Abstract
During kidney development many proteases are involved with the remodeling process of the extracellular matrix (ECM) during nephrogenesis. This study used embryonic kidneys culture, tridimensional cell culture, and reverse transcriptase-polymerase chain reaction (RT-PCR) techniques in order to investigate the expression of cathepsins S (CS) and cathepsin H (CH) during metanephrogenesis and their functional interface with hepatic growth factor (HGF) and nerve growth factor (NGF). Results have shown that cathepsin S has been expressed early than the cathepsin H in the nephrogenesis. NGF antibody in the embryonic kidney cultures, in a dose-dependent mechanism inhibited the CS but not CH genic expression by RT-PCR. The tridimensional cells culture with MDCK and IMCD cells confirmed the interface between HGF and CS and CH once their inhibitors added to the culture, reduced the fancy branching formation induced by this growth factor. In summary, this study suggests that CS and CH are differently expressed during nephrogenesis and also that they are involved with the tubulogenesis probably mediating specific growth factors such as NGF and HGF.
Introduction
Mammalian nephrogenesis is modulated by a series of regulatory components with diverse interlinked cellular and molecular actions. Cellular interactions with the extracellular matrix (ECM) play a definite role in this phase.
The most likely assumption is that the matrix may be responsible for providing substrates for cell migration, adhesion, and differentiation, which define the pattern of growth and its ramifications. These morphogenetic events, which are activated in nephrogenesis, characterize a complex creative process and involve effects combined with the proteolytic remodeling of the ECM itself and the activity of growth factors (GF). The direct or indirect control of the expression and activity of a specific group of proteases by GF, modifying the ECM and interfering with the final embryonic result, is one of the hypotheses of this study.Citation[1-4]
Growth factors have been expressively investigated and, over the last two decades, their involvement in renal development has been confirmed. Among the GFs most extensively investigated in nephrogenesis, we emphasize the hepatic growth factor (HGF) and the neural growth factor (NGF) in the view of their confirmed representatively in studies on renal embryonic development.Citation[5-10]
The promoting effect of HGF and its c-met receptor in nephrogenesis and in the structural and functional maintenance of the adult kidney have been convincingly defined before.Citation[7], Citation[11] The major biological responses triggered in embryogenesis, including growth, dispersal, motility, and anti-apoptotic effect on tubular cells characterize the pro-genesis activity of HGF.Citation[7] In addition to its significant involvement during nephrogenesis, exogenously administered HGF has shown in vivo activity by attenuating ECM deposition, reducing the interstitial fibrosis process, and suppressing the profibrogenic activities of TGF-β1 and its receptor.Citation[12]
Suprisingly, NGF has been related to nephrogenesis in studies on cell culture and embryonic kidneys. It was observed that, even though the ureteral bud did not express NGF, ramification was inhibited by its antisense oligonucleotide.Citation[5] In addition, the presence of receptors for this growth factor from the earliest phases of nephrogenesis until the formation of permanent nephric unitsCitation[13] and the absence of NGF expression by the ureteral bud and mesenchyme suggest that secretion of diffusible neurotrophic factors may occur at a certain time in order to optimize nephrogenesis, confirming the stimulatory role of NGF. This result supports the idea that the spinal cord is a powerful inducer of the metanephric mesenchyme, demonstrating that this effect can occur even in the absence of functional or genetic similarity and does not depend on a direct contact with the embryonic structure.Citation[14]
Despite the large number of studies available about the role of these GF in nephrogenesis, there are questions still to be clarified. One of them is their mechanism of action.
It should be pointed out that the action of proteases and, no less important, the action of their inhibitors in this phase are fundamental and are defined as a uniform movement for the formation of the organ. Indeed, the fact that the dynamic stability of the ECM is crucial for cell control during nephrogenesis and that this characteristic depends not only on its structural components but also on the regulatory expression of proteases.
However, the expression of matrix-degrading proteases during nephrogenesis, which reaffirms their remodeling role, is studied even less than the expression of the GF themselves. There are studies describing the role of metalloproteins in endothelial and epithelial tubulogenesis, in podocyte differentiation, and particularly in pathologic events such as diabetic glomerulopathy and transplant rejection, among others.Citation[15-22]
However, even less has been reported about the probable mediating effect of cysteine proteinases on the role of GF during nephrogenesis. It should be pointed out that the preferential concentration of these proteases in renal tissue seems to be related to the clearance activity of the formed organ. Their concentration in the permanent organ suggests that the activity of these proteases may start during embryogenesis at different times and with different substrates, and therefore, similar to other proteases known to play a remodeling role, cystine proteases may represent a relevant signaling system in renal development.
The premise of the present study was that a balance must exist in nephrogenesis between the levels of cystine proteases and structural ECM proteins as well as between their stimulatory and inhibitory factors. We question here whether this equilibrium may be modulated by GF.
Experimental procedures
Obtaining Embryonic Kidneys
Pregnant rats were submitted to laparotomy for embryo removal at the gestational ages of 13, 14, 15, 16, and 18 days and then sacrificed. The embryos and their metanephron were aseptically removed under a DF Vasconcelos stereomicroscope at 10 × magnification. The metanephron of different ages were destined to the study of gene expression of cathepsins S and H. Other metanephrons aged 13 days, were used for experiments of embryonic kidney culture.
The metanephron to be submitted to reverse transcriptase-polymerase chain reaction (rt-PCR) were kept in phosphate-buffered saline (PBS) prepared with diethylpyrocarbonate (DEPC) water free of RMAse in order to preserve total tissue ribonucleic acid (RNA).
PCR Techniques
rt-PCR
Embryonic kidneys identified according to the gestational age of the dam were submitted to rt-PCR for the identification of the gene sequences of cathepsins S and H.
The primers for actin and cathepsins S and H used for the present study were obtained from the nitrogenated-base sequences of their respective complementary DNAs in rats (actin: S:5′ CGTGACATTAAGGAGAAGCTG 3′ e AS: 5′ CTCAGGAGGAGCAATGATCTTGA 3′, 375 pb; cathepsin S: S 5′TGTGGTTCCTGCTGGGCTTTC 3 and AS 5′ GAGTGTATTCCGTCTTTGTGA 3′, 638 pb; cathepsin H: S 5′ GGCATCATGGGAGAGGACAGC 3′ and AS 5′ GGGATGGGGTAGGAGGCAGAG 3′, 406 pb) published previouslyCitation[23&24] and manufactured by National Biosciences Lab., Inc (Plymouth, Minnesota, USA).
β-Actina served as a marker for the presence of RNA in the samples. The kidney of the dam (adult rat) was used as positive control.
Embryonic Kidney Culture
Culture of Embryonic Kidney with Protease Inhibitors
The objective of the experiments with embryonic kidney culture was to determine the expression of cathepsins S and H in explants treated with anti-NGF or anti-TGF-β antibodies.
The organ culture technique used was that elaborated by Avner.Citation[14] Thirteen-day-old kidneys were cultured individually on 24-well plates (Sigma) on filter paper (Costar, 0.8 µm pores), and fed with specific medium consisting of Dulbecco's Modified Eagle's medium-DMEM (Sigma Chemical CO., St. Louis, MO. USA) and Nutrient Mixture F-12 (Sigma) at the 1:1 proportion, supplemented with 5 μg/mL insulin (Biobrás), 5 μg/mL transferrin (Sigma), 25 ng/mL PGE1 (Sigma), 3.2 pg/mL triiodothyronine pg/mL (Sigma), and 18 ng/mL hydrocortisone (Sigma). Mycostatin (50 U/mL) (Sigma), 50 U/mL penicillin (Sigma), 50 mg/mL gentamicin (Sigma), 1.1 mg/mL NaHCO3 (Merck), and 10 mM HEPES (Sigma) were also added, as described by Avner.Citation[14]
After the first 24 hours of culture under the described conditions, the medium was supplemented with antineuronal growth factor (NGF) antibody (Sigma) at concentrations of 1:1000 and 1:5000.
A total of approximately 10 metanephron were used for each assay and 1 μg RNA was obtained from each group. We used 10 control kidneys, and 10 kidneys treated with 1:1000 NGF.
Three-Dimensional Cell Culture on Collagen Gel
In order to determine the tubulogenic effect of HGF and its association with cathepsins S and H, we used the technique of cell culture on collagen gel, which represents thus far the most reliable method of investigation of the growth-promoting role of this GF.
The collagen gel was obtained from a mixture of eight parts of liquid collagen solution (rat tail type I collagen) with one part of sterilized DMEM concentrated 10x and one part sterilized 0.2 M HEPES, as previously described.Citation[7&8] The pH of the final solution was 7.4 and the solution was kept on ice to prevent changes in consistency.
Immortalized rat MDCK (ATCC, USA) and IMCD (inner medullary collector duct) cells (Stebe Guilers) were left in a monolayer culture with DMEM supplemented with 5% fetal calf serum (GIBCO BRL, Life Technologies, NY, USA) and antibiotics until reaching semiconfluence, when they were trypsinized with trypsin-EDTA. Free cells were counted and dispersed on previously prepared collagen gel. At that time, this solution was known to contain 5 × 104 cells/mL and 100 μL aliquots of this collagen solution were added to each well of a 96-well plate. After 10 minutes at 37°C, the solution acquired a gelatinous consistency and the medium was added at that time. The gel was then maintained in a humidified 5% CO2 and 95% air atmosphere. Hepatic growth factor (Sigma, 10 ng/mL) and the cathepsin inhibitors were added to the culture. The medium was changed daily.
In each experiment the ramified cell colonies and the tubular structures were counted daily for 5 days starting from the first day of culture. Twenty cell colonies in each experiment were monitored for the presence of cell projections or tubules by two investigators who qualified the complexity of the tubular structures formed on each plate without being aware of the type of treatment applied to the assay (double-blind study).
The cultures were photographed with an inverted Nikon Diaphot microscope using lenses with 10 × or 40 × magnification. The images presented are those that showed more clearly the effect observed by the investigators.
Results and Discussion
Morphogenesis is influenced by growth factors that control the gene expression or the activity of proteases that directly or indirectly modify the ECM components.Citation[25-27] Among the GF more classically related to nephrogenesis there are IGF-1 and IGF-II, TGF-α and TGF-β, LIF, EGF, HGF, and NGF.Citation[10] The growth factors HGF and NGF were the target of the present study, in which, embryonic organ culture, cell culture on type I collagen gel, and RT-PCR were used to characterize the participation of these factors in nephrogenesis and to investigate their probable association with the proteolytic activity of cathepsins S and H.
There are at least four classes of neutral proteases potentially involved in ECM remodeling, with the metalloproteins and the serine proteases having been best characterized thus far among them.Citation[27] Also important are plasmin and the lysosomal proteases, among them cathepsins S and H.
The present results showed that cathepsins S and H exhibit different patterns of gene expression during nephrogenesis. Cathepsin S is expressed later during the embryonic period (), a fact probably associated with processes of preservation of the morphological stability of the functional cell unit, almost complete during this phase. In contrast, the earlier expression of cathepsin H () may be related to immediate postinductive morphogenetic events, which in this species occur by about the thirteenth day of embryonic development. Previous studies permit us to infer that the temporal distribution of cathepsins S and H during nephrogenesis observed in the present study follows a pattern similar to that of other ECM proteins of some GF.Citation[28] Likely, it was recently observed in a spatio temporal distribution of insulin-like growth factor during nephrogenesis in normal or streptozotocin-induced diabetic rats.Citation[11]
Figure 1 Image of the gel with the gene products concerning the embryonic kidneys at the gestational ages of 13, 14, 15, 16, and 18 days, and the kidneys of the mother (MK). The “M” line refers to the digested plasmid used as a marker.
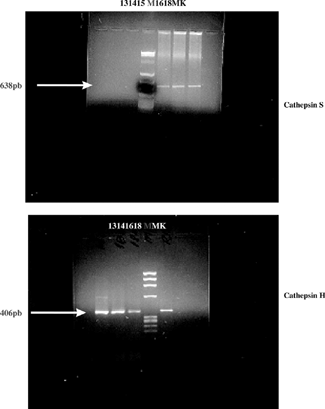
Figure 2 Image of the gel with gene products for cathepsin S (CS) and cathepsin H (CH) obtained by RT-PCR from 14-day embryonic kidneys cultured in vitro for two days under normal conditions with anti-NGF (1:1000 and 1:5000) treatment. The “M” line refers to the digested plasmid used as a marker.
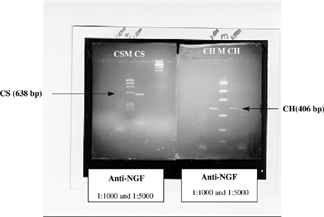
Analysis of assays involving the anti-NGF antibody and its association with cathepsins S and H permitted us to observe that the administration of this antibody inhibited the expression of both cathepsins by an apparently dose-dependent mechanism. Thus, culture media prepared with high concentrations of anti-NGF antibodies (1:1000) fully abolished the gene expression of cathepsin S, a fact that was not observed when the same antibody was added at lower doses (l:5000) (). In contrast, cathepsin H was not inhibited by anti-NGF antibody even at high concentrations.
These data indicated that NGF may participate in the nephrogenesis mediated by cathepsin S, stimulating the synthesis or inhibiting the release of its inhibitors, all of these actions being probably under the selective control of its substrates.
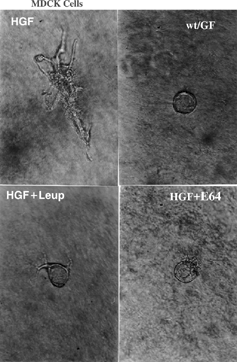
The results obtained with the model of cell culture on collagen gel and the inhibition of cathepsins by E-64 and leupeptin in the presence of HGF were intriguing. Tubular structures with relevant lumen and ramifications formed in the MDCK cell culture, confirming the renal morphogenic role of this GF. However, when the inhibitors were added, impairment of the tubular network stimulated with HGF was observed, giving origin to a cystic cell structure as an antithesis of the tubulogenic effect previously confirmed for HGF (). It has been reported that HGF inhibition with anti-HGF antibody in cultures of MDCK cells co-cultured with the embryonic kidney resulted in a significant reduction in the number of cells that developed tubular cell processes.Citation[8] The tubulogenic role of HGF confirms early HGF participation in nephrogenesis, probably associated with events occurring after induction of the metanephric mesenchyme. However, considering that in this study, proteolytic inhibition was not sufficiently specific to determine whether there was a preferential action on one cathepsin in particular, it is not possible to state whether the negative mediation of HGF occurred by inhibition of cathepsin H or S, which are expressed at embryonic times that do not coincide. Moreover, it should be pointed out that the HGF role in nephrogenesis depends on its endogenous activator, c-Met, which also requires a proteolytic clavation to initiate signaling, as shown previously (van Adelsberg J. et al. 2001). Thus, that addition of proteases inhibitors can have inhibited HGF activator and so, it the role of this growth factor was eclipsed in this model.
Figure 3 Three-dimensional culture of MDCK cells in the presence of HGF (10 ng/mL) (HGF) or in its absence (wt/GF), or treated with HGF (10 ng/mL) + leupeptin (10 μg/mL) (HGF + Leup) or HGF (10 ng/mL) + E-64 (10 μg/mL) (HGF + E-64). Photograph taken on the third day of culture with the same magnification.
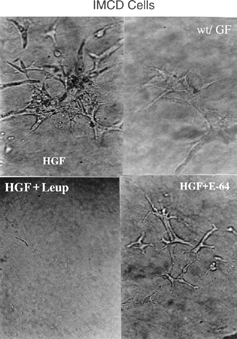
The IMCD cells showed a behavior similar to that of MDCK cells, with some peculiarities. In that experiment, the absence of HGF did not fully contain ramification, but a smaller number of branches was observed. The addition of leupeptin abolished ramification, while E-64 reduced the complexity of the culture (). However, the ability of IMCD cells to branch out in a three-dimensional culture on collagen type I gel without the need for exogenous stimuli in addition to the specific culture medium has been well defined. Thus, cathepsins may be related to the cell response to HGF, particularly in these cells.Citation[29]
Figure 4 Three-dimensional culture of IMCD cells in the presence of HGF (10 ng/mL) (HGF) or in its absence (wt/GF), or treated with HGF (10 ng/mL) + leupeptin (10 μg/mL) (HGF + Leup) or HGF (10 ng/mL) + E-64 (10 μg/mL) (HGF + E-64). Photograph taken on the third day of culture with the same magnification.
Taken together, our cell culture data confirm the direct participation of cathepsins S and H, cysteine-proteases, in nephrogenesis. Additionally, the hypothesis of an association of these proteases with GF was confirmed, indicating that they may play a role in the functional mediation of these polypeptides during the events of proliferation and differentiation triggered during the formation of the normal organ. It is a fact that GF participate in nephrogenesis, but there are no data detailing the mechanisms of activation of growth movements. However, it is known that the direct and isolated autocrine activity is unlikely and ill defined and that the modes of cell sensitization for development is variable, as demonstrated in the present study. Previous studies obtained results similar to ours concerning the functional interface between cathepsin and GF by confirming the mitogenic co-activation of interleukin I and platelet-derived growth factor (PDGF) and the stimulation of lysosomal proteolysis by chaperones or the suppression of cathepsin activity by transforming growth factor-beta 1.Citation[30&31] However, there is the need for an unequivocal definition of the causal effect of GF on the regulation of the levels and the expression of ECM components, proteases, and inhibitors in a developmental process.
In summary, the present findings permit us to suggest that cathepsins present a pattern of gene expression that varies temporally along metanephrogenesis and that their expression may play a role by mediating the activity of growth factors such as NGF and HGF, which are known to be involved in kidney embryogenesis.
References
- Ekblom, P. Renal development. In Kidney Physiology and Pathophysiology; Seldin, D W., Giebish, G., Eds. New York, 1991; 72.
- Ekblom, P. Basement membranes in development. In Molecular and Cellular Aspects of Basement Membranes; Rohrbach, D H., Timpl, R., Eds.; Academic Press: New York, 1993; 359.
- Ekblom, P. Renal development. In The Kidney; Seldin, D W., Giebisch, G., Eds.; Raven Press: New York, 1992; 475.
- Saxen, L. Organogenesis of the kidney. In Developmental and Cell Biology; Barlow, P W., Green, P B., White, C C., Eds.; Cambridge University Press: New York, 1987.
- Sariola, H.; Saarma, M.; Sainio, K.; , et al. Dependence of kidney morphogenesis on the expression of nerve growth factor receptor. Science 1991, 254 (5031), 571–573. [PUBMED], [INFOTRIEVE], [CSA]
- Taub, M.; Wang, Y.; Szczesny, T M.; Kleinman, H K. Epidermal growth factor or transforming growth factor α is required for kidney tubulogenesis in matrigel cultures in serum-free medium. Proc. Natl. Acad. Sci. U. S. A. 1990, 87 4002–4006. [PUBMED], [INFOTRIEVE], [CSA]
- Santos, O F.; Moura, L A.; Rosen, E M.; Nigam, S K. Modulation of HGF-induced tubulogenesis and branching by multiple phosphorylation mechanisms. Dev. Biol. 1993, 159 535–548. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Santos, O F.P.; Nigam, S K. HGF-induced tubulogenesis and branching of epithelial cells modulated by extra cellular matrix and TGF-beta. Dev. Biol. 1993, 160 293–302. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Santos, O F.; Barros, E J.; Yang, X M.; , et al. Involvement of hepatocyte growth factor in kidney development. Dev. Biol. 1994, 163 525–529. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kanwar, Y S.; Carone, F A.; Kumar, A.; Wada, J.; Ota, K.; Wallner, E L. Role of extracellular matrix, growth factors and proto-oncogenes in metanephric development. Kidney Int. 1997, 52 589–606. [PUBMED], [INFOTRIEVE], [CSA]
- Duong Van Huyen, J P.; Amri, K.; Belair, M F.; Vilar, J.; Merlet-Benichou, C.; Lelievre-Pegorier, M. Spatio-temporal distribution of insulin-like growth factor receptor during nephrogenesis in fetuses from normal and diabetic rats. Cell Tissues Res. 2003, 31 (4), 367–379. [CSA], [CROSSREF]
- Liu, Y. Hepatocyte growth factor and the kidney. Curr. Opin. Nephrol. Hypertens. 2002, 11 (1), 23–30. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Durbec, P.; Marcos-Gutierrez, C V.; Kilkenny, C.; , et al. GNDF signalling through the RET receptor tyrosine kinase. Nature 1996, 381 789–793. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Avner, E D. Serum-free organ culture of embryonic mouse metanephros. In Methods for Serum-Free Culture of Epithelial and Fibroblast Cells; Alan R. Inc: New York, 1984; 33.
- Matrisian, L M.; Hogam, B L. Growth factor regulated proteases and extracellular matix remodeling during mammalian development. Curr. Top. Dev. Biol. 1990, 24 219–259. [PUBMED], [INFOTRIEVE], [CSA]
- Paczek, L.; Teschner, M.; Schaefer, R M.; Kovar, J.; Romen, W.; Heidland, A. Proteinase activity in isolated glomeruli of Goldblatt hypertensive rats. Clin. Exp. Hypertens. 1992, 13 (3), 339–356. [CSA]
- Paczek, L.; Teschner, M.; Schaefer, R M.; Kovar, J.; Romen, W. Intraglomerular proteinase activity in adryamicin-induced nephropathy. Nephron 1992, 60 81–86. [PUBMED], [INFOTRIEVE], [CSA]
- Schaeffer, L.; Lorenz, T.; Daemmirich, J.; Heidland, A.; Schaefer, R M. Role of proteinases in renal hypertrophy and matrix accumulation. Nephrol. Dial. Transplant. 1995, 10 801–807. [CSA]
- French, H A. Pathways of proteolysis affecting renal cell growth. Curr. Opin. Nephrol. Hypertens. 2002, 11 (4), 445–450. [CSA], [CROSSREF]
- La Fleur, M A. Endothelial tubulogenesis within fibrin gels specifically required for the activity of membrane type matrix metalloprotease (MTMMPs). J. Cell. Sci. 2002, 115 (117), 3427–3438. [CSA]
- Asanuma, K. Selective modulation of the secretion of proteinases and theri inhibition by growth factors in cutured differentiated podocytes. Kidney Int. 2002, 62 (3), 822–831. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lelongst, B.; Ronco, P. Role of matrix metalloproteinases in kidney development and glomerulopathy: lessons from transgenic mice. Nephrol. Dial. Transplant. 2002, 17 (Suppl 9), 28–31. [CSA], [CROSSREF]
- Petanceska, S.; Burke, S.; Watson, S J.; Devi, L. Differential distribution of mesenger RNAs for cathepsins B, L and S in the adult rat brain: na in situ hibridization sutdy. Neuroscience 1994, 59 (3), 729–738. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Qian, F. Molecular cloning of rat precursor cathepsin H and the expression of five lysossomal cathepsins in normal tissues and in a rat carcinosarcoma. Int. J. Biochem. 1990, 22 (12), 1457–1464. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Laurie, G W.; Horikoshi, S.; Killen, P D.; Segui-Real, B.; Yamada, Y. In situ hybridization reveals temporal and spatial changes in cellular expression of mRNA for laminin receptor, laminin and basement membrane (type IV) collagen in the developing kidney. J. Cell Biol. 1989, 109 (3), 1351–1362. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Inaguna, Y.; Kusabe, M.; Mackie, E J.; Pearson, C A.; Chiquet-Chrismann, R.; Sakakura, T. Epithelial induction of stromal tenascin in the mouse mammary gland from embryogenesis to carcinogenesis. Dev. Biol. 1988, 128 (2), 245–255. [CSA], [CROSSREF]
- Hunter, D D.; Murphy, M D.; Olsson, C V.; Brunken, W J. S-laminin expression in adult and developing retina: a potential cell for photoreceptor morphogenesis. Neuron 1992, 8 399–413. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kleiner, D E., Jr.; Stetler-Stevenson, W G. Structural biochemistry and activation of matrix metalloproteinases. Curr. Opin. Cell Biol. 1993, 5 891–897. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Safirstein, R. Gene expression in nephrotoxic and ischemic acute renal failure. J. Am. Soc. Nephrol. 1994, 4 (7), 1387–1395. [PUBMED], [INFOTRIEVE], [CSA]
- Wang, A Z.; Ojakian, G K.; Nelson, W J. Steps in the morphogenesis of a polarized epithelium I: uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J. Cell. Sci. 1990, 95 (1), 137–151. [PUBMED], [INFOTRIEVE], [CSA]
- Robertson, T E.; Nikolic-Paterson, D J.; Hurst, L A.; Atkins, R C.; Chadban, S J. IL-10 induces mesangial cell proliferation via PDGF-dependent mechanism. Clin. Exp. Immunol. 2002, 130 (2), 241–244. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]