?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Breast cancer is a serious disease, and therefore early detection is crucial for successful treatment and patient management. Unfortunately, globally, the number of breast cancer cases is increasing due to various multifaceted factors. It is currently one of the leading causes of cancer deaths in women, worldwide. Cancerous cells in the breast can form lumps that impact the patient’s health, and even seemingly harmless tumors could be fatal if undiagnosed early enough. Fortunately, artificial intelligence techniques have proven effective in detecting diseases, and doctors can therefore use them to effectively and accurately diagnose breast cancer early. This paper explores the use of genetic algorithms, ant colony optimization, and Hybrid Hopfield Neural Network-E2SAT (HHNN-E2SAT) models, for breast cancer prediction. The HHNN-E2SAT models outperform standard algorithms like the Random Forest and Support Vector Machines, achieving over 98% on all performance metrics (i.e. Accuracy, F1-score, Sensitivity, Specificity, and Precision).
Introduction
A fundamental challenge in bioinformatics or clinical research is the inability to accurately identify important information (Embi and Payne Citation2009). As such, diagnostics is an active and evolving field of medicine (Bolboacă Citation2019). Breast cancer is scored highly among various cancers because of its associated effects on a patient (Ataollahi et al. Citation2015). Many lives could potentially be saved from breast cancer deaths if intelligent data-driven methods are developed to a level of real-world application (Ahn et al. Citation2023). Previous studies highlight the use of feature-based data mining (DM) techniques in the prediction of various diseases, breast cancer inclusive (Ahn et al. Citation2023; Amin, Kia Chiam, and Dewi Varathan Citation2019; Amrane et al. Citation2018; Ganggayah et al. Citation2019b). To analyze raw data such as the primary causes of mortality in cancer patients, and to offer fresh perspectives on illness prevention with precise forecasts, data mining methods generally offer a promising alternative (Ahn et al. Citation2023; Amin, Kia Chiam, and Dewi Varathan Citation2019; Ganggayah et al. Citation2019b). Being able to identify any type of cancer, particularly breast cancer, as early as possible is essential for proper patient management, thus minimizing cancer fatalities. Like most types of cancer, breast cancer, is a complex disease often influenced by a combination of various factors (Zhang et al. Citation2020). These include genetic, environmental, and lifestyle, among other factors. shows key factors associated with cancer as extracted from (Ataollahi et al. Citation2015; CDC Citation2023; Petrucelli, Mary, and Tuya Citation1993).
Table 1. A description of cancer-associated factors as established from literature.
As a result of advancements in digital data storage and processing, large amounts of clinical diagnostic data are nowadays available from various diagnostic centers, hospitals, research centers, as well as electronic repositories accessible via the world wide web. As data availability continues to be seamless digitally, it is imperative that intelligent data-driven methods for classification and rapid detection of diseases, breast cancer inclusive, be explored (Ahn et al. Citation2023). It should be noted, however, that medical diagnosis ought to be based not only on the medical practitioner’s training but also their experience (Bolboacă Citation2019). This is a premise on which data-driven intelligent methods for diagnosis become a feasible alternative, as it is possible to aggregate both the expert knowledge and experience in the design of intelligent diagnostic systems (Arbaiy et al. Citation2017; Huang et al. Citation2023; Kattan Citation2001; Pietro Citation1985). Advancements in diagnostics notwithstanding, numerous difficulties, such as erroneous diagnosis, are still prevalent in intelligent medical diagnostic systems (Balogh, Miller, and Ball Citation2015). As the growing medical databases contain multidimensional heterogeneous data such as examination records, measurements, tests, prescriptions, etc., there is a need for more adaptive and advanced methods for extraction of meaningful information needed to achieve intelligent feature-based medical diagnosis (Ellis, Sander, and Limon Citation2022; Reyes et al. Citation2021). This work thus conducts a comprehensive comparative analysis of feature-based machine learning methods for breast cancer prediction.
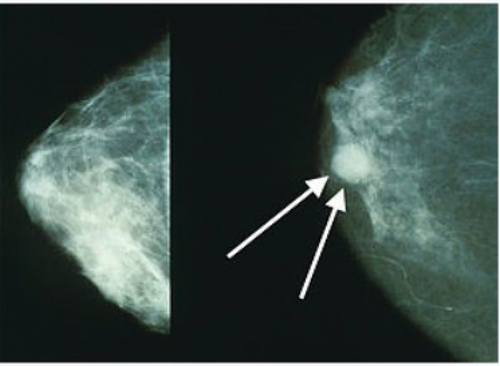
Clinically, a multitude of procedures can be used to accurately identify breast cancer, including mammography, magnetic resonance imaging (MRI), breast examination, thermography, and tissue sampling, among new emerging others (Bethesda Citation2023). These clinical procedures generate data used for training Artificial Intelligence (AI) based breast cancer diagnosis systems. The need for AI-based methods can also be justified by the inherent changes faced by these traditional detection methods (Budh and Sapra Citation2024). For instance, whereas X-rays-based methods have commonly been used to detect cancer, because the number of extracellular carcinomas is so small, it is extremely difficult to diagnose breast cancer at an early phase (Jaglan, Dass, and Duhan Citation2019). On the other hand, mammography can detect cancer in its earliest stages, with the procedure taking only a few minutes (Takkar et al. Citation2017). shows the difference between normal and malignant images.
Early discovery of malignant breast cancer can lead to better treatment and reduce fatalities in breast cancer patients. This study explores various machine learning techniques for breast cancer prediction, through the extraction of significant traits (features) from complex data sets.
Using PCA (Principal Component Analysis) and hybrid machine learning classifiers, this study aims to reduce the dataset’s size by focusing on the most important aspects for accurate diagnosis. This study aims to detect breast cancer in the early stage in order to improve survival chances of the patient. The remainder of the paper is arranged as follows: Section 2 presents a review of previous related work, while Section 3 explains the methodology proposed in the current study. Section 4 describes the validation of the proposed hybrid machine learning classifier using existing techniques on the breast cancer dataset. Section 5 concludes with the study’s findings and recommendations for further research.
Literature Review
As the quantity and scope of databases that store medical data continues to expand quickly, conventional learning approaches can no longer suffice to analyze and look for unique patterns and knowledge concealed inside this large volume of medical data. Hence, there is an increasing need for innovative tools and methods to find meaningful information from large data repositories. Previous studies have utilized various machine learning methods in the detection or forecast or classification of breast cancer (Hajiabadi et al. Citation2020; Mümine Citation2019; Serhat et al. Citation2020; Uddin et al. Citation2023; Vikas, Pal, and Tiwari Citation2018; Vivek et al. Citation2020). By eliminating the less useful factors in detection, classification, and forecasting methods (Arunadevi and Ganeshamoorthi Citation2019), PCA reduces the number of unrelated parameters into a collection of principal variables (Amin, Kia Chiam, and Dewi Varathan Citation2019). Variable selection is therefore important in prediction and decision analysis, particularly when working with medical information. Rather than predicting cancer patient survival using all dataset’s features, which can result in complex and unintelligible outcomes and representations, parameter selection is crucial to creating a better prediction model that only uses an integral parameter set (Amrane et al. Citation2018; Dana and Shubair Citation2016; Ganggayah et al. Citation2019b).
To identify breast cancer, popular classifiers like random forest (RF), support vector machines (SVM) and Decision Tree (DT) have been commonly employed (Dana and Shubair Citation2016; Mümine Citation2019; Uddin et al. Citation2023). Additionally, dimensionality reduction using PCA and other methods has also been previously used for selecting salient features (Khandezamin, Naderan, and Javad Rashti Citation2020). In a previous study (Khandezamin, Naderan, and Javad Rashti Citation2020), researchers developed a system that used SVM, distinguishing between benign and malignant tumors with excellent classification accuracy and little computing effort. The authors utilized hybridized PCA (principal component analysis) and a variety of classifiers on several datasets of breast cancer that obtained great performance. Similarly, to detect breast cancer, the author in presented a method built on memetic Pareto artificial neural networks (Muhammad et al. Citation2019).
Artificial Neural Networks (ANN) are a computing method created to simulate how the nervous system executes a function using widely dispersed multiprocessing of the different basic nodes. Such components, also known as nodal or nerve cells for computing, are characterized neurologically because they retain facts or develop capabilities and make them accessible to the user by modifying values. Several experts suggested and invented various ways to use ANN to detect and treat cancer in the breast due to the efficient measurement that it provided and then for their good precision toward an accurate diagnosis of disease (Jalal Abdullah, Mohammed Hasan, and Waleed Citation2019). By their fundamental design, neural networks are particularly adept at processing intricate datasets, such as those from breast cancer research, in which the input data and goal predictions interact unpredictably (Uddin et al. Citation2023).
Support Vector Machine (SVM) is a supervised training technique for developing regression and classification principles using information. It is a supervised machine learning system that performs well in pattern recognition applications. If there are numerous characteristics and samples, SVM performs well in classification tasks. The SVM algorithm creates a binary classifier. The SVM method has frequently been utilized for prediction and classification of breast cancer (Akay Citation2009; Huang et al. Citation2017). The common application use-case is for the SVM classifier to identify malignant growths as part of the cancer diagnosis process, particularly of the breast in females (Akay Citation2009; Huang et al. Citation2017; Jalal Abdullah, Mohammed Hasan, and Waleed Citation2019).
Random Forest (RF) algorithm (RFA) has been described as a generic rule for randomized predictors (Tomislav et al. Citation2018). The binary tree is typically divided into identical vertices by iteratively updating to create an RF. The information propagation from the root node increases the success or node’s resemblance to the parent node. The source information is gathered using bootstrapping sample size to produce many trees for growing RF. Regarding collecting predictive variables as feed to some trees, every branch inside the RF leads to activation. The dataset’s independent variables can be effectively handled by RF (Dana and Shubair Citation2016; Uddin et al. Citation2023). A group of various separate randomized decision trees collaborate to form RF. These trees are produced by bootstrapping given information. Any individual tree inside the random forest throws one value based on the set of predictor values entered. The category that receives the most results determines the model’s forecast in categorizing a training set. RF is produced by repeatedly splitting the tree structure into similar nodes. Through transmission, the tree’s root influences the child node’s resemblance.
Decision Tree (DT), a non-linear supervised algorithm, could be applied to classification and regression problems and is often employed in health care (Elhazmi et al. Citation2022). The root node of a Decision Tree (DT) architecture is one of the multiple vertices. All vertices are connected by edges, except for the root, and each node contains a single initial. Certain vertices, known as internal nodes or sample vertices, provide one or even more external connections, but others do not. Vertices, often known as exit or endpoint, are all in this category. Every intermediate node in a decision tree is responsible for dividing the example space into two or more subsets, and it does so by a specific discontinuity function determined by the input similarity measure. Many diseases, like cancer, diabetes, and heart disease, have previously been diagnosed using DT (Fatin Kadhim Citation2022). DT is a robust and accurate training algorithm that may address classification and regression issues. That employs the highest, tree-based advancement technique. A layered division approach is used to split the data among several groups at every stage to ensure that the information within every category is similar. Each internal network of the DT ties to a trial parameter, ties to something like the results obtained at each route, and ties to a distinct category for every sub-tree. A tree can grow from either parent node by choosing an “optimal feature” or “perfect feature” from the available characteristics utilizing unpredictability or knowledge to obtain measurements before performing “dividing”.
David (Omondiagbe, Veeramani, and Sidhu Citation2019) proposed breast cancer diagnosis based on feature selection and an SVM Classifier. Haji Abadi et al (Hajiabadi et al. Citation2020) suggested that linear discrimination analysis was applied using a typical attitude to minimize the quantity of features, before ANN applying three loss functions (i.e. the current cup, a hinge, and cross-entropy) was trained to evaluate the dataset at different volume levels. SVM, Logistic regression, and KNN classifiers have equally been recommended for the analysis of breast cancer (Omondiagbe, Veeramani, and Sidhu Citation2019; Rabiei et al. Citation2022). SVM was found to be one of the best classifiers. Breast cancer’s prognostic qualities have not changed at all. Mogana Darshini Ganggayah et al. (Citation2019a) investigated using supervised machine-learning algorithms to identify breast cancer, making use of breast cancer datasets from the UCI repository (UCI Citation2019). On their dataset, they used Logistic Regression, SVM, and KNN. In addition, their efficiency was estimated and compared. They determined that SVM was the best predictive analytic technique, with a 92.7% accuracy.
More recently, away from classical machine learning, application of deep learning methods in the diagnosis of cancer and other human-related health illnesses has gained increasing popularity (Anari et al. Citation2022). Recent literature indicates that Convolutional Neural Networks (CNN) are particularly better fitted model for cancer diagnosis tasks compared to other deep learning models (Kasgari et al. Citation2023; Nazanin et al. Citation2022; Ramin et al. Citation2023). CNN has been successful for breast tumor segmentation and detection using mammograms (Ramin et al. Citation2023). Brain tumor localization and segmentation from magnetic resonance imaging (MRI) are difficult and crucial challenges for a variety of medical analysis applications (Nazanin et al. Citation2022)
summarizes the literature reviewed, taking into account; datasets utilized, number of classification classes, methods and techniques used, as well as the accuracy or outcome obtained from the classification/prediction/forecast task.
Table 2. Summary of related literature reviewed.
Materials And Methods
Description of Dataset
The breast cancer database is taken from the UCI repository (UCI Citation2019). A total of 699 tumor cases in the database were reduced to 659 after data preprocessing. Benign tumor cases are 458 (65.5%), and malignant cases are 241 (34.5%). The nine dataset attributes are presented in , excluding code number and category level. Each feature was measured in the domain range from 1 to 10, where value 1 represents benign and 10 represents malignant cases.
Table 3. Dataset attributes.
In the current research work, instances are classified into two types: benign as positive and malignant patients as negative. Linear correlation describes straight-line correlations in the range 1 to +1 for two variables, where 1 represents the ideal negative association and +1 represents the perfect positive relationship. The Pearson correlation between positive and negative classes is established by determining the link between nine features of benign and malignant classes.
Data Pre-Processing
Data pre-processing replaces missing values, identifies and eliminates external factors, and resolves subjective discrepancies. For example, the code number form has been deducted from the database because it does not affect diseases. As a result, the database has 16 missing value values. Medium replaces attributes that do not exist for that class. In addition, the database uses random selection to ensure proper data rotation.
Correlation Function in Feature Selection
The proposed model deals with 569 breast cancer samples with 32 attributes, of which only 11 are isolated. The features are designated using correlation-based feature selection (CFS) based on PCA-based trait assessment. This attribute removes duplicate or inappropriate features in the validation dataset. PCA also solves the redundant matching problem by removing additional variables or alternatives that combine two or more variables. The scale from 0 to 1 identifies the variables that need not be removed. It improves the model’s effectiveness and precision. The research examined 32 attributes, of which 11 were considered key attributes are mentioned in .
Table 4. Selected features from the breast cancer dataset.
The extraction of 11 attributes was achieved using the WEKA tool’s multifactor method. Training and test data are in the data set, with the covariate control to be performed as shown in the equation. (1) Principal component analysis (PCA) is used for statistical analysis and effective pre-treatment to assess the scattering plot of breast cancer cells and analyze their characteristics. The process is performed using the call function training and testing data sets.
Here, t is the test data, E is the anticipated sample, and T is the breast cancer data set. To identify which qualities are appropriate, it compares training and testing occurrences. Two data sets are correlated linearly, and Pearson’s correlation coefficient is calculated using the covariance between the two models multiplied by the constant variance of each sample of genetic data. This process of normalization is denoted by Eq. (2).
HYBRID Hopfield Neural Network-E2SAT MODELS for Classification
For achieving the correct synaptic weight in HNN-E2SAT models, HNN-E2SATGA and HNN-E2SATACO (Kasihmuddin, Sathasivam, and Asyraf Mansor Citation2017) are used in the learning phase and each neuron will receive a consistent interpretation, with minimal clausal inconsistencies used to describe the cost function for the HNNE2SAT model (Hikmatul et al. Citation2020). HNN – the following algorithms replace HNN-E2SATGA, E2SATES, and HNN-E2SATACO (Hikmatul et al. Citation2020). The satisfiability problem is explained in detail with relevant examples in (Ali et al. Citation2017; Barrett, Dill, and Stump Citation2002; Sathasivam, Tajuddin, and Abdullah Citation2011; Velev Citation2004).
Algorithm of the Hybrid Hopfield Neural Network-E2SAT Model
Translate E2 Satisfiability clauses into the Boolean calculation
Assign neurons in E2 Satisfiability clauses to each variable
Initialize to zero any synaptic weight (Hikmatul Fadhilah Sianipar et al. 2020).
Check the E2 Satisfiability logic’s inconsistency.
Derive E2 Satisfiability Cost Function by assigning.
The neuron states that it is true when Sx = 1 and false when Sx = −1
Derive all cost functions of E2 Satisfiability clauses on behalf of multiplication (Hikmatul et al. Citation2020).
(6) Check clauses on gratification through the use of EA, GA, and ACO (Hikmatul Fadhilah Sianipar et al. 2020).
(7) Obtained synaptic weight relates to the E2 Satisfiability logic.
(8) Calculate equation H to calculate the prediction of breast cancer.
(8) Apply Equation
To find the neuron state in manipulation of the corresponding local field.
(9) Final Energy inspection means a prediction of breast cancer.
Experiment Results And Discussion
We individually used five machine learning methods: RF, DT, SVM, and ANN, to predict whether a cell is harmful or normal (i.e. benign or malignant). Processing was carried out using an Intel Core i7 with 32GB of RAM, using Python and its associated open-source libraries, in Jupiter Notebook.
In this particular way, taxonomic analysis is based on the exact traits that define the benign and fatal categories of tumor cases. In the early stages, symptoms are divided into seven stages and nine bad aspects of breast cancer. Principal Component Analysis (PCA) is used for dimensionality reduction, and Classification techniques are used to anticipate breast cancer cases. The results of the experiments demonstrate that the suggested model properly classified 521 of the 569 occurrences while wrongly classifying 48 of them.
Performance Measure Parameters
A few performance parameters can be used to gauge how well machine learning approaches perform. Four types of confusion matrixes are used to evaluate the parameters: one for actual data and one for predicted data. The following is a breakdown of the meanings of the terms:
True Positive = TP
False Positive = FP
True Negative = TN
False Negative = FN
Our study employs the following parameters extensively to evaluate some phrases using their related formulae to measure the study’s performance. The following formulae are used to gauge the effectiveness of the different classification methods in this current comparative study:
Accuracy (AC) is the ratio of properly classified samples from total samples:
Sensitivity (SE): Recall is another term for sensitivity. Percent of all positive cases that are considered to be “perceived” as positive:
Specificity (SP): The rate at which breast cancer is projected to be present in all examples is known as specificity, defined as the correlation between any one set of observed negative criteria and any other group of observed negative examples.
Precision (P):
Negative predictive value (NPV): The percentage of negative situations that remain true negatives is called NPV:
F1 score: Harmonic mean of precision and sensitivity is distinct as F1 score:
provides the prediction outcome of techniques, respectively.
Performance Analysis of Proposed Model without Feature Selection
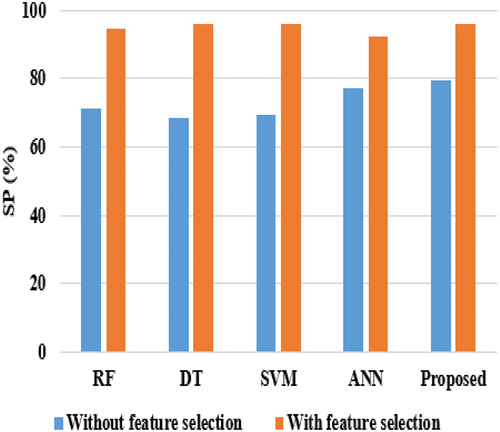
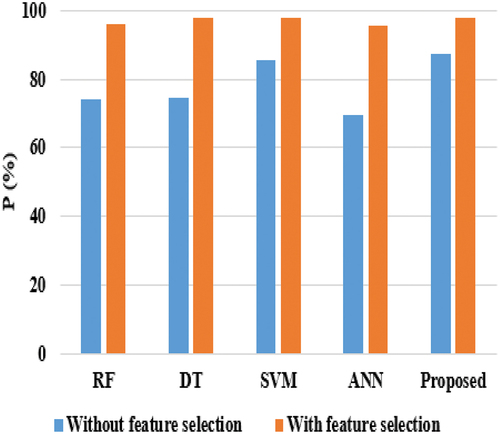
Here, two types of analyses (i.e., with feature and without feature selection) are carried out to test the efficiency of the suggested model with functioning techniques. provides the validation analysis of all methods without feature selection in terms of Precision, Sensitivity, and Specificity. show the graphical comparison of techniques based on Specificity (SP) and Precision (P).
Table 5. LR-confusion matrix for ten-fold cross-validation.
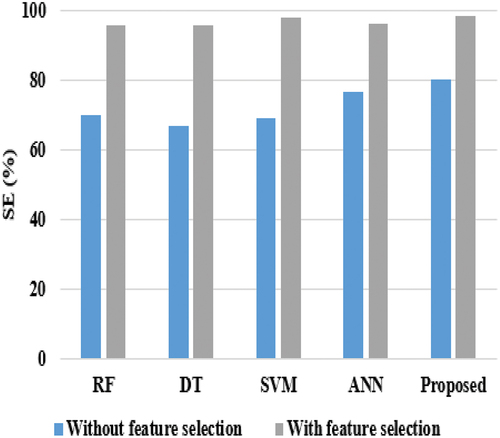
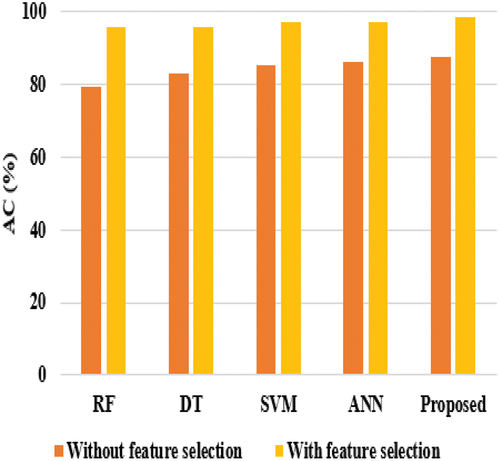
Considering precision, RF, and DT achieved nearly 74%, ANN reached 69.33%, SVM achieved 85.52%, and the proposed model achieved 87%. DT and SVM techniques achieved nearly 67% on sensitivity, while RF & ANN attained 70% and 76%, respectively, on sensitivity, and the proposed model achieved the highest sensitivity score of 80.21%. The proposed model’s better performance is that the HNN-E2SAT uses ACO and GA for gaining the synaptic weight, where DT is unstable, i.e., the structure of the optimal tree is highly affected by small changes in the training data. Hence, it shows low performance. In the analysis of specificity, DT and SVM achieved nearly 69%, RF gained 71.33%, ANN reached 77.27%, and the proposed model achieved 79.27%. shows the experimental evaluation of the suggested model with existing techniques in terms of AC and F1-score.
Table 6. Analysis of the model without feature selection.
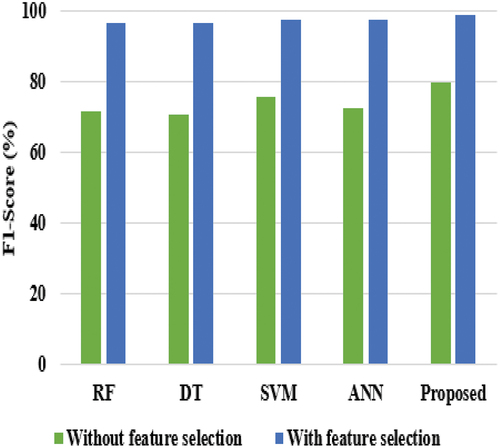
In the analysis of the F1-score, the SVM, ANN, and RF achieved nearly 71% to 74%, DT reached 70.85%, and the proposed model achieved only 79.88%. The reason for the poor results of the proposed model is that it is tested without feature selection, and all attributes are considered. However, the proposed model achieved better performance than existing techniques. While analyzing the experiments on AC, the RF achieved low performance, i.e., 79.20%, DT, SVM, and ANN achieved nearly 84% to 86% of AC, and the proposed model achieved 87.45% of AC. The RF requires more sophisticated techniques for high classification accuracy, which is usually inferior to gradient-boosted trees. The comparative graphical representation of all classifiers in terms of SE is shown in .
Performance Analysis of Proposed Model with Feature Selection
All the ML techniques, including the proposed model, are tested with feature selection, and the results are considered in .
Table 7. Comparison of the proposed model without feature selection.
When comparing and , the performance of all classifiers achieved better performance, as shown in . This is achieved because the feature selection technique plays a major role and uses only 11 attributes for breast cancer classification. In the analysis of sensitivity, RF and DT achieved nearly 95%, ANN and SVM achieved almost 97%, and the proposed model achieved 98.27%. When considering specificity, DT and SVM gained nearly 95%, ANN earned 92%, RF achieved 94%, and the proposed model reached 96%. When compared with all techniques, ANN and RF achieved nearly 95% at precision, SVM and DT achieved nearly 97%, while the proposed model achieved 98.02%. Even though SVM achieved better performance than other existing techniques, it equally performs lower than the proposed model. The reason is that SVM does not perform well when the data is large and contains more noise, i.e., overlapping target classes. provides the comparative results of the proposed model in terms of Accuracy and F1-score. and provide the comparison results of the proposed classifier in terms of AC and F1-score.
Table 8. Performance evaluation of various classification techniques with feature selection.
On accuracy comparison, the proposed model achieved 98.57%, RF and DT achieved nearly 95%, while SVM and ANN achieved almost 97% as per . It shows that feature selection plays a major role in refining the performance of the all-classifier technique in diagnosing breast cancer. In the analysis of the F1-score, the SVM and ANN achieved nearly 97%, RF and DT reached almost 96%, and the proposed model achieved 98.90% as per .
Table 9. Evaluation of the proposed model with feature selection.
While comparing with previous related studies utilizing feature-based machine learning methods (Dana and Shubair Citation2016; Omondiagbe, Veeramani, and Sidhu Citation2019), the results of the current study emphasize the fact that optimizing input data through feature-selection enhances the performance of classification and prediction models of breast cancer. Additionally, the proposed HNN-E2SAT algorithm outperforms several classical machine learning methods with and without feature selection as demonstrated in the current study as well as in previous related studies (Akay Citation2009; Ganggayah et al. Citation2019b; Huang et al. Citation2017; Muhammad et al. Citation2019). Moreover, future work could further explore hyperparameter optimization in breast cancer diagnostic systems as experimented in (Ogundokun et al. Citation2022) in order to further achieve real-time breast cancer diagnosis, cloud computing platforms could be leveraged as explored in (Lahoura et al. Citation2021).
Conclusion And Future Scope Of Work
Diagnosis methods in the medical industry are both expensive and time-consuming. Machine-learning approaches can be used as a clinical aid to detect breast cancer, especially early on in order to increase survival chances. This can be particularly helpful for new physicians and medical practitioners as misdiagnosis is quite common in the absence of highly experienced personnel. The main goal of this research was to explore feature-based machine learning techniques for early detection of breast cancer, especially in its early stages. Salient feature selection was performed on the basis of correlation coefficients. The attribute evaluator then used a PCA-based ranking algorithm to determine relevant characteristics, with the top-ranking attributes being selected for use in breast cancer categorization. The suggested classifier was used to improve the accuracy of the prediction approach. Out of 569 cases, 559 were accurately categorized. Overall, the suggested model proved effective in identifying benign and malignant breast cancer class labels, as confirmed by statistical analysis of all comparative methodologies. The proposed model (HNN-E2SAT) achieved an accuracy of 98.57% and a precision of 98.02%, whereas the SVM approach achieved a precision of 97.82% and an accuracy of 97.14%. However, this study is limited to a single dataset. In the future, it is recommended that extension of this study involves conducting experiments with larger datasets and combining deep learning methods to further optimize breast cancer prediction and classification methods. Moreover, extended experiments to determine the robustness and interpretability of the proposed HNN-E2SAT model ought to be conducted, for the model to be deemed generalizable beyond the present evaluation scope.
Acknowledgement
Our sincere thanks to VIT Bhopal University, Bhopal (MP) for providing infrastructure, lab facilities and resources to pursue this research work.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
References
- Aavula, R., and R. Bhramaramba. 2019. XBPF: An extensible breast cancer prognosis framework for predicting susceptibility, recurrence and survivability. International Journal of Engineering and Advanced Technology 8(5):159–23. https://www.ijeat.org/portfolio-item/C5808028319/.
- Abdar, M., M. Zomorodi-Moghadam, X. Zhou, R. Gururajan, X. Tao, P. D. Barua, and R. Gururajan. 2020. A new nested ensemble technique for automated diagnosis of breast cancer. Pattern recognition letters 132 (April):123–31. doi:10.1016/j.patrec.2018.11.004.
- Ahn, J. S., S. Shin, S.-A. Yang, E. Kyung Park, K. Hwan Kim, S. Ick Cho, C.-Y. Ock, and S. Kim. 2023. Artificial intelligence in breast cancer diagnosis and personalized medicine. Journal of Breast Cancer 26 (5):405–35. doi:10.4048/jbc.2023.26.e45.
- Akay, M. F. 2009. Support vector machines combined with feature selection for breast cancer diagnosis. Expert Systems with Applications 36 (2, Part 2):3240–47. doi:10.1016/j.eswa.2008.01.009.
- Ali, M., R. Ali, M. Ali, and R. Ali. 2017. Satisfiability in big boolean algebras via boolean-equation solving. Journal of King Abdulaziz University Engineering Sciences 28 (1):3–18. doi:10.4197/Eng.28-1.1.
- Ali, H., K. Shaban, A. Erradi, A. Mohamed, S. Khan Rumi, and F. D. Salim. 2021. ‘Spatiotemporal data mining: A survey on challenges and open problems’. Artificial Intelligence Review 55 (2):1441–88. https://link.springer.com/article/10.1007/s10462-021-09994-y.
- Amin, M. S., Y. Kia Chiam, and K. Dewi Varathan. 2019. Identification of significant features and data mining techniques in predicting heart disease. Telematics and Informatics 36 (March):82–93. doi:10.1016/j.tele.2018.11.007.
- Amrane, M., S. Oukid, I. Gagaoua, and T. Ensari. 2018. Breast cancer classification using machine learning. In 2018 Electric Electronics, Computer Science, Biomedical Engineerings’ Meeting (EBBT), 1–4. Istanbul, IEEE. doi:10.1109/EBBT.2018.8391453.
- Anand, P., A. B. Kunnumakara, C. Sundaram, K. B. Harikumar, S. T. Tharakan, O. S. Lai, B. Sung, and B. B. Aggarwal. 2008. Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical Research 25 (9):2097–116. doi:10.1007/s11095-008-9661-9.
- Anari, S., T. S. Nazanin, M. Negin, D. Shadi, and R. Amirali. 2022. Review of deep learning approaches for thyroid cancer diagnosis. In Mathematical Problems in Engineering, ed. A. Darba, (August): 1–8. doi:10.1155/2022/5052435.
- Arbaiy, N., S. Eliza Sulaiman, N. Hassan, and Z. Afizah Afip. 2017. Integrated knowledge based expert system for disease diagnosis system. In IOP Conference Series: Materials Science and Engineering 226 (August):012097. doi:10.1088/1757-899X/226/1/012097.
- Arunadevi, J., and K. Ganeshamoorthi. 2019. Feature selection facilitated classification for breast cancer prediction. In 2019 3rd International Conference on Computing Methodologies and Communication (ICCMC), 560–63. doi:10.1109/ICCMC.2019.8819752.
- Ataollahi, M. R., J. Sharifi, M. R. Paknahad, and A. Paknahad. 2015. Breast cancer and associated factors: A review. Journal of Medicine and Life 8 (Spec Iss 4):6–11.
- Balogh, E. P., B. T. Miller, and J. R. Ball. 2015. Committee on diagnostic error in health care, board on health care services, institute of medicine, and engineering the national academies of sciences. In Improving Diagnosis in Health Care, National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK338594/. ‘Overview of Diagnostic Error in Health Care’
- Barrett, C. W., D. L. Dill, and A. Stump. 2002. Checking satisfiability of first-order formulas by incremental translation to SAT. In Computer Aided Verification, ed. E. Brinksma and K. G. Larsen, 236–49. US: Springer. doi:10.1007/3-540-45657-0_18.
- Bethesda. 2023. ‘PDQ Breast Cancer Screening’. pdqCancerinfosummary. Nciglobal,ncienterprise. 24 November 2023. https://www.cancer.gov/types/breast/patient/breast-screening-pdq.
- Bolboacă, S. D. 2019. Medical diagnostic tests: A review of test anatomy, phases, and statistical treatment of data. Computational and Mathematical Methods in Medicine 2019 (May):1891569. doi:10.1155/2019/1891569.
- Budh, D. P., and A. Sapra. 2024. Breast cancer screening. In StatPearls, US: StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK556050/.
- CDC. 2023. Breast and Ovarian Cancers and Family Health History | CDC. Accessed October 11, 2023. https://www.cdc.gov/genomics/disease/breast_ovarian_cancer/breast_ovarian_cancer.htm.
- Dana, B., and R. Shubair. 2016. Comparative study of machine learning algorithms for breast cancer detection and diagnosis. 2016. https://ieeexplore.ieee.org/document/7818560.
- Elhazmi, A., A. Al-Omari, H. Sallam, H. N. Mufti, A. A. Rabie, M. Alshahrani, A. Mady, A. Alghamdi, A. Altalaq, M. H. Azzam, et al. 2022. Machine learning decision tree algorithm role for predicting mortality in critically Ill adult COVID-19 patients admitted to the ICU. Journal of Infection and Public Health 15(7):826–34. doi:10.1016/j.jiph.2022.06.008.
- Ellis, R. J., R. M. Sander, and A. Limon. 2022. Twelve key challenges in medical machine learning and solutions. Intelligence-Based Medicine 6 (January):100068. doi:10.1016/j.ibmed.2022.100068.
- Embi, P. J., and P. R. O. Payne. 2009. Clinical research informatics: challenges, opportunities and definition for an emerging domain. Journal of the American Medical Informatics Association 16 (3):316–27. doi:10.1197/jamia.M3005.
- Fatin Kadhim, N. 2022. ‘Breast cancer detection using decision tree and k-nearest neighbour classifiers’. 2022. https://ijs.uobaghdad.edu.iq/index.php/eijs/article/view/5386.
- Ganggayah, M. D., N. Aishah Taib, Y. Cheng Har, P. Lio, and S. Kaur Dhillon. 2019a. Predicting factors for survival of breast cancer patients using machine learning techniques. BMC Medical Informatics & Decision Making 19 (1):48. doi:10.1186/s12911-019-0801-4.
- Ganggayah, M. D., N. Aishah Taib, Y. Cheng Har, P. Lio, and S. Kaur Dhillon. 2019b. Predicting factors for survival of breast cancer patients using machine learning techniques. BMC Medical Informatics & Decision Making 19 (1):48. doi:10.1186/s12911-019-0801-4.
- Gopal, V. N., F. Al-Turjman, R. Kumar, L. Anand, and M. Rajesh. 2021. Feature selection and classification in breast cancer prediction using IoT and machine learning. Measurement 178 (June):109442. doi:10.1016/j.measurement.2021.109442.
- Hajiabadi, H., V. Babaiyan, D. Zabihzadeh, and M. Hajiabadi. 2020. Combination of loss functions for robust breast cancer prediction. Computers & Electrical Engineering 84 (June):106624. doi:10.1016/j.compeleceng.2020.106624.
- Hartama, D., A. Perdana Windarto, and A. Wanto. 2019. The application of data mining in determining patterns of interest of high school graduates. Journal of Physics: Conference Series 1339 (1):012042. doi:10.1088/1742-6596/1339/1/012042.
- Hikmatul, F. S., N. Nazifah Yu Zaini, M. Shareduwan, M. Kasihmuddin, M. A. Mansor, and S. Sathasivam. 2020. Hybrid ant colony optimization for even-2 Satisfiability Programming in Hopfield Neural Network | AIP Conference Proceedings, https://pubs.aip.org/aip/acp/article/2266/1/040014/1007336/Hybrid-ant-colony-optimization-for-even-2.
- Huang, S., C. NIanguang, P. P. Pacheco, S. Narandes, Y. Wang, and X. Wayne. 2017. Applications of Support Vector Machine (SVM) learning in cancer genomics. Cancer Genomics & Proteomics 15 (1):41–51. doi:10.21873/cgp.20063.
- Huang, X., X. Tang, W. Zhang, S. Pei, J. Zhang, M. Zhang, Z. Liu, R. Chen, and Y. Huang. 2023. A generic knowledge based medical diagnosis expert system. arXiv. doi:10.48550/arXiv.2110.04439.
- Jaglan, P., R. Dass, and M. Duhan. 2019. Breast Cancer Detection Techniques: Issues and Challenges. Journal of the Institution of Engineers (India): Series B 100 (March):379–86. doi:10.1007/s40031-019-00391-2.
- Jalal Abdullah, A., T. Mohammed Hasan, and J. Waleed. 2019. An expanded vision of breast cancer diagnosis approaches based on machine learning techniques. https://ieeexplore.ieee.org/document/8950530.
- Kasgari, A. B., S. Safavi, M. Nouri, J. Hou, N. Tataei Sarshar, and R. Ranjbarzadeh. 2023. Point-of-interest preference model using an attention mechanism in a convolutional neural network. Bioengineering 10 (4):495. doi:10.3390/bioengineering10040495.
- Kasihmuddin, M. S. M., S. Sathasivam, and M. Asyraf Mansor. 2017. Hybrid genetic algorithm in the hopfield network for maximum 2-satisfiability problem’ 1870. (August):050001. doi:10.1063/1.4995911.
- Kattan, M. W. 2001. Expert systems in medicine. In International Encyclopedia of the social & behavioral sciences, ed. J. S. Neil and B. B. Paul, 5135–39. US: Pergamon. doi:10.1016/B0-08-043076-7/00556-8.
- Khan, N., F. Afaq, and H. Mukhtar. 2010. Lifestyle as risk factor for cancer: evidence from human studies. Cancer Letters 293 (2):133–43. doi:10.1016/j.canlet.2009.12.013.
- Khandezamin, Z., M. Naderan, and M. Javad Rashti. 2020. Detection and classification of breast cancer using logistic regression feature selection and GMDH Classifier. Journal of Biomedical Informatics 111 (November):103591. doi:10.1016/j.jbi.2020.103591.
- Lahoura, V., H. Singh, A. Aggarwal, B. Sharma, M. Abed Mohammed, R. Damaševičius, S. Kadry, and K. Cengiz. 2021. Cloud computing-based framework for breast cancer diagnosis using extreme learning machine. Diagnostics (Basel, Switzerland) 11 (2):241. doi:10.3390/diagnostics11020241.
- Mughal, M. J. H. 2018. Data mining: Web data mining techniques, tools and algorithms: An overview. International Journal of Advanced Computer Science and Applications (IJACSA) 9 (6). doi:10.14569/IJACSA.2018.090630.
- Muhammad, H. M., J. Ping Li, A. Ul Haq, M. Hunain Memon, and W. Zhou. 2019. Breast cancer detection in the iot health environment using modified recursive feature selection’. https://www.hindawi.com/journals/wcmc/2019/5176705/.
- Mümine, K. K. 2019. Breast cancer prediction and detection using data mining classification algorithms: A comparative study. Tehnicki Vjesnik – Technical Gazette 26:1. doi:10.17559/TV-20180417102943.
- National Council Institute. 2015. Risk factors: Hormones – NCI. cgvArticle. Nciglobal,ncienterprise. Accessed April 29, 2015. https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones.
- Nazanin, T. S., R. Ranjbarzadeh, S. Jafarzadeh Ghoushchi, G. G. de Oliveira, S. Anari, M. Parhizkar, and M. Bendechache. 2022. ‘Glioma brain tumor segmentation in four MRI modalities using a convolutional neural network and based on a transfer learning method’. 2022. https://link.springer.com/chapter/10.1007/978-3-031-04435-9_39.
- Ogundokun, R. O., S. Misra, M. Douglas, R. Damaševičius, and R. Maskeliūnas. 2022. Medical Internet-of-things based breast cancer diagnosis using hyperparameter-optimized neural networks. Future Internet 14 (5):153. doi:10.3390/fi14050153.
- Omondiagbe, D. A., S. Veeramani, and A. S. Sidhu. 2019. Machine learning classification techniques for breast cancer diagnosis. In IOP Conference Series: Materials Science and Engineering 495 (June):012033. doi:10.1088/1757-899X/495/1/012033.
- Parsa, N. 2012. Environmental factors inducing human cancers. Iranian Journal of Public Health 41 (11):1–9. doi:10.1186/1479-5876-4-14.
- Petrucelli, N., B. D. Mary, and P. Tuya 1993. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In GeneReviews®, ed. P. Margaret. J. F. Adam, G. M. Mirzaa, R. A. Pagon, S. E. Wallace, L. J. Bean, K. W. Gripp, and A. Amemiya, US: University of Washington, Seattle. http://www.ncbi.nlm.nih.gov/books/NBK1247/.
- Pietro, T. 1985. Knowledge based expert systems for medical diagnosis. Statistics in Medicine 4 (3):317–25. doi:10.1002/sim.4780040311.
- Rabiei, R., S. Mohammad Ayyoubzadeh, S. Sohrabei, M. Esmaeili, and A. Atashi. 2022. Prediction of breast cancer using machine learning approaches. Journal of Biomedical Physics & Engineering 12 (3):297–308. doi:10.31661/jbpe.v0i0.2109-1403.
- Ramin, R., S. Jafarzadeh Ghoushchi, N. Tataei Sarshar, E. Babaee Tirkolaee, S. Samar Ali, T. Kumar, and M. Bendechache. 2023. ‘ME-CCNN: Multi-encoded images and a cascade convolutional neural network for breast tumor segmentation and recognition’. Artificial Intelligence Review 56 (9):10099–136. https://link.springer.com/article/10.1007/s10462-023-10426-2.
- Reyes, L. T., J. Klöckner Knorst, F. Ruffo Ortiz, and T. Machado Ardenghi. 2021. Scope and challenges of machine learning-based diagnosis and prognosis in clinical dentistry: A literature review. Journal of Clinical and Translational Research 7 (4):523–39.
- Sathasivam, S., W. A. Tajuddin, and W. Abdullah. 2011. Logic mining in neural network: Reverse analysis method. Computing 91 (2):119–33. doi:10.1007/s00607-010-0117-9.
- Serhat, S., U. Kursuncu, E. Kibis, M. Anis Abdellatif, and A. Dag. 2020. ‘A hybrid data mining approach for identifying the temporal effects of variables associated with breast cancer survival’. Expert Systems with Applications 139:112863. https://www.sciencedirect.com/science/article/abs/pii/S0957417419305731.
- Shadi, A., A. Anguera, J. William Atwood, J. A. Lara, and D. Lizcano. 2019. Particularities of data mining in medicine: lessons learned from patient medical time series data analysis. EURASIP Journal on Wireless Communications and Networking 2019 (1):260. doi:10.1186/s13638-019-1582-2.
- Shravya, C., K. Pravalika, and S. Subhani. 2019. Prediction of breast cancer using supervised machine learning techniques. International Journal of Innovative Technology and Exploring Engineering 8(6):1106–1110. https://www.ijitee.org/portfolio-item/F3384048619/.
- Sohail, M. N., R. Jiadong, M. Musa Uba, and M. Irshad. 2019. A comprehensive looks at data mining techniques contributing to medical data growth: a survey of researcher reviews. In Recent developments in intelligent computing, communication and devices, ed. S. Patnaik and V. Jain, 21–26. US: Springer. doi:10.1007/978-981-10-8944-2_3.
- Takkar, N., S. Kochhar, P. Garg, A. K. Pandey, U. R. Dalal, and U. Handa. 2017. Screening methods (clinical breast examination and mammography) to detect breast cancer in women aged 40–49 years. Journal of Mid-Life Health 8 (1):2–10. doi:10.4103/jmh.JMH_26_16.
- Tan, X., A. T. Su, H. Hajiabadi, M. Tran, and Q. Nguyen. 2021. Applying machine learning for integration of multi-modal genomics data and imaging data to quantify heterogeneity in tumour tissues. In Artificial neural networks, ed. H. Cartwright, 209–28. US: Springer US. doi:10.1007/978-1-0716-0826-5_10.
- Tomislav, H., M. Nussbaum, M. N. Wright, G. B. M. Heuvelink, and B. Gräler. 2018. ‘Random forest as a generic framework for predictive modeling of spatial and spatio-temporal variables’. 2018. https://pubmed.ncbi.nlm.nih.gov/30186691/.
- UCI. 2019. ‘UCI machine learning repository’. https://archive.ics.uci.edu/.
- Uddin, K., M. Mohi, N. Biswas, S. Tasmin Rikta, and S. Kumar Dey. 2023. Machine learning-based diagnosis of breast cancer utilizing feature optimization technique. Computer Methods and Programs in Biomedicine Update 3 (January):100098. doi:10.1016/j.cmpbup.2023.100098.
- Velev, M. N. 2004. Efficient translation of boolean formulas to cnf in formal verification of microprocessors. In Proceedings of the 2004 Asia and South Pacific Design Automation Conference, 310–15. ASP–DAC ’04. Yokohama, Japan, IEEE Press.
- Vikas, C., S. Pal, and B. B. Tiwari. 2018. ‘Prediction of benign and malignant breast cancer using data mining techniques’. SSRN Electronic Journal 2018. https://journals.sagepub.com/doi/10.1177/1748301818756225.
- Vivek, K., B. Kishore Mishra, M. Mazzara, D. N. H. Thanh, and A. Verma. 2020. ‘Prediction of malignant and benign breast cancer: A data mining approach in healthcare applications’. 2020. https://link.springer.com/chapter/10.1007/978-981-15-0978-0_43.
- Wang, S., J. Cao, and P. S. Yu. 2022. ‘Deep learning for spatio-temporal data mining: a survey |. IEEE Journals & Magazine | IEEE Xplore’ 2022. https://ieeexplore.ieee.org/document/9204396.
- William, W., and O. Mangasarian. 1993. ‘Breast cancer Wisconsin (Diagnostic)’. [ object Object]. doi:10.24432/C5DW2B.
- Yang, J., Y. Li, Q. Liu, L. Li, A. Feng, T. Wang, S. Zheng, A. Xu, and J. Lyu. 2020. Brief introduction of medical database and data mining technology in big data era. Journal of Evidence-Based Medicine 13 (1):57–69. doi:10.1111/jebm.12373.
- Yusupova, N., O. Smetanina, A. Agadullina, and E. Sazonova. 2020. Knowledge Identification by structured data for decision making in project teams 385–90. Atlantis Press. doi:10.2991/aisr.k.201029.072.
- Zhang, Y.-B., X.-F. Pan, J. Chen, A. Cao, Y.-G. Zhang, L. Xia, J. Wang, H. Li, G. Liu, and A. Pan. 2020. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies |. British Journal of Cancer’ 2020. https://www.nature.com/articles/s41416-020-0741-x.