Abstract
Mycophenolate mofetil (MMF) is a potent immunosuppressive agent used in renal transplantation. Gastrointestinal and hematological side effects are commonly observed, but hepatotoxicity has not been reported. In this study, we assessed MMF-related hepatotoxicity in renal transplant recipients. A total of 124 renal transplantation recipients (RTRs) were evaluated for elevated liver enzymes associated with MMF, and 79 patients were enrolled to the study. Patients used MMF 2 g/day. The patients who had progressive increase in liver enzymes after renal transplantation and their AST, ALT, GGT, ALP, bilirubin levels, hepatitis, cytomegalovirus (CMV), abdominal ultrasonography, duration of hepatotoxicity, and decreased dosage or withdrawal of MMF were recorded. Also, we evaluated their liver enzymes while the patients were on the waiting list. Of the 79 patients, 11 patients (13.9%) had a progressive increase in liver enzymes. The median (min–max) age of the patients with MMF-hepatotoxicity was 29 (19–54) and 72.7% of them were male. None of the patients had hepatitis B or C, CMV infection, or other possible causes for elevated liver enzymes and their abdominal ultrasonography were normal. High liver enzyme levels regressed after the withdrawal (n = 6) or reduce dosage (n = 5) of MMF. The median time of the increase in liver enzymes was 28 (4–70) days and after 50% reduction or withdrawal of MMF, returned to normal values in 16 (4–210) days. The median levels of ALT in waiting list (I), before (II), and after (III) reduction dosage or withdrawal of MMF were 22.0 (3–22), 222.0 (51–508), and 33.0 (21–64) U/L, respectively (p I–II = 0.004, p I–II = 0.013, and p II–III = 0.005). There were no differences for ALP, GGT, total bilirubin, and direct bilirubin levels. Also, the correlation between recovery time of ALT and persistence time of ALT elevation before adjustment of MMF was significant (r = 0.739, p = 0.009). Consequently, after renal transplantation, hepatotoxicity can occur due to a lot of reason including MMF usage. If hepatotoxicity related to MMF is not considered, especially in the early period of renal transplantation, resolution of hepatotoxicity can be required long term.
Introduction
Mycophenolate mofetil is a potent immunosuppressive agent used in combination with calcineurin inhibitors or in renal transplantation. The mycophenolate mofetil (MMF) targets the de novo purine biosynthesis pathway by noncompetitive inhibition of inosine monophosphate dehydrogenase, acting against the proliferation of T and B lymphocytes.Citation[1] Clinical trials have shown that MMF was generally well tolerated. The most prominent adverse effects of MMF are nausea, vomiting, diarrhea, and hematological side effects.Citation[2&3] In the literature, although hepatotoxicity has not been reported, Sollinger et al. documented elevated liver enzymes in several (n = 5) patients receiving MMF.Citation[4]
In this study, we aimed to evaluate the hepatotoxicity of MMF in renal transplanted patients.
Material and Method
A total of 124 renal transplantation recipients (RTRs) were evaluated for elevated liver enzymes associated with MMF. The patients with hepatitis (n = 6), hepatitis (n = 6), tuberculosis (n = 2), active cytomegalovirus (CMV) infection (n = 1), pancreatitis (1), congestive heart failure (1), azathioprine therapy (21), and therapy that did not include MMF immunosuppressive regimens (n = 7) were excluded from this study. We assessed the postrenal transplantation period for elevated liver enzymes. Seventy-nine patients were enrolled in the study, and the patients who had a progressive increase in liver enzymes after renal transplantation were recorded. In case of a temporary and slight increase or possible other etiologies (infection, antibiotics, antihyperlipidemics, antihypertensive agents etc,) for high levels of liver enzymes were not take into consideration. The patients whose elevated liver enzymes returned to normal liver enzymes levels after withdrawal or reducing dosage of MMF were considered. We noted the levels of AST, ALT, GGT, ALP, bilirubins, renal functions, hepatitis, hepatitis, CMV, abdominal ultrasonography, detection time, and duration of high liver enzymes and decreased dosage or withdrawn of MMF. Liver enzymes were assessed after reduced dosage or withdrawal of MMF. Also, we evaluated the liver enzymes of the study patients while they were in waiting list. We did not change the dosage of other immunosuppressives and drugs except MMF.
Results
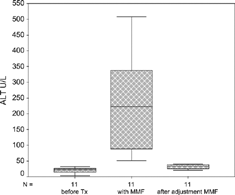
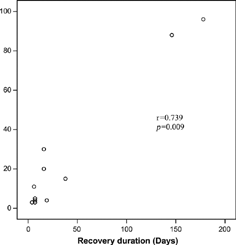
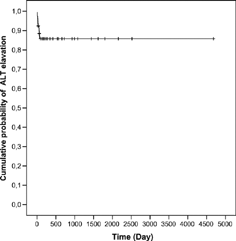
Of the 79 patients, 11 patients (13.9%) had a progressive increase in liver enzymes after renal transplantation. The median (min–max) age of the patients with MMF hepatotoxicity was 29 (19–54) years and 72.7% of them were male. The median age of the patients without MMF hepatotoxicity were 33 (15–66) years and 69.1% of them were male. There were no statistical differences for age and sex between patients with and patients without elevated liver enzymes (p = 0.185, p = 1.00, respectively). The median renal transplantation period of RTRs without MMF hepatotoxicity was 20.50 (1–156) months. The median levels of liver function tests in waiting list (I), before (II) and after (III) reduction or withdrawal of MMF are shown in . shows the levels of ALT I, II, and III. Also, liver enzymes, hepatitis B-C, CMV, detection time and duration of elevated liver enzymes, and abdominal ultrasonography of the patients are shown in . None of the patients had blood transfusion during the operation or after renal transplantation. All of the patients used MMF, 2 g/day. The median time of the increase in liver enzymes was 28 (4–70) days. After a reduction of 50% or withdrawal of MMF, liver enzymes returned to normal values in 16 (4–210) days. The correlation between recovery time of ALT and persistence time of ALT elevation before adjustment of MMF was significant (r = 0.739, p = 0.009) (). The risk of ALT elevation according to the time after renal transplantation was shown in .
Table 1. Liver function tests of the patients
Table 2. The laboratory values of the patients with elevated liver enzymes
Discussion
MMF is generally administered as 2–3 g/day in divided doses. However, some patients cannot tolerate the full dose. The incidence of adverse events is higher in patients treated with MMF 3 g/day compared to patients receiving MMF 2 g/day, suggesting a dose dependent-effect.Citation[5] Our patients used MMF 2 g/day after renal transplantation.
Gastrointestinal complications of MMF including nausea, vomiting, diarrhea, esophagitis, and gastritis are more prominent than its more serious complications such as cholestasis, hemorrhagic gastritis, pancreatitis, and large bowel perforation. Bone marrow depression (leucopenia, thrombocytopenia, and anemia) has been reported in renal transplant patients with a frequency of 7%–35%.Citation[6] Myelosuppression generally improves approximately 1 week after discontinuation of MMF.Citation[6] The incidence of malignancy during the MMF therapy in patients with psoriasis was not significantly different than that of age-matched controls.Citation[6] However, it is important to remember that the frequency of malignancy may actually be increased in transplant patients receiving concomitant immunosuppressive drugs. A slight increase in infections, especially tissue-invasive CMV, with the usage of MMF has been reported.Citation[4], Citation[6] In our patients with MMF-related hepatotoxicity:, other side effects such as infection, malignancy, gastrointestinal adverse effect, and myelosuppression did not develop. In the literature, to our knowledge, there was only one report about increased liver enzymes with the usage of MMF in RTRs. In 1992, Sollinger et al. noted increased liver enzymes in 5 of 75 renal transplant patients who used MMF.Citation[4] We did not find a distinctive cause for elevated liver enzymes in our patients and liver enzymes did not return to normal values unless we reduced the dosage of MMF or withdraw it. In 10 of our patients, after elevation of liver enzymes, first we changed cyclosporine to tacrolimus, than stopped tacrolimus and other medications except MMF and steroids, but elevation of liver enzyme levels persisted until we withdrew MMF. Also in 11 patients the period between the beginning of hepatotoxicity and stopping of MMF was longer so that hepatoxicity improved in 6 and 5 months, respectively. As seen on resolution of drug hepatotoxicity required longer time if drug administration is continued.
Most symptoms are either self-limiting or resolve with reducing the dose of MMF or changing the dosing interval.Citation[2],Citation[6] The MMF was reduced to 1 g/day in five of our patients. Although, it was reported that MMF did not occasion permanent organ toxicity,Citation[6] six of our patients could not tolerate MMF, because restarting of MMF caused recurrence of elevation in liver enzymes. In clinical practice, MMF dose reductions, interruptions, or discontinuations are often undertaken because of gastrointestinal symptoms.Citation[7] The impact of MMF dose changes on clinical outcome has been assessed in a few retrospective studies. And, it was concluded that the overall incidence of acute rejections was significantly higher in patients with gastrointestinal adverse events and patients who underwent MMF dosage adjustments or discontinuations compared with those that did not suffer gastrointestinal adverse events.Citation[8] Although hepatotoxicity was more prominent in the first 3 months, clinically overt acute rejection or loss of renal function did not develop for 2 years in our patients. But it can be important to reduce or withdraw MMF for rejection in some patients. To evaluate the elevated liver enzymes in the first 3 months of renal transplantation, MMF-associated hepatotoxicity must be kept in mind before further investigations.
Consequently, in the postrenal transplantation period, hepatotoxicity can occur for a lot of reasons including MMF usage. If hepatotoxicity related to MMF is not considered, especially in the early period, resolution of hepatotoxicity can be required long-term.
References
- Mourad, M.; Malaise, J.; Eddour, D C.; , et al. Pharmacokinetic basis for the efficient and safe use of low-dose mycophenolate mofetil in combination with tacrolimus in kidney transplantation. Clin. Chem. 2001, 47, 1241–1248. [PUBMED], [INFOTRIEVE], [CSA]
- Mourad, M.; Malaise, J.; Eddour, D C.; De Meyer, M.; , et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin. Chem. 2001, 47, 88–94. [PUBMED], [INFOTRIEVE], [CSA]
- Sollinger, H W. Mycophenolate in transplantation. Clin. Transplant. 2004, 18, 485–492. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sollinger, H W.; Belzer, F O.; Deierhoi, M H.; , et al. RS-61443 (Mycophenolate Mofetil). A multicenter study for refractory kidney transplant rejection. Ann. Surg. 1992, 216 (4), 513–519. [PUBMED], [INFOTRIEVE], [CSA]
- European Mycophenolate Mofetil Study Group. Placebo controlled study of mycophenolate mofetil combined with cyclosporine and corticosteroids for prevention of acute rejection. Lancet 1995, 345, 1321–1325. [CSA]
- Mele, T S.; Halloran, P F. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology 2000, 47, 215–245. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pelletier, R P.; Akın, B.; Henry, M L.; , et al. The impact of mycophenolate mofetil dosing patterns on clinical outcome after renal transplantation. Clin. Transplant. 2003, 17, 200–205. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Knoll, G A.; McDonald, I.; Khan, A.; Van Walraven, C. Mycophenolate mofetil dose reduction and risk of acute rejection after renal transplantation. J. Am. Soc. Nephrol. 2003, 14, 238. [CSA]