stage (Moyes and Houlis Citation2003); the residence time for the first leaching stage was not stated. The second leaching stage, termed the “Halex leach”, involves the extraction of un-leached Au, Pt, Pd and remaining base metals from the first stage leach residue. Leaching is carried out at 100°C using spent electrolyte from the Cu- and Ni-electrowinning circuits, where electrogenerated BrCl2- serves as the oxidant (Moyes and Houlis Citation2003). Base metal recoveries in the second stage leach were again reported to be greater than 95%, with 86% of the Au, and 87% of the Pd also being leached; Pt recoveries were poor however with only 54% extraction (Moyes and Houlis Citation2003). Recovery of precious metals post-leaching is by precipitation using either sodium hydrogen sulfide (NaHS) or pyrrhotite-rich nickel concentrate, and copper and nickel are recovered in separate electro-winning circuits as cathode.
3.3.2. Albion ProcessTM
The Albion ProcessTM is an atmospheric oxidative leaching process which is preceded by an ultra-fine grinding stage. The process was developed to treat a range of sulfide concentrates containing base and precious metals and was originally developed by MIM Holdings/Xstrata (Hourn and Turner Citation2012; Hourn, Turner, and Holzberger Citation1996) with Glencore Technology (a subsidiary of Glencore) being the licensor of the process/technology after the purchase of Xstrata. The original patent for the Albion ProcessTM covered the ultra-fine grinding of base metal sulfide concentrate or ore to a P80 of 20 µm or less followed by leaching in a sulfuric acid medium containing dissolved ferric and ferrous iron with sparging of air, oxygen-enriched air or pure oxygen at temperatures between 60°C and the boiling point of the solution (Hourn, Turner and Holzberger Citation1996). The first step in the Albion ProcessTM is the fine grinding of the sulfide-bearing material in an IsaMillTM to a P80 of 10–12 µm followed by leaching of the milled material in specially designed agitated leaching tanks at 93–98°C with oxygen injection using Hypersparge™ technology which can deliver oxygen at supersonic velocities (450–550 m.s−1) (Hourn and Turner Citation2012). No external heating or cooling requirements are required for the leaching process, the heat being generated from oxidation of sulfidic sulfur and cooling being achieved via water evaporation. Sulfidic sulfur is oxidized to elemental sulfur and sulfate, with the degree of elemental sulfur oxidation to sulfate being controlled by the pulp pH. Processing of base metal sulfide concentrate via the Albion ProcessTM is carried out under acidic conditions whilst the processing of refractory gold concentrates is carried out under neutral conditions (pH 5–7) to oxidize elemental sulfur to sulfate. The Albion ProcessTM has been commercially deployed for the processing of sphalerite, refractory gold concentrates (Hourn and Turner Citation2012) and most recently, chalcopyrite concentrate at Sable Zinc (Zambia) (Stieper Citation2018). The process has not been industrially applied to the processing of nickel sulfide concentrates and information on processing of nickel sulfide concentrates is limited; the original patent by Hourn, Turner, and Holzberger (Citation1996) only had one application of the Albion ProcessTM to the processing of a low-grade nickel concentrate (1.7% Ni as pentlandite) which was milled to a P80 of 5 µm prior to leaching at atmospheric pressure.
3.4. Chloride-based leaching processes
Chloride leaching involves the dissolution of base metal sulfide concentrates, ores or mattes exclusively in chloride media as opposed to sulfate-media with the addition of halide ions such as in the CESL, Voisey’s Bay or Intec processes which were previously described. Advantages of chloride lixiviants have been stated to be a high recovery of metals during leaching, ability to regenerate and recycle the lixiviants, inertness of pyrite in chloride media and reduced formation of sulfate during leaching (Dutrizac Citation1992). Against these advantages however are several limitations such as the lack of selectivity of chloride lixiviants during leaching which can complicate downstream liquor purification and lixiviant regeneration/recycling and the corrosivity of chlorides toward many common materials of construction (Dutrizac Citation1992; Forward and Warren Citation1960; Honey, Muir, and Hunt Citation1997). Despite the disadvantages, chloride-based leaching processes have been successfully utilized for processing nickel mattes with these processes in current use at the Falconbridge Nikkelverk refinery (Norway), the Niihama nickel refinery (Japan) and Sandouville-Le Havre (France) (Kerfoot and Weir Citation1988; Makino et al. Citation1996; Stensholt, Zachariasen, and Lund Citation1986). A common theme to all three matte leach processes is the use of chlorine gas as an oxidant in conjunction with either Cu(II) (Falconbridge, Niihama) or Fe(III) (Sandouville) (Muir and Ho Citation2006).
Despite the successful application of chloride leaching in nickel matte refining, the only chloride-based leaching process developed at scale for processing nickel sulfide concentrates is the HydroNic process developed by Outotec Oyj (Karonen, Tiihonen, and Haavanlammi Citation2009) and key details of the process are summarized in . HydroNic employs a two-stage leaching process to dissolve Ni, Co and Cu from base metal sulfide concentrates. In the first stage, nickel is leached from pentlandite by reaction with copper(II) chloride dissolved in a sodium chloride solution recycled from the second leach stage, with Ni and Fe being leached into solution (as their chlorides) whilst copper is precipitated as copper(I) sulfide (Cu2S). This leaching stage is non-oxidative and is a cementation process. In the second stage of leaching, the concentrate is leached under oxidative conditions using oxygen or chlorine gas as the oxidant. Iron is hydrolyzed and precipitated from solution during leaching whilst sulfide in the concentrate is oxidized to elemental sulfur and sulfate. The solution from the second leaching stage is recycled to the first stage to remove copper from solution via cementation as Cu2S. After copper removal, the solution is treated further with caustic (NaOH) and limestone to precipitate iron (as oxyhydroxide) and sulfate (as gypsum) from solution. After iron/sulfate removal, Ni and Co are precipitated from solution as a mixed hydroxide by neutralization with MgO. The patent for the HydroNic process was filed by Outotec Oyj in 2006 (Haavanlammi et al. Citation2006). Interestingly, another patent was filed by Outotec Oyj in the same year for a chloride leach process for treatment of nickel sulfide ore and concentrates, key differences in this process were the absence of a non-oxidative leaching step, copper(II) was precipitated from the leach liquor as atacamite (Cu2(OH)3Cl) which was returned to the leaching stage and copper recovery after nickel hydroxide precipitation in the form of cuprous hydroxide (CuOH) from which copper metal was recovered via hydrogen reduction (Krebs et al., Citation2006). The hydronic process has been tested on a range of nickel concentrate grades; however, it does not appear to have been implemented at an industrial scale.
3.5. Bioleaching processes
Bioleaching generally involves the dissolution of sulfide ores/concentrates in an acidic sulfate medium where ferric ions act as an oxidant, oxidizing the sulfide minerals to elemental sulfur with release of metal ions into solution (Watling Citation2006). The microorganisms in bioleaching serve a dual purpose of regenerating ferric ions by oxidation of ferrous iron and generation of sulfuric acid via oxidation elemental sulfur (Watling Citation2006). Some reasons for considering bioleaching for the processing of sulfide ores/concentrates stated by Miller (Citation1997) are simple equipment requirements and flexibility of bioleach processes with respect to variation in concentrate/ore head grade and mineralogy (Miller Citation1997). Potential disadvantages of bioleaching are the slow reaction kinetics and sensitivity of the microorganisms to temperature, mineral pulp density, agitation rate and chloride concentration in process water (Astudillo and Acevedo Citation2008; Bailey and Hansford Citation1993; Honey, Muir, and Hunt Citation1997; Nemati and Harrison Citation2000). Many of these limitations are dependent on the bacterial strain and in the case of chloride concentration, can be addressed through the development of halotolerant bacterial strains such as in the BioHeapTM process described below (Section 3.5.2.3). A summary of piloted bacterial leaching processes and commercial operations employing bioleaching for processing of nickel sulfide flotation concentrates is described. After sulfuric acid pressure oxidation, bioleaching processes are second with respect to the number of developed technologies and industrial deployment. The key features of the piloted processes are presented in whilst a summary of commercial operations utilizing bioleaching technologies are presented in .
3.5.1. Piloted processes
Three bacterial leaching processes have been developed for the processing of nickel sulfide bearing material; the processes are BioNIC® (BHP Limited), BacTech (BacTech Limited) and the BioHeapTM process (BioHeap Limited, a subsidiary of Western Areas Limited). The BioNIC® and BacTech processes are stirred tank bacterial leaching processes that have been successfully piloted on a range of nickel concentrates and a review of these processes and pilot plant operations has been covered by Watling (Citation2008) in a review on the bioleaching of nickel-copper sulfides. Neither of these processes progressed beyond pilot plant scale. The BioHeapTM process developed by BioHeap Limited, a subsidiary of Western Areas Limited, is in current use at Western Areas Limited’s Cosmic Boy nickel concentrator in Western Australia and the process and operations are described below.
3.5.2. Commercial bioleach operations
3.5.2.1. Talvivaara heap bioleaching operation, Finland
The operation at Talvivaara is the only Ni sulfide heap bioleaching operation, processing a black schist ore from the Sotkamo deposit (Finland), containing 0.23–0.27% Ni, 0.5–0.56% Zn, 0.13–0.14% Cu and 0.02% Co (Crundwell et al. Citation2011b; Riekkola-Vanhanen Citation2013; Watling Citation2008). The principal sulfide minerals in the ore are pyrrhotite, pyrite, sphalerite, pentlandite, violarite and chalcopyrite whilst non-sulfidic gangue phases are graphite, quartz, mica, anorthite and microcline (Riekkola-Vanhanen Citation2007, Citation2013; Watling Citation2008). Ore from the mine is subjected to four stages of crushing to a P80 of 8 mm followed by agglomeration with bacteria and sulfuric acid, stacking of the agglomerates to form heaps and leaching of the heaps with a sulfuric acid solution at pH 1.8 (Crundwell et al. Citation2011b; Riekkola-Vanhanen Citation2007, Citation2013; Watling Citation2008). Metal values (Zn, Ni, Co, Cu) are recovered from the pregnant leach solution by sequential precipitation as their sulfides using H2S (Crundwell et al. Citation2011b; Riekkola-Vanhanen Citation2013). A simplified process flow diagram of the Talvivaara heap bioleaching operation is presented in . The mine is still operational and is currently being run by Terrafame Limited, a Finnish state-owned mining company which purchased the operation from the parent company (Talvivaara Mining Company) after declaring bankruptcy (Sairinen, Tiainen, and Mononen Citation2017). Recently, Terrafame plans on exploiting another nearby black schist ore body (Kolmisoppi) which is currently undergoing environmental assessment (Törmänen and Tuomela Citation2021). Terrafame intends to begin production of battery grade Ni and Co sulfates starting from the MSP produced from the heap leach operation, with pressure oxidation of the MSP being the first step in the process (Törmänen and Tuomela Citation2021). Based on a recent company announcement, production ramp-up of the battery chemicals plant started in June 2021 and first delivery of products to customers in July 2021 (Anon Citation2021).
Figure 8. Simplified process flow diagram for the hydrometallurgical processing of black schist ore from the Sotkamo deposit at the Talvivaara heap bioleaching operation (Finland) including expected leaching recoveries for the base metals and product grades. a copper product had not been produced at the time of reporting by Riekkola-Vanhanen (Citation2013). Adapted from Riekkola-Vanhanen (Citation2013).
3.5.2.2. Mondo nickel project, Finland
Talc processing operations at Sotkomo and Vuonos in Finland operated by Mondo Minerals (now Elementis) produce nickel sulfide concentrate grading 8.70% Ni as a by-product (Neale et al. Citation2015). The primary sulfide minerals in order of decreasing abundance in the concentrate are pyrrhotite, pentlandite, pyrite and gersdorffite (Neale et al. Citation2015). In the past the concentrate was typically sold to toll smelters, however the high arsenic content (1.56% As) had made this option less feasible (Neale et al. Citation2015). To produce a more valuable product and increase revenue, a hydrometallurgical plant was built to process 12,000 tonnes per annum of concentrate and recover nickel and cobalt as a mixed hydroxide precipitate (Neale et al. Citation2015); a simplified process flow diagram of the hydrometallurgical process in use is depicted in . Nickel concentrate from both operations is combined and processed in a beneficiation circuit where feed is re-ground to a P80 of 30–40 µm followed by magnetic separation to reject pyrrhotite, and flotation to remove talc and magnesite, to produce an upgraded concentrate containing 12.4% Ni (Laukka et al. Citation2018; Neale et al. Citation2015, Citation2016). The nickel concentrate is subjected to a bioleaching process which was developed in conjunction with Mintek; the leaching conditions are a temperature range of 41–49°C pH range of 1.3–1.6 and a pulp density of 15–17% solids (Laukka et al. Citation2018; Neale et al. Citation2015). The overall residence time in the bioleaching circuit is 9.5 days and nickel and cobalt extractions are reported to be 87.8% and 90.7%, respectively (Laukka et al. Citation2018). The leach slurry is neutralized to pH 3.5 using limestone to precipitate iron and arsenic from solution followed by solid–liquid separation (Laukka et al. Citation2018; Neale et al. Citation2015). The nickel-rich solution is subsequently treated with magnesia (MgO) slurry to precipitate Ni and Co as a mixed hydroxide precipitate (MHP) (Neale et al. Citation2016). The raffinate produced from MHP precipitation is treated with lime (CaO) to precipitate residual Ni and the precipitate is recycled to iron-arsenic precipitation circuit to re-dissolve residual Ni (Neale et al. Citation2016). Törmänen and Tuomela (Citation2021) have indicated that the bioleach plant has only been operational periodically with the nickel sulfide concentrate by-product presumably being sold on the market at other times.
Figure 9. Simplified process flow diagram for the bioleaching of nickel concentrate produced as a by-product of talc mining at Mondo Minerals (now Elementis) Vuonos talc concentrator operations in Finland. the beneficiation circuit has been omitted for simplicity. Operating conditions of major unit operations and final nickel product grade during processing of nickel concentrate grading 10.49% Ni are presented. Adapted from Neale et al. (Citation2015), Neale et al. (Citation2016).
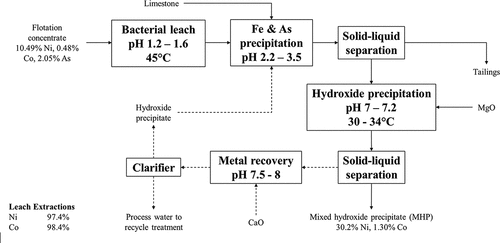
3.5.2.3. Cosmic boy nickel concentrator, Western Australia
The Cosmic Boy nickel concentrator in Western Australia is owned and operated by Western Areas Limited (Fewings and Seet Citation2014). The concentrator batch processes high grade nickel sulfide ore from the Flying Fox (5.1% Ni grade) and Spotted Quoll (5.6% Ni grade) underground nickel mines in Western Areas Limited’s Forrestania operations (Fewings and Seet Citation2014; Fewings et al. Citation2015). The output from the concentrator is sold to nickel smelters; however, the processing of ore from the Spotted Quoll mine presents challenges as the ore has a high arsenic content and the arsenic is present as niccolite and gersdorffite (Fewings and Seet Citation2014; Fewings et al. Citation2015). Niccolite and gersdorffite are rejected during flotation using cyanide as a depressant at pH 9.8 to 10.5, though this results in a loss of Ni to the tailings (Fewings et al. Citation2016). The cleaner tailings arising from processing Spotted Quoll ore grade 18.3% Ni and 6.4% As (Fewings et al. Citation2016). To recover Ni values from the cleaner tailings, Western Areas Limited has implemented a bioleach process based on proprietary BioHeapTM technology, whereby Ni is leached from the flotation cleaner tailings whilst As and Fe are rejected as an environmentally stable precipitate (Fewings and Seet Citation2014; Fewings et al. Citation2015, Citation2016).
A key feature of the BioHeapTM process is the use of proprietary bacterial cultures which are tolerant to arsenic and high concentrations of total dissolved salts (TDS) (up to 200 g/L TDS) with a wide temperature range of operation (15–95°C) (Fewings and Seet Citation2012). Leaching using proprietary BioHeapTM cultures is reported to take place at elevated pH (above pH 2) (Fewings and Seet Citation2012) unlike the more common acid ferric sulfate leaching processes utilizing mesophilic and thermophilic microorganisms. The reported benefits of bioleaching above pH 2 is lower acid consumption and simpler downstream neutralization due to reduced dissolution of gangue minerals and rejection of iron during leaching (Fewings and Seet Citation2012). The operating conditions of the leach circuit are reported to be a pH between 2.8 and 3.5 at 55°C in stirred tank reactors using treated process water containing 50 g/L TDS (Fewings et al. Citation2016); the residence time in the bioleaching circuit is reported to be 5–7 days (Fewings et al. Citation2015). The process water is treated with Caro’s acid (H2SO5) to destroy cyanide which was reported to cause some retardation of Ni leaching (Fewings et al. Citation2015). The Ni is subsequently recovered from the leach liquor by precipitation using sodium sulfide (Na2S) to produce a nickel sulfide precipitate grading 45–65% Ni, which is blended with the final flotation concentrate (Fewings et al. Citation2016). A process flow diagram of the BioHeapTM leach process implemented at the Cosmic Boy nickel concentrator is presented in .
Figure 10. Simplified process flow diagram for bacterial leaching (BioHeaptm) of high arsenic nickel flotation cleaner tailings at the Cosmic Boy nickel concentrator, Forrestania Nickel Project (western Australia), owned and operated by Western Areas Limited. Adapted from Fewings et al. (Citation2016).
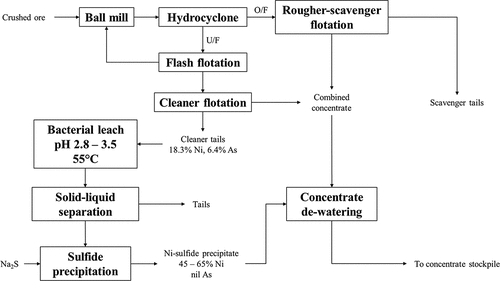
3.6. Roast-leach processes
Roasting of nickel bearing sulfide concentrates followed by leaching has been practised at two operations in Canada, the Falconbridge iron ore plant and the Inco iron ore recovery plant, both treating nickeliferous pyrrhotite concentrates from their mill operations and one operation in Finland, the Kokkola refinery, treating pyrite-pyrrhotite concentrates. It appears there was an economic incentive at the time for these operations to extract iron and base metal values from iron sulfide concentrates produced as a by-product of copper/nickel mining, rather than diverting them to tailings. The Falconbridge and Inco pyrrhotite processing plants have been closed since 1972 and 1982, respectively (Rezaei et al. Citation2017), whilst the pyrite roaster at the Kokkola refinery in Finland shut in 1987 (Sauni et al. Citation2010), however brief descriptions are provided for historical purposes. Since the closure of the Canadian plants, nickeliferous pyrrhotite is rejected to the tailings at Canadian mill operations and there is a considerable amount of pyrrhotite in tailings storage areas, where it is a potential source of acid mine drainage but is also of potential economic value due to the contained Ni (Peek, Barnes, and Tuzun Citation2011; Rezaei et al. Citation2017).
3.6.1. Falconbridge iron ore plant
The Falconbridge iron ore plant was operated between 1955 and 1972 and processed pyrrhotite concentrate via a sulfation roast-leach process to recover Fe in the form of a hematite product and base metals (Ni, Cu, Co) as a sulfide precipitate which is subsequently smelted (Rezaei et al. Citation2017). Pyrrhotite concentrate was subjected to oxidative roasting in the presence of Na2SO4 (4–5% by weight of the pyrrhotite feed) at 680°C (Rezaei et al. Citation2017; Thornhill Citation1961) which converted the base metals to their respective sulfates and iron to hematite. There were no provisions to capture SO2 from the roasters which was discharged directly into the atmosphere (Rezaei et al. Citation2017). The calcine from the roasters was leached in water to dissolve the base metal salts and recover an iron oxide leach residue which was dried and pelletized, producing an iron oxide product grading 66% Fe, 0.1% Ni, 2.2% SiO2 and 0.5% S (Rezaei et al. Citation2017). The leach liquor is treated with pyrrhotite concentrate to reduce ferric iron in the liquor to the divalent state, followed by precipitation of base metals as their sulfides via addition of elemental sulfur, Na2S and iron borings which produces a sulfide precipitate typically grading 20.28–20.91% Ni, 1.85–2.30% Cu and 0.44–0.49% Co at an overall Ni recovery of 82.2% (Thornhill Citation1961). The plant was shut in 1972 due to the introduction of environmental regulations which imposed annual limits on SO2 emissions from smelters (Potvin and Negusanti Citation1995). Further operational details of the Falconbridge iron ore process are provided by Thornhill (Citation1961) whilst technical details of the roasting process and sulfide precipitation method are provided in the relevant patents (Thornhill Citation1954, Citation1960; Thornhill and Coulter Citation1966).
3.6.2. Inco iron ore recovery plant
Inco (now Vale) operated a modified Caron process between 1956 and 1982 and extracted iron, sulfur, Ni and Cu from pyrrhotite concentrate recovered from the Copper Cliff or Clarabelle mills in the form of iron oxide pellets, sulfuric acid, nickel oxide (NiO) and copper sulfide (CuS) (Conard Citation2013; Rezaei et al. Citation2017). The reduction roast/leach sectio n of the plant was shut in 1982 amid an economic downturn whilst the pyrrhotite roasters continued to operate supplying sulfuric acid to the market until 1991 (Rezaei et al. Citation2017). Conard (Citation2013) indicated that production of NiO continued until the mid-1990s, though with the shutdown of the reduction kiln, the feed was metallic Ni powder from Inco’s Copper Cliff nickel refinery.
The first stage in the process was an oxidizing roast of the pyrrhotite concentrate at 760°C to produce an iron oxide calcine and SO2; the SO2 stream was sent to a sulfuric acid plant whilst the calcine was transferred to the reduction kiln (Conard Citation2013). The Ni present in pyrrhotite was oxidized to nickel ferrite (NiFe2O4) (Conard Citation2013; Rezaei et al. Citation2017). In the reduction kiln, Ni in nickel ferrite was reduced to a mixture of Fe-Ni alloy and nickel sulfide whilst hematite in the calcine was reduced to magnetite using partially combusted natural gas (Conard Citation2013; Rezaei et al. Citation2017). The reduced calcine was leached in aerated ammonia-ammonium carbonate lixiviant at 55°C to dissolve Ni and other base metals in the calcine (Conard Citation2013). The iron oxide leach residue was sent to a pelletizing plant to produce hematite pellets grading 67% Fe (Conard Citation2013). Copper (along with some Co) was recovered from the leach liquor by precipitation as a sulfide using sodium hydrosulfide (NaHS) with the precipitate being sent to a smelter (Conard Citation2013). Nickel was recovered from the leach liquor after Cu removal by precipitation as a basic nickel carbonate using soda ash (Na2CO3) and calcination at 550°C to produce an acid-soluble nickel oxide product grading 77% Ni and 0.15% Co (Conard Citation2013).
3.6.3. Kokkola refinery, Finland
The Kokkola refinery operated by Outokumpu Oyj processed pyrite-pyrrhotite flotation concentrate recovered as a by-product from the Outokumpu copper mine for recovery of base metals between 1966 and 1987 (Palperi and Aaltonen Citation1971; Sauni et al. Citation2010). The pyrite-pyrrhotite concentrate graded 0.67% Co, 0.37% Ni, 0.28% Cu and 0.4% Zn (Palperi and Aaltonen Citation1971). A two-stage roasting process was employed whereby part of the concentrate was roasted to produce an oxide calcine (termed dead roasting) which was then combined with fresh sulfide concentrate and sodium sulfate in the second roasting stage where sulfation of the base metals takes place at 680°C (Deng Citation1992; Palperi and Aaltonen Citation1971). The rationale for this two-stage roasting approach was (a) finely divided ferric oxide produced from dead roasting catalyses SO2 oxidation to SO3 which reacts with metal oxides to form their sulfates and (b) addition of fresh concentrate to the second stage maintained a desirable atmosphere for sulfation and supplied heat for roasting (Palperi and Aaltonen Citation1971). The SO2 produced from roasting was sent to a sulfuric acid plant whilst the iron oxide leach residue (termed purple ore) grading 64.3% Fe (Palperi and Aaltonen Citation1971) was presumably sold. Nickel, Cu and Zn were recovered from the leach liquor by precipitation as their sulfides, with the sulfide precipitate being smelted and refined at Harjavalta (Finland) (Palperi and Aaltonen Citation1971). Metallic cobalt was recovered by hydrogen reduction of a cobalt-ammonium sulfate solution (Palperi and Aaltonen Citation1971). The sulfation roasting plant reportedly closed in 1987 (Sauni et al. Citation2010), ahead of the closure of the Outokumpu copper mine in 1989, though the production of cobalt products at Kokkola continues to this day and a cobalt refinery is currently operated by Umicore (Törmänen and Tuomela Citation2021).
4. Summary
A summary of the advantages, disadvantages, and commercial progress of each of the various leaching systems that have been employed for direct extraction of nickel from flotation concentrates is presented in . It can be seen from that sulfate-based leaching technologies, particularly pressure oxidation and bioleaching, are the most advanced with regards to the number of processes that have been piloted and commercially deployed at nickel sulfide processing operations. Both technologies are well established, having enjoyed success in other industries and therefore pose a low technical risk relative to other less established leaching technologies. Other factors to consider in selection of a process/technology for direct leaching of nickel sulfide concentrates besides the track record/experience in other industries is the flexibility/adaptability of the process to changes in feed composition and the types of finished nickel products that can be produced i.e. intermediates such as MHP or MSP, or class 1 nickel (>99.8% Ni) products such as electrowon cathode, metallic Ni briquettes or powder, or high-purity nickel sulfate. Some of the processes are summarized in such as nitric acid leaching have not progressed beyond laboratory scale investigation and therefore remain unproven at large scale. It is interesting to note that despite the success of chloride-based leaching processes in the commercial processing of nickel mattes, chloride-based leaching has not seen use for direct processing of nickel sulfide concentrates. Despite the number of technologies that have been developed for direct leaching of nickel sulfide concentrates, the adoption of direct leaching processes for treatment of nickel sulfide concentrates/ores remains low. However, the expected demand for class 1 nickel in lithium-ion batteries and electric vehicles could provide renewed interest in direct leaching of nickel sulfide concentrates for the following reasons; firstly, Ni extraction from sulfidic resources is likely to be lower cost relative to lateritic resources and secondly, production of class 1 nickel or intermediates such as MHP could enable nickel sulfide miners to better capture the value of their sulfide concentrates through hydrometallurgical processing.
Table 6. Comparison of leaching processes that have been evaluated for the direct leaching of nickel sulfide flotation concentrates and commercial progress.
Acknowledgements
The authors are grateful for the support provided by CSIRO Mineral Resources to Nebeal Faris through the ResearchPlus CERC Postdoctoral Fellowship program. The authors would like to thank Dr. Robbie McDonald and Mr. David McCallum of CSIRO Mineral Resources for taking time to review and provide valuable feedback during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adams, M., and G. Johnson, 2001. Advances in nickel sulfide process development. In ALTAc 2001 Nickel/Cobalt Conference, 18. Melbourne, Victoria, Australia: ALTA Metallurgical Services.
- Adams, M., K. Liddell, and T. Holohan. 2011. Hydrometallurgical processing of Platreef flotation concentrate. Minerals Engineering 24 (6):545–50. doi:10.1016/j.mineng.2010.09.009.
- Adams, M. D., K. S. Liddell, and L. A. Smith. 2015. The KellGold hydrometallurgical process for cyanide-free extraction of gold from refractory concentrates and feedstocks-a preliminary assessment, World Gold Conference 2015, 141–54. Johannesburg: The Southern African Institute of Mining and Metallurgy.
- Agar, G. E. 1991. Flotation of chalcopyrite, pentlandite, pyrrhotite ores. International Journal of Mineral Processing 33 (1–4):1–19. doi:10.1016/0301-7516(91)90039-L.
- Anon. 2015. The first cargo of nickel from the processing plant at Long Harbour is transported to the USA. Accessed August 27, 2021. http://www.vale.com/canada/EN/aboutvale/news/Pages/primeiro-carregamento-niquel-usina-processamento-long-harbour-transportado-eua.aspx
- Anon, 01 October 2021 BHP delivers first crystals from Kwinana nickel sulphate plant https://www.bhp.com/news/media-centre/releases/2021/10/bhp-delivers-first-crystals-from-kwinana-nickel-sulphate-plant Accessed 05 May 2022 () (BHP)
- Anon, July 29, 2021. Terrafame’s net sales in the first half of 2021 amounted to EUR 185.0 million – Battery chemicals plant’s ramp-up started. Accessed August 27, 2021. https://www.terrafame.com/news-from-the-mine/news/2021/07/terrafames-net-sales-in-the-first-half-of-2021-amounted-to-eur-185.0-million-battery-chemicals-plants-ramp-up-started.html
- Astudillo, C., and F. Acevedo. 2008. Adaptation of Sulfolobus metallicus to high pulp densities in the biooxidation of a flotation gold concentrate. Hydrometallurgy 92 (1–2):11–15. doi:10.1016/j.hydromet.2008.02.003.
- Bailey, A., and G. Hansford. 1993. Factors affecting bio‐oxidation of sulfide minerals at high concentrations of solids: A review. Biotechnology and Bioengineering 42 (10):1164–74. doi:10.1002/bit.260421006.
- Barnes, S. J., S. Barnes, and C. Perring. 2007. Komatiite-hosted nickel sulfide deposits: Geology, geochemistry, and genesis. Society of Economic Geologists Special Publication 13:51–97.
- Barnes, S.-J., and P. C. Lightfoot Hedenquist, J. W., Thompson, J. F. H., Goldfarb, R. J., Richards, J. P. 2005. Formation of Magmatic Nickel Sulfide Deposits and Processes Affecting Their Copper and Platinum Group Element Contents. Economic Geology 100th Anniversary. Littleton, Colorado, USA: Society of Economic Geologists. 179–213.
- Barnes, S. J., J. E. Mungall, M. Le Vaillant, B. Godel, C. M. Lesher, D. Holwell, P. C. Lightfoot, N. Krivolutskaya, and B. Wei. 2017. Sulfide-Silicate textures in magmatic Ni-Cu-PGE sulfide ore deposits: Disseminated and net-textured ores. American Mineralogist 102 (3):473–506. doi:10.2138/am-2017-5754.
- Barnes, S. J., S. Staude, M. Le Vaillant, R. Piña, and P. C. Lightfoot. 2018. Sulfide-Silicate textures in magmatic Ni-Cu-PGE sulfide ore deposits: Massive, semi-massive and sulfide-matrix breccia ores. Ore Geology Reviews 101:629–51. doi:10.1016/j.oregeorev.2018.08.011.
- Berezowsky, R., 2000. The pressure acid leaching of nickel sulphide concentrates. In TMS 129th Annual Meeting, 32. Nashville, TN: The Minerals, Metals & Materials Society (TMS).
- Berezowsky, R. M. G. S., M. Collins, D. Kerfoot, and N. Torres. 1991. The commercial status of pressure leaching technology. JOM 43 (2):9–15. doi:10.1007/BF03220132.
- Bide, T., L. Hetherington, and G. Gunn. 2008. Nickel – Mineral profile, 1–24. Keyworth, Nottingham, UK: British Geological Survey.
- Björkman, M., 2019. Hitura nickel mine cleanup effort to cost €16m for the Finnish state, Mining Metalnews.com. Accessed September 21, 2021. https://www.miningmetalnews.com/index.php/20190828/1272/hitura-nickel-mine-cleanup-effort-cost-eu16m-finnish-state
- Brown, J. A., and V. G. Papangelakis. 2005. Interfacial studies of liquid sulphur during aqueous pressure oxidation of nickel sulphide. Minerals Engineering 18 (15):1378–85. doi:10.1016/j.mineng.2005.02.008.
- Bulatovic, S. M. 2007. Flotation of nickel and nickel-copper ores. In Handbook of flotation reagents: Chemistry, theory and practice, volume 1: Flotation of sulfide ores, 401–42. Amsterdam, Netherlands: Elsevier.
- Campagnol, N., K. Hoffman, A. Lala, and O. Ramsbottom, 2017. The future of nickel: A class act, McKinsey & Company, 16 pp. Downloaded August 31, 2021. https://www.mckinsey.com/~/media/McKinsey/Industries/Metals%20and%20Mining/Our%20Insights/The%20future%20of%20nickel%20A%20class%20act/The%20future%20of%20nickel%20A%20class%20act.pdf
- Caron, M. 1950. Fundamental and practical factors in ammonia leaching of nickel and cobalt ores. JOM-Journal of the Minerals, Metals and Materials Society 2 (1):67–90. doi:10.1007/BF03398981.
- Cole, P. M., A. E. Norton, G. Benetis, D. W. Dew, and D. M. Miller. 1997. The BioNIC process: Description of the process and presentation of pilot plant results. In Proceedings of the Nickel-Cobalt 97 International Symposium, ed. W. C. Cooper and I. Mihaylov, 98–110. Sudbury, ON: The Metallurgical Society of CIM.
- Conard, B. R. 2013. Processing history at Vale Canada’s (Inco’s) iron ore recovery plant. Canadian Institute of Mining, Metallurgy and Petroleum 4 (4):61–68.
- Cooke, P. 2021. The lithium wars: From Kokkola to the Congo for the 500 mile battery. Sustainability 13 (8):4215. doi:10.3390/su13084215.
- Corrans, I., G. Johnson, and J. Angove. 1993. The recovery nickel and gold from sulphide concentrates. In XVIII International Mineral Processing Congress, ed. J. T. Woodcock, 1227–31. Sydney, Australia: Australasian Institute of Mining and Metallurgy (AusIMM).
- Crossley, B., T. Gerrans, D. Honey, and D. Muir, inventor(s); WMC Resources Ltd, assignee. 1998. Leaching of a nickel sulphide concentrate. AU Patent 8077698A. Filed August 18, 1998.
- Crundwell, F., M. S. Moats, V. Ramachandran, T. Robinson, and W. G. Davenport. 2011a. Converting – Final oxidation of iron from molten matte. In Extractive metallurgy of nickel, cobalt and platinum-group metals, 233–46. Amsterdam, Netherlands: Elsevier.
- Crundwell, F., M. S. Moats, V. Ramachandran, T. Robinson, and W. G. Davenport. 2011b. Extraction of nickel and cobalt from sulfide ores. In Extractive metallurgy of nickel, cobalt and platinum-group metals, 147–58. Amsterdam, Netherlands: Elsevier.
- Crundwell, F., M. S. Moats, V. Ramachandran, T. Robinson, and W. G. Davenport. 2011c. Flash smelting of nickel sulfide concentrates. In Extractive metallurgy of nickel, cobalt and platinum-group metals, 215–32. Amsterdam, Netherlands: Elsevier.
- Crundwell, F., M. S. Moats, V. Ramachandran, T. Robinson, and W. G. Davenport. 2011d. Separation of the platinum-group metals from base metal sulfides, and the refining of nickel, copper and cobalt. In Extractive metallurgy of nickel, cobalt and platinum-group metals, 457–87. Netherlands: Elsevier.
- Crundwell, F., M. S. Moats, V. Ramachandran, T. Robinson, and W. G. Davenport. 2011e. Slow cooling and solidification of converter matte. In Extractive metallurgy of nickel, cobalt and platinum-group metals, pp. 259–67. Amsterdam, Netherlands: Elsevier.
- Crundwell, F., M. S. Moats, V. Ramachandran, T. Robinson, and W. G. Davenport. 2011f. Smelting of nickel sulfide concentrates by roasting and electric furnace smelting. In Extractive metallurgy of nickel, cobalt and platinum-group metals, 199–214. Amsterdam, Netherlands: Elsevier.
- Dalvi, A., G. Bacon, and R. Osborne. 2004. The past and the future of nickel laterites. In PDAC 2004 International Convention trade show and investors exchange, Prospectors and Developers Association of Canada, Toronto, Canada, (7–10 March, 2004).
- Defreyne, J., W. Grieve, D. Jones, and K. Mayhew. 2006. The role of iron in the CESL process. In 3rd International Symposium on Iron Control in Hydrometallurgy, ed. J. E. Dutrizac and P. A. Riveros, 205–20. Montreal, Canada: Canadian Institute of Mining, Metallurgy, and Petroleum.
- Deng, T. 1992. Chemical oxidation of iron sulfide minerals for metals recovery. Mineral Processing and Extractive Metullargy Review 10 (1):325–45. doi:10.1080/08827509208914094.
- Deng, T. 1993. Aqueous pressure oxidation of minerals – A salient development in hydrometallurgy. Mineral Procesing and Extractive Metallurgy Review 12 (2–4):185–222. doi:10.1080/08827509508935258.
- Deng, T. 1994. Nickel and cobalt extraction by pressure hydrometallurgy, 9. Government Resesrch Announcement Index.
- Dew, D., C. Van Buuren, K. McEwan, and C. Bowker. 1999. Bioleaching of base metal sulphide concentrates: A comparison of mesophile and thermophile bacterial cultures. Process Metallurgy 9:229–38.
- Dowling, S., S. J. Barnes, R. Hill, and J. Hicks. 2004. Komatiites and nickel sulfide ores of the Black Swan area, Yilgarn Craton, Western Australia. 2: Geology and genesis of the orebodies. Mineralium Deposita 39 (7):707–28. doi:10.1007/s00126-004-0438-8.
- Dreisinger, D., W. Murray, D. Hunter, K. Baxter, J. Ferron, and C. Fleming, 2005. The application of the PLATSOL™ process to copper-nickel-cobalt-PGE/PGM concentrates from PolyMet Mining’s NorthMet deposit. In ALTA 2005 Nickel/Cobalt Conference, . May 16-18, 2005. Rendezvous Observation City Hotel, Perth, Australia: Melbourne, Victoria, Australia. ALTA Metallurgical Services.
- Dutrizac, J. 1992. The leaching of sulphide minerals in chloride media. Hydrometallurgy 29 (1–3):1–45. doi:10.1016/0304-386X(92)90004-J.
- Duyvesteyn, W. P., and B. Sabacky. 1993. The Escondida process for copper concentrates. In The Paul E. Queneau International Symposium - Extractive Metallurgy of Copper, Nickel and Cobalt. Volume I: Fundamental Aspects, ed. R. G. Reddy and R. N. Weizenbach, 881–910. Denver, Colorado, USA: The Minerals, Metals and Materials Society (TMS).
- Eckstrand, O. R., and L. J. Hulbert. 2007. Magmatic nickel-copper-platinum group element deposits. In Mineral deposits of Canada: A synthesis of major deposit types, district metallogeny, the evolution of geological provinces, and exploration methods , ed. W. D. Goodfellow, 5: 205–22. St John‘s, Newfoundland and Labrador, Canada: Geological Association of Canada.
- Eksteen, J., E. Oraby, and V. Nguyen. 2020. Leaching and ion exchange based recovery of nickel and cobalt from a low grade, serpentine-rich sulfide ore using an alkaline glycine lixiviant system. Minerals Engineering 145:106073. doi:10.1016/j.mineng.2019.106073.
- Elias, M. 2002. Nickel laterite deposits-geological overview, resources and exploitation. Giant ore deposits: Characteristics, genesis and exploration. CODES Special Publication 4:205–20.
- Eltham, J. A., and P. A. Tilyard, 1973. An approach to the flotation of Western Australian nickel ores. In Australian Institute of Mining and Metallurgy Conference, Perth, WA, 417–29.
- Fassell, W. M. 1962. Hyperatmospheric extractive metallurgy: Its past, present and future. Pure and Applied Chemistry 5 (3–4):683–700. doi:10.1351/pac196205030683.
- Feng, B., Q. Feng, Y. Lu, and P. Lv. 2012. The effect of conditioning methods and chain length of xanthate on the flotation of a nickel ore. Minerals Engineering 39:48–50. doi:10.1016/j.mineng.2012.05.022.
- Ferron, C., and C. Fleming, 2001. PLATSOL treatment of the Northmet copper-nickel-pgm bulk concentrate- pilot plant results. In ALTA 2001 Nickel/Cobalt-7: 2001. May 15-17, 2001. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Rendezvous Observation City Hotel, Perth, Australia.
- Ferron, C., and C. Fleming. 2002. Pilot plant demonstration of the Platsol process for the treatment of the NorthMet copper-nickel-PGM deposit. Mining Engineering (Colorado) (USA) 54 (12):33–39.
- Ferron, C. J., C. Fleming, D. B. Driesinger, and P. T. O’-Kane, 2000. Single-step pressure leaching of base and precious metals(gold and PGM’s) using the platsol process. In ALTA 2000 Nickel/Cobalt-6, 193–210.
- Ferron, C., C. Fleming, P. O’-Kane, and D. Dreisinger. 2002. High temperature chloride assisted leach process to extract simultaneously Cu, Ni, Au and the PGM’s from various feedstocks. In Proceedings of the 32nd Annual Hydrometallurgy Meeting and International Conference on the Practice and Theory of Chloride/Metal Interaction, ed. E. Peek and G. van Weert, 11–28. Montreal, Quebec, Canada: Canadian Institute of Mining, Metallurgy and Petroleum.
- Fewings, J., and S. Seet, 2012. Bacterial leaching at elevated pH using BioHeap™ technology. In ALTA 2012 Nickel-Cobalt-Copper Proceedings May 28-30, 2012. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Burswood Convention Centre, Perth, Australia, 365–72.
- Fewings, J., and S. Seet, 2014. BioHeap® application at cosmic boy nickel concentrator. In ALTA 2014 Nickel-Cobalt-Copper Proceedings 24-31 May, 2014. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Perth, Australia, 423–30.
- Fewings, J., S. Seet, T. McCredden, and C. Fitzmaurice, 2015. BioHeap® application at cosmic boy nickel concentrator – An update. In ALTA 2015 Nickel-Cobalt-Copper Proceedings, 25-27 May, 2015. Melbourne, Victoria, Australia: ALTA Metallurgical Services). Perth, Australia, 356–72.
- Fewings, J., S. Seet, T. McCredden, and C. Fitzmaurice, 2016. BioHeap® – Cosmic boy mill recovery enhancement project. An update. In ALTA 2016 Nickel-Cobalt-Copper Proceedings, 23-25 May, 2016. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Perth, Australia, 366–74.
- Fewings, J., and A. Wilathgamuwa. 1998. Pilot plant experience on the bacterial leaching of Ni/Co sulfide concentrates at BacTech (Australia) Ltd, Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, 13. Melbourne, Victoria, Australia: ALTA Metallurgical Services.
- Fleming, C., C. J. Ferron, D. Dreisinger, and P. T. O’-Kane. 2000. A process for the simultaneous leaching and recovery of gold, platinum group metals and base metals from ores and concentrates. In EPD congress 2000, ed. P. R. Taylor, 419–31. Nashville, TN: The Minerals, Metals and Materials Society (TMS).
- Forward, F. 1953. Ammonia pressure leach process for recovering nickel, copper, and cobalt from Sherritt Gordon nickel sulphide concentrate. Transactions of the Canadian Institute of Mining and Metallurgy and the Mining Society of Nova Scotia 56:363–70.
- Forward, F., and V. Mackiw. 1955. Chemistry of the ammonia pressure process for leaching Ni, Cu, and Co from Sherritt Gordon sulphide concentrates. JOM 7 (3):457–63. doi:10.1007/BF03377530.
- Forward, F., and I. Warren. 1960. Extraction of metals from sulphide ores by wet methods. Metallurgical Reviews 5 (1):137–64. doi:10.1179/mtlr.1960.5.1.137.
- Gilbertson, B. 2000. Creating value through innovation: Biotechnology in mining. Mineral Processing and Extractive Metallurgy 109 (2):61–67. doi:10.1179/mpm.2000.109.2.61.
- Grassi, R. A., A. G. Clausdorff, R. C. Harrison, and K. J. Osten, inventor(s); IGO Limited, assignee. Method for preparing a high-purity hydrated nickel sulphate. WO Patent 2020061639A1. Filed September 26, 2019.
- Grguric, B., N. Rosengren, C. Fletcher, and J. Hronsky. 2007. Type 2 deposits: Geology, mineralogy, and processing of the Mount Keith and Yakabindie orebodies, Western Australia. Special Publication-Society of Economic Geologists 13:119.
- Haavanlammi, L., O. Hyvarinen, J. Karonen, and D. Krebs, inventor(s); Outotec Oyj, assignee. Method for processing nickel bearing raw material in chloride-based leaching. AU Patent 2006298627B2. Filed March 10, 2006.
- Heinzle, T., D. Miller, and V. Nagel, 1999. Results of an integrated pilot plant operation using the BioNIC® process to produce nickel metal. In Proceedings Biomine ‘99 and water management in metallurgical operations ‘99, 16–25. Glenside, SA: Australian Mineral Foundation.
- Hoatson, D. M., S. Jaireth, and A. L. Jaques. 2006. Nickel sulfide deposits in Australia: Characteristics, resources, and potential. Ore Geology Reviews 29 (3–4):177–241. doi:10.1016/j.oregeorev.2006.05.002.
- Holwell, D. A., Z. Adeyemi, L. A. Ward, D. J. Smith, S. D. Graham, I. McDonald, and J. W. Smith. 2017. Low temperature alteration of magmatic Ni-Cu-PGE sulfides as a source for hydrothermal Ni and PGE ores: A quantitative approach using automated mineralogy. Ore Geology Reviews 91:718–40. doi:10.1016/j.oregeorev.2017.08.025.
- Honey, D. J., D. M. Muir, and P. R. Hunt. 1997. Hydrometallurgical processing options for nickel sulphide concentrates. In Proceedings of the Nickel-Cobalt 97 International Symposium, ed. W. C. Cooper and I. Mihaylov, 15–29. Sudbury, ON, Canada: The Metallurgical Society of CIM.
- Hourn, M., and D. Turner, 2012. Commercialisation of the Albion process. In Proceedings of the ALTA 2012 gold conference, 19. May 31 - June 1 2012 Burswood Convention Centre, Perth, Australia: Melbourne, Victoria, Australia: ALTA Metallurgical Services.
- Hourn, M. M., D. W. Turner, and I. R. Holzberger, inventor(s); M.I.M. Holdings Limited, Highlands Frieda Pty Ltd, assignee(s). 1996. Atmospheric mineral leaching process. US Patent 5993635A. Filed March 22, 1996.
- IGO Limited, 2020. Annual report 2020. Downloaded August 25 , 2021. https://www.igo.com.au/site/PDF/892ef7e5-efdb-4abc-a241-2e9605018439/2020AnnualReporttoShareholders
- Inco Staff. 1956. Development of the Inco iron ore recovery process. Transactions of the Canadian Institute of Mining and Metallurgy and the Mining Society of Nova Scotia, 59, 201–07.
- Johnson, G. D., and H. A. Evans, 1998. Activox® and the Yakabindie process for treatment of nickel sulfide concentrates. In ALTA 1998 Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, 25-27 May, 1998. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Hyatt Regency Hotel, Perth, Australia.
- Johnson, G., H. Evans, and J. Turner, 2000. Autoclave availability: Activox® the advantages of operating at reduced temperatures and pressures. In Proceedings of the International Conference ALTA 2000 Nickel/Cobalt, 15-17 May, 2000. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Rendezvous Hotel, Perth, Australia, 306–16.
- Jones, D. L., inventor; Cominco Engineering Services Ltd, assignee. 1996. Process for the recovery of nickel and/or cobalt from an ore or concentrate. US Patent 5855858A. Filed June 7, 1996.
- Jones, D., J. Hestrin, and R. Moore. 1998. CESL process for nickel/cobalt/copper sulphides, testing in an integrated pilot plant . In ALTA 1998 Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, 12. Melbourne, Australia: ALTA Metallurgical Services.
- Jones, D. L., K. Mayhew, R. Mean, and M. Neef, 2010. Unlocking disseminated nickel sulphides using the CESL nickel process. In ALTA 2010 Nickel/Cobalt/Copper Conference, 14. Melbourne, Victoria, Australia: ALTA Metallurgical Services.
- Jones, D., K. Mayhew, and L. O’-Conner, 2009. Nickel and cobalt recovery from a bulk copper-nickel concentrate using the CESL process, Teck Resources Limited, 12 pp. Downloaded February 2 , 2021. https://www.teck.com/media/CESL-Publication-Nickel-Cobalt-Recovery-Bulk-Copper-Nickel-Concentrate-CESL-Process.pdf
- Jones, D. L., R. Moore, and S. K. Stocker, inventor(s); Cominco Engineering Services Ltd, assignee. 2003. Pressure oxidation leaching in the presence of an acidic solution of halide and sulfate ions from copper and base metal containing ore/concentrate. US Patent 7438874B2. Filed May 22, 2003.
- Karonen, J., M. Tiihonen, and L. Haavanlammi. 2009. Hydronic – A novel nickel refining method for nickel concentrates. In Hydrometallurgy of nickel and cobalt 2009, ed. J. J. Budac, R. Fraser, I. Mihaylov, V. G. Papangelakis, and D. J. Robinson, 17–26. Sudbury, Ontario, Canada: The Metallurgy and Materials Society (MetSoc).
- Keele, R., and E. Nickel. 1974. The geology of a primary millerite-bearing sulfide assemblage and supergene alteration at the Otter Shoot, Kambalda, Western Australia. Economic Geology 69 (7):1102–17. doi:10.2113/gsecongeo.69.7.1102.
- Kerfoot, D. 1989. The development of the Sherritt ammonia pressure leach process. Canadian Mining and Metallurgical Bulletin 82 (926):136–41.
- Kerfoot, D. G. E., and P. D. Cordingley, 1997. The acid pressure leach process for nickel and cobalt laterite. Part II: Review of operations at Fort Saskatchewan In Proceedings of the Nickel-Cobalt 97 International Symposium, ed. W. C. Cooper and C. A. Levac, 355–70. Sudbury, Canada.
- Kerfoot, D., E. Krause, B. Love, and A. Singhal, inventor(s); Vale Canada Ltd, assignee. 2001. Recovery of nickel and cobalt values from a sulfidic flotation concentrate by chloride assisted oxidative pressure leaching in sulfuric acid. WO Patent 2002024966A1. Filed February 26, 2001.
- Kerfoot, D. G., and D. R. Weir. 1988. The hydro and electrometallurgy of nickel and cobalt. In Extractive metallurgy of nickel and cobalt, ed. G. P. Tyroler and C. A. Landolt, 241–67. Arizona: The Metallurgical Society Phoenix.
- Kerr, A. 2002. An overview of recent developments in flotation technology and plant practice for nickel ores. In Mineral Processing, Plant Design, Practice and Control Proceedings, ed. A. L. Mular, D. N. Halbe, and D. J. Barratt, 1142–58. Vancouver, Canada: Society for Mining, Metallurgy and Exploration.
- King, A., and J. Budden, 1996. The bacterial oxidation of nickel and cobalt polymetallic concentrates. In Proceedings of the Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, Perth, Australia, 13–14.
- Kitay, A., V. Mechev, and V. Volkov. 1991. Ecological pyro-hydrometallurgical technology of nickel pyrrhotite concentrate treatment with non-traditional and reagentless recovery of sulphur dioxide from low concentrated gases. In EMC’91: Non-Ferrous Metallurgy—Present and Future, ed. J. Vereecken, 149–55. England, Brussels, Belgium: Elsevier Science Publishers Ltd.
- Klyushnikov, A. M., R. I. Gulyaeva, E. N. Selivanov, and S. M. Pikalov. 2021. Kinetics and mechanism of oxidation for nickel-containing pyrrhotite tailings. International Journal of Minerals, Metallurgy and Materials 28 (9):1469–77. doi:10.1007/s12613-020-2109-x.
- Kofluk, R. P., and G. K. W. Freeman. 2006. Iron control in the Moa Bay laterite operation. In Iron Control in Hydrometallurgy, ed. J. E. Dutrizac and P. A. Riveros Montreal, Quebec, Canada: Metallurgical Socierty of the Canadian Institute of Mining, Metallurgy and Petroleum, 573–89.
- Kozyrev, S. M., M. Z. Komarova, L. N. Emelina, O. I. Oleshkevich, O. A. Yakovleva, D. V. Lyalinov, V. I. Maximov, and L. J. Cabri. 2002. The mineralogy and behaviour of PGM during processing of the Noril’Sk-Talnakh PGE-Cu-Ni ores. In The geology, geochemistry, mineralogy, mineral beneficiation of the platinum-group elements, ed. L. Cabri, vol. 54, 757–91. Montreal, Quebec, Canada: Canadian Institute Mining, Metallurgy and Petroleum Special.
- Krebs, D., O. Hyvarinen, and D. Dreisinger, inventor(s); Outotec Oyj, assignee. 2006. Processing nickel sulphide ore or concentrates with sodium chloride. CA Patent 2624609C. Filed October 3, 2006.
- Kuhn, M., N. Arbiter, and H. Kling. 1974. Anacondas Arbiter process for copper. Canadian Mining and Metallurgical Bulletin 67 (742):62–71.
- Laukka, A., J. Seppälä, J. Neale, P. van Aswegen, S. Barnett, A. Taskinen, and K. Koistinen, 2018. Mondo minerals nickel sulfide bioleach project: Maximising value through gold recovery. In ALTA 2018 Nickel-Cobalt-Copper Proceedings, 21-23 May, 2018.Melbourne, Victoria, Australia: ALTA Metallurgical Services. Perth, Australia, 507–24.
- Liddell, K. S., inventor. 1999. Hydrometallurgical treatment process for extraction of platinum group metals obviating the matte smelting process. WO Patent 1999060178A1. Filed May 19, 1999.
- Liddell, K. S., and M. D. Adams, inventor(s); Lifezone Limited, assignee. Hydrometallurgical treatment process for extraction of metals from concentrates. WO Patent 2014009928A1. Filed July 12, 2013.
- Liddell, K., and M. Adams. 2012. Kell hydrometallurgical process for extraction of platinum group metals and base metals from flotation concentrates. Journal of the Southern African Institute of Mining and Metallurgy 112 (1):31–36.
- Liddell, K., M. Adams, L. Smith, and B. Muller. 2019. Kell hydrometallurgical extraction of precious and base metals from flotation concentrates-Piloting, engineering, and implementation advances. Journal of the Southern African Institute of Mining and Metallurgy 119 (6):585–94. doi:10.17159/2411-9717/602/2019.
- Liddell, K., T. Newton, M. Adams, and B. Muller. 2011. Energy consumption for Kell hydrometallurgical refining versus conventional pyrometallurgical smelting and refining of PGM concentrates. Journal of the Southern African Institute of Mining and Metallurgy 111 (2):127–32.
- Lotter, N., D. Bradshaw, M. Becker, L. Parolis, and L. Kormos. 2008. A discussion of the occurrence and undesirable flotation behaviour of orthopyroxene and talc in the processing of mafic deposits. Minerals Engineering 21 (12–14):905–12. doi:10.1016/j.mineng.2008.02.023.
- Lu, Z., M. Jeffrey, Y. Zhu, and F. Lawson. 2000. Studies of pentlandite leaching in mixed oxygenated acidic chloride-sulfate solutions. Hydrometallurgy 56 (1):63–74. doi:10.1016/S0304-386X(00)00067-0.
- Ma¨ Kinen, T., and P. Taskinen. 2008. State of the art in nickel smelting: Direct Outokumpu nickel technology. Mineral Processing and Extractive Metallurgy 117 (2):86–94. doi:10.1179/174328508X290867.
- Mackiw, V., W. Lin, R. Benoit, and T. Benz. 1958. Nickel-Cobalt separation at Sherritt Gordon. Jom 10 (12):800–02. doi:10.1007/BF03398274.
- Makino, S., M. Sugimoto, F. Yano, and N. Matsumoto. 1996. Operation of the MCLE (matte chlorine leach electrowinning) plant for nickel refining at Sumitomo Metal Mining Co., Ltd. In EPD Congress 1996, ed. G. W. Warren, 297–311. Anaheim, CA: The Minerals, Metals and Materials Society.
- Mani, H., M. Xu, P. Quinn, and R. Stratton-Crawley. 1997. The effect of ultramafic mineralogy on pentlandite flotation. In Proceedings of the Second UBC-McGill Bi-annual International Symposium on Fundamentals of Mineral Processing and the Environment, ed. J. A. Finch, S. R. Rao, and I. Holubec, 63–76. Sudbury, ON: Canadian Institute of Mining, Metallurgy and Petroleum.
- Marsh, E. E., E. D. Anderson, and F. Gray, 2013. Nickel-cobalt laterites: A deposit model: Chapter H in mineral deposit models for resource assessment, Scientific Investigations Report 2010-5070-H, 38. Reston, Virginia, USA: U.S. Geological Survey.
- Marston, R., D. Groves, D. Hudson, and J. Ross. 1981. Nickel sulfide deposits in Western Australia; a review. Economic Geology 76 (6):1330–63. doi:10.2113/gsecongeo.76.6.1330.
- Matousek, J. 1982. The pyrometallurgical winning of cobalt. CIM Bulletin (Canadian Institute of Mining, Metallurgy and Petroleum) 75 (848):121–27.
- Mayhew, K., T. McCoy, R. Mean, and A. Miller, 2013. Teck’s CESL nickel process: Advancing towards a commercial ready hydromet solution for low grade disseminated nickel sulphides. In ALTA 2013 Nickel-Cobalt-Copper Conference, 259–69.
- McDonald, R. G., and J. Li. 2020. The high temperature co-processing of nickel sulfide and nickel laterite sources. Minerals 10 (4):351. doi:10.3390/min10040351.
- McDonald, R., and D. Muir. 2007. Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 86 (3–4):191–205.
- McRae, M. E., 2018. Nickel. In Metals and minerals: U.S. Geological Survey minerals yearbook 2015. Reston, Virginia, USA: U.S. Geological Survey, 1–18. Downloaded October 22, 2021. https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/nickel/myb1-2015-nicke.pdf
- McRae, M. E. 2020a. Nickel. In Mineral commodity summaries 2020, 112–13. Reston, Virginia, USA: U.S. Geological Survey.
- McRae, M. E. 2020b. Nickel. In Metals and minerals: U.S. Geological Survey minerals yearbook 2016, 1–18. Reston, Virginia, USA: U.S. Geological Survey. Downloaded September 21, 2020b. https://prd-wret.s3.us-west-2.amazonaws.com/assets/palladium/production/atoms/files/myb1-2016-nickel.pdf.
- Meddings, B., and D. J. I. Evans. 1971. The changing role of hydrometallurgy. CIM Bulletin (Canadian Institute of Mining, Metallurgy and Petroleum 64 (706):48–57.
- Meng, X., and K. N. Han. 1996. The principles and applications of ammonia leaching of metals—A review. Mineral Processing and Extractive Metullargy Review 16 (1):23–61. doi:10.1080/08827509608914128.
- Miller, P. C. 1997. The design and operating practice of bacterial oxidation plant using moderate thermophiles (the BacTech Process). In Biomining – Theory, microbes and industrial processes, ed. D. E. Rawlings, 81–102. Berlin, Heidelberg: Springer.
- Miller, P., M. Rhodes, R. Winby, A. Pinches, and P. Van Staden. 1999. Commercialization of bioleaching for base-metal extraction. Mining, Metallurgy & Exploration 16 (4):42–50. doi:10.1007/BF03403233.
- Mishra, G., K. Viljoen, and H. Mouri. 2013. Influence of mineralogy and ore texture on pentlandite flotation at the Nkomati nickel mine, South Africa. Minerals Engineering 54:63–78. doi:10.1016/j.mineng.2013.04.009.
- Mitchell, J. S. 1957. Cobalt pressure leaching and reduction at Garfield. JOM (The Journal of the Minerals, Metals & Materials Society (TMS)) 9 (3):343–45. doi:10.1007/BF03398496.
- Moskalyk, R., and A. Alfantazi. 2002. Nickel sulphide smelting and electrorefining practice: A review. Mineral Processing and Extractive Metallurgy Review 23 (3–4):141–80. doi:10.1080/08827500306893.
- Moyes, J., and F. Houlis. 2003. The development of the Intec Nickel Process to treat a low-grade Ni/Cu/Co/PGM concentrate. In ALTA Ni-Co 2003 Proceedings, 19-20 May, 2003. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Perth, Western Australia, 11.
- Mudd, G. M. 2010. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore Geology Reviews 38 (1–2):9–26.
- Muir, D., and E. Ho. 2006. Process review and electrochemistry of nickel sulphides and nickel mattes in acidic sulphate and chloride media. Mineral Processing and Extractive Metallurgy 115 (2):57–65. doi:10.1179/174328506X109031.
- Naldrett, A. 1999. World-Class Ni-Cu-PGE deposits: Key factors in their genesis. Mineralium Deposita 34 (3):227–40. doi:10.1007/s001260050200.
- Naldrett, A. J. 2004. The Jinchuan deposit, China. In Magmatic sulfide deposits, 373–404. Berlin, Heidelberg, Germany: Springer.
- Neale, J., M. Gericke, C. Pawlik, P. van Aswegen, S. Barnett, and J. Seppälä, 2015. The MONDO minerals nickel sulfide bioleach project: From test work to design. In Nickel-Cobalt-Copper Conference Proceedings, 25-27 May, 2015. Perth, Australia, 373–96, Melbourne, Victoria, Australia: ALTA Metallurgical Services.
- Neale, J., J. Seppälä, A. Laukka, P. van Aswegen, and S. Barnett, 2016. The MONDO minerals nickel sulfide bioleach project: Constrution Commissioning and Early Plant Operation. In ALTA 2016 Nickel-Cobalt-Copper Proceedings, 23-25 May. Melbourne, Victoria, Australia: ALTA Metallurgical Services. Perth, Australia, 375–411.
- Nemati, M., and S. T. L. Harrison. 2000. Effect of solid loading on thermophilic bioleaching of sulfide minerals. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology 75 (7):526–32. doi:10.1002/1097-4660(200007)75:7<526:AID-JCTB249>3.0.CO;2-4.
- Nickel, E., J. Ross, and M. Thornber. 1974. The supergene alteration of pyrrhotite-pentlandite ore at Kambalda, Western Australia. Economic Geology 69 (1):93–107. doi:10.2113/gsecongeo.69.1.93.
- Norton, A., J. Coetzee, and C. Barnett, 1998. BioNIC®: An economically competitive process for the biological extraction of nickel. In Proceedings of the Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, Perth, Australia, 25–27.
- Nyman, B., A. Aaltonen, S.-E. Hultholm, and K. Karpale. 1992. Application of new hydrometallurgical developments in the Outokumpu HIKO process. Hydrometallurgy 29 (1–3):461–78. doi:10.1016/0304-386X(92)90027-W.
- Oxley, A., M. E. Smith, and O. Caceres. 2016. Why heap leach nickel laterites? Minerals Engineering 88:53–60. doi:10.1016/j.mineng.2015.09.018.
- OZ Minerals, 2020. Annual and sustainability report 2020. Downloaded August 31, 2021. https://www.ozminerals.com/uploads/media/210218_OZ_Minerals_2020_Annual_and_Sustainability_Report.pdf
- Palmer, C., and G. Johnson. 2005. The Activox® process: Growing significance in the nickel industry. JOM 57 (7):40–47. doi:10.1007/s11837-005-0251-6.
- Palperi, M., and O. Aaltonen. 1971. Sulfatizing roasting and leaching of cobalt ores at Outokumpu Oy. JOM (The Journal of the Minerals, Metals & Materials Society (TMS)) 23 (2):34–38. doi:10.1007/BF03355685.
- Peek, E., A. Barnes, and A. Tuzun. 2011. Nickeliferous pyrrhotite – “Waste or resource?”. Minerals Engineering 24 (7):625–37. doi:10.1016/j.mineng.2010.10.004.
- Pietrobon, M. C., S. Grano, S. Sobieraj, and J. Ralston. 1997. Recovery mechanisms for pentlandite and MgO-bearing gangue minerals in nickel ores from Western Australia. Minerals Engineering 10 (8):775–86. doi:10.1016/S0892-6875(97)00056-3.
- Potvin, R. R., and J. J. Negusanti. 1995. Declining industrial emissions, improving air quality, and reduced damage to vegetation. In Restoration and Recovery of an Industrial Region Progress in Restoring the Smelter-Damaged Landscape Near Sudbury, Canada, Gunn, J. M. Springer series on environmental management, 51–65. New York: Springer.
- Queneau, P. E., W. K. Sproule, and A. Illis, inventor(s); Inco Limited, assignee. 1949. Method of producing high-grade iron oxide from ores rich in nickeliferous pyrrhotite. US Patent 2556215A. Filed March 5, 1949.
- Rezaei, S., F. Liu, S. Marcuson, M. Muinonen, V. L. Lakshmanan, R. Sridhar, and M. Barati. 2017. Canadian pyrrhotite treatment: The history, inventory and potential for tailings processing. Canadian Metallurgical Quarterly 56 (4):410–17. doi:10.1080/00084433.2017.1361671.
- Riekkola-Vanhanen, M. 2007. Talvivaara black schist bioheapleaching demonstration plant. In Advanced Materials Research, 30–33. Zurich: Trans Tech Publ.
- Riekkola-Vanhanen, M. 2013. Talvivaara mining company–from a project to a mine. Minerals Engineering 48:2–9. doi:10.1016/j.mineng.2013.04.018.
- Robles, E., I. Cronje, and G. Nel. 2009. Solvent extraction design consideration for the Tati Activox® plant. Journal of the Southern African Institute of Mining and Metallurgy 109 (6):383–91.
- Rorke, G., P. Basson, and D. Miller, 2001. Advancements in thermophile bioleaching technology. In ALTA 2001 Nickel/Cobalt Conference, 17. Melbourne, Victoria, Australia: ALTA Metallurgical Services.
- Sairinen, R., H. Tiainen, and T. Mononen. 2017. Talvivaara mine and water pollution: An analysis of mining conflict in Finland. The Extractive Industries and Society 4 (3):640–51. doi:10.1016/j.exis.2017.05.001.
- Sauni, R., A. Linna, P. Oksa, H. Nordman, M. Tuppurainen, and J. Uitti. 2010. Cobalt asthma—A case series from a cobalt plant. Occupational Medicine 60 (4):301–06. doi:10.1093/occmed/kqq023.
- Senior, G. D., L. K. Shannon, and W. J. Trahar. 1994. The flotation of pentlandite from pyrrhotite with particular reference to the effects of particle size. International Journal of Mineral Processing 42 (3–4):169–90. doi:10.1016/0301-7516(94)00031-X.
- Senior, G., and S. Thomas. 2005. Development and implementation of a new flowsheet for the flotation of a low grade nickel ore. International Journal of Mineral Processing 78 (1):49–61. doi:10.1016/j.minpro.2005.08.001.
- Sizgoric, M. 1981. Alteration of nickel sulfide ores and its effect on their flotation Process mineralogy : extractive metallurgy, mineral exploration, energy resources ; proceedings of a symposium sponsored by TMS-AIME Process Mineralogy Committee at the 110th AIME Annual Meeting, 22-26 February, 1981. Chicago, Illinois, USA, Hausen, D. M., Park, W. C. Warrendale, Pensylvania, USA: The Metallurgical Society of AIME. 225–32.
- Song, X., Y. Wang, and L. Chen. 2011. Magmatic Ni-Cu-(PGE) deposits in magma plumbing systems: Features, formation and exploration. Geoscience Frontiers 2 (3):375–84. doi:10.1016/j.gsf.2011.05.005.
- Stensholt, E. O., H. Zachariasen, and J. H. Lund. 1986. Falconbridge chlorine leach process. Transactions of the Institution of Mining and Metallurgy: Section C: Mineral Processing & Extractive Metallurgy 95 (3):10–16.
- Stevens, D., G. Bishop, B. Shinghal, B. Love, and I. Mihaylov, 2009. Operations of the pressure oxidative leach process for Voisey’s Bay nickel concentrate at Vale Inco’s Hydromet demonstration plant. In Proceedings of the 48th Annual Conference of Metallurgists of CIM, Sudbury, Canada, 3–16.
- Stieper, G., 2018. First chalcopyrite copper concentrate leaching using Albion processTM Technology (Conference presentation). In 10th International Seminar on Process Hydrometallurgy, Santiago, Chile.
- Subramanian, K. N., Ferrajuolo, R. 1976. Oxygen pressure leaching of Fe-Ni-Cu sulfide concentrates at 110°C — effect of low chloride addition. Hydrometallurgy, 2 (2): 117–125.
- Taylor, A., H. Fairley, and R. Winby. 1997. Re-opening of the radio hill nickel project, Western Australia using bacterial leaching of nickel sulphide concentrates. In ALTA 1997 Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, 57. Melbourne, Victoria, Australia: ALTA Metallurgical Services.
- Thornhill, P. G., inventor; Falconbridge Nickel Mines Ltd, assignee. Recovery of metals from solutions. US Patent 3103414A. Filed February 25, 1960.
- Thornhill, P. G., inventor; Falconbridge Ltd, assignee. 1954. Method of roasting metal sulfide concentrates in a fluidized bed. US Patent 2813015A. Filed April 30, 1954.
- Thornhill, P. G. 1961. The Falconbridge iron ore process. Transactions of the Canadian Institute of Mining and Metallurgy and the Mining Society of Nova Scotia 64:337–44.
- Thornhill, P. G., and E. H. Coulter, inventor(s); Falconbridge Nickel Mines Ltd, assignee. 1966. Method of precipitating metals as sulphides. US Patent 3450495A. Filed September 16, 1966.
- Toguri, J. 1975. A review on the methods of treating nickel-bearing pyrrhotite; with special reference to the Sudbury Area pyrrhotite. Canadian Metallurgical Quarterly 14 (4):323–38. doi:10.1179/cmq.1975.14.4.323.
- Toguri, J. M., H. Babaie, and R. Sridhar, 1995. Effect of arsenic on the Cu-Ni matte separation process. In Proceedings Second International Symposium on Quality in Non-ferrous Pyrometallurgy, 20-24 August, 1995. Vancouver, British Columbia, Canada, Kozlowski, M. A., McBean, R. W., Argyropoulos, S. A. (Montréal, Canada: Metallurgical Society of the Canadian Institute of Mining, Metallurgy and Petroleum, 311–18.
- Tong, L., and D. Dreisinger. 2009. The adsorption of sulfur dispersing agents on sulfur and nickel sulfide concentrate surfaces. Minerals Engineering 22 (5):445–50. doi:10.1016/j.mineng.2008.12.006.
- Törmänen, T., and P. Tuomela, 2021. Analysis of Finnish battery mineral deposits with special emphasis on cobalt. Open File Research Report, 29/2021. Geological Survey of Finland, 63 pp. Downloaded August 27 , 2021. https://tupa.gtk.fi/raportti/arkisto/29_2021.pdf.
- Warner, A. E. M., C. M. Díaz, A. D. Dalvi, P. J. Mackey, A. V. Tarasov, and R. T. Jones. 2007. JOM world nonferrous smelter survey Part IV: Nickel: Sulfide. JOM 59 (4):58–72. doi:10.1007/s11837-007-0056-x.
- Watling, H. 2006. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 84 (1–2):81–108. doi:10.1016/j.hydromet.2006.05.001.
- Watling, H. 2008. The bioleaching of nickel-copper sulfides. Hydrometallurgy 91 (1–4):70–88. doi:10.1016/j.hydromet.2007.11.012.
- Watmuff, I. 1974. Supergene alteration of the Mt Windarra nickel sulphide ore deposit, Western Australia. Mineralium Deposita 9 (3):199–221. doi:10.1007/BF00203996.
- Wishaw, B. 1993. Nickel matte refining at the Kwinana, WA, refinery of Western Mining Corporation Limited. Australasian Mining and Metallurgy, AusImm 2:1203–06.
- Woodward, T., 2014. On the operability of the Sherritt-Gordon ammonia leach at the Kwinana nickel refinery. Doctoral dissertation, Murdoch University, 407 pp.
- Woodward, T. M., and P. A. Bahri. 2007. Steady-state optimisation of the leaching process at Kwinana Nickel refinery. In 17th European Symposium on Computer Aided Process Engineering. , Pleşu, V., Agachi, P. S., Computer Aided Chemical Engineering, 24: 557–62. Amsterdam, The Netherlands: Elsevier.
- Xiao, Z., and A. Laplante. 2004. Characterizing and recovering the platinum group minerals – A review. Minerals Engineering 17 (9–10):961–79. doi:10.1016/j.mineng.2004.04.001.
