Abstract
Background. Studies conducted in several countries have indicated that the survival of patients undergoing renal replacement therapy (RRT) depends on the attributed cause of end-stage renal disease (ESRD). Objectives. This study was conducted to evaluate the association between attributed cause of ESRD and mortality risk in RRT patients in Brazil. Methods. We analyzed 88,881 patients from the Brazilian Ministry of Health Registry who were undergoing RRT between April 1997 and July 2000. Cox proportional hazards models were used to estimate the relative risk (RR) of death in patients with ESRD secondary to diabetes mellitus (DM), polycystic kidney disease (PKD), and primary glomerulopathies (GN) compared with a reference group comprised of patients with ESRD caused by hypertensive nephropathy. Patient's age, gender, and length of time (years) in RRT before inclusion in the registry (vintage) were included in the adjusted Cox model. Results. Compared with the reference group, the mortality risk was 27% lower in patients with PKD (RR = 0.73, 95% CI: 0.65–0.83, p< 0.0001); 29% lower in patients with GN (RR = 0.71, 95% CI: 0.68–0.74, p< 0.0001); and 100% greater in DM patients (RR = 2.00, 95% CI: 1.92–2.10, p< 0.0001). These relative risks remained statistically significant after adjustment for age, gender, and length of time in RRT before inclusion in the registry. Conclusions. Our data indicate that compared with the patients with hypertensive nephrosclerosis as attributed cause of ESRD, patients undergoing RRT in Brazil with idiopathic glomerulopathy and polycystic kidney disease have a lower risk of mortality, and patients with diabetes mellitus have a greater risk of mortality.
Introduction
The life expectancy of patients with end-stage renal disease (ESRD) is much lower as compared with age-matched groups in the general population. The mortality risk of ESRD patients, however, varies largely depending on several demographic and clinical characteristics, including the underlying kidney disease. Several studies have reported that patients receiving renal replacement therapy (RRT) are at an increased risk of mortality when the cause of their ESRD is diabetes mellitus (DM), apparently because of the large number of comorbidities associated with diabetes, especially cardiovascular diseases.Citation[1]
In contrast, a lower mortality risk has been reported for patients whose ESRD was caused by polycystic kidney disease (PKD) and idiopathic glomerulopathy (GN), as well as higher risk for ESRD patients with DM.Citation[2-4]
Logistic regression analysis of mortality from Latin American registries shows that patients who had diabetes and/or were older than 65 suffered greater mortality, whereas patients who had GN and/or were younger than 65 experienced reduced mortality.Citation[2] In addition, PKD and idiopathic GN were the most frequently diagnosed causes of ESRD in patients who survived more than 10 years in contrast with DM.Citation[5&6]
This study was developed to assess by Cox models whether the reported cause of ESRD is associated with mortality risk in Brazil, with and without adjustments for age, gender, and year on RRT (vintage).
Subjects and Methods
Our study population was comprised of all ESRD patients receiving RRT (dialysis and kidney transplantation) who were reported to the Brazilian Ministry of Health between April 1, 1997 and July 31, 2000. At the time of starting RRT and each 3 months of continuing RRT for ESRD, the Ministry of Health receives a report from the nephrology units around the country as a requirement for payment. The first and last registers of each patient were evaluated, without considering the type of treatment received.
Patients with any of the following characteristics were excluded for this analysis: those who spent less than 90 days in RRT, those younger than 12 years or older than 95, and those with unclassified or unknown causes of ESRD or with rare pathologies and/or diseases with expected short survival times as causes of ESRD (e.g., multiple myeloma, urogenital neoplasia).
Patients were classified according to the cause of their ESRD: hypertensive nephropathy (HN), DM, idiopathic GN, and PKD. To estimate the risk of death associated with cause of ESRD, a reference group was formed consisting of patients diagnosed with hypertensive nephropathy (REF HN).
For the main analysis, both incident and prevalent patients were included. Specific analysis were performed for incident cases (i.e., new patients who have started RRT during the study period), and for prevalent cases (i.e., those already receiving RRT in April 1997).
The Cox proportional hazards model was used to estimate the relative risk (RR) of death by ESRD cause, with and without adjustments for gender, age, and previous duration of RRT. Variables were excluded with p< 0.10 but entered when p< 0.05. Statistical analyses were performed by using software SPSS® Version 10.0 for Windows®.
Results
During the study period, data from 88,881 patients were available for analysis. Three percent of these patients had received renal transplants, 14% were receiving peritoneal dialysis, and 83% were receiving hemodialysis. Patients' average age was 49.7 ± 17.5 years, the mean follow-up time was 560 ± 440 days, and 56.8% were male. The observed mortality was 16.6 deaths per 100 patient-years.
The characteristics of the 44,240 patients that remained after applying the exclusion criteria are detailed in .
Table 1. Characteristics of study patients
Compared with the reference group, the mortality risk was 27% lower in patients with PKD (RR = 0.73, 95% CI: 0.65–0.83, p< 0.0001); 29% lower in patients with GN (RR = 0.71, 95% CI: 0.68–0.74, p< 0.0001); and 100% greater in DM patients (RR = 2.00, 95% CI: 1.92–2.10, p< 0.0001). After adjusting for age, gender, and length of time in RRT before inclusion in the registry (vintage), the relative risk of mortality was still greater for patients with DM and smaller for patients with PKD and GN compared with the reference group ( and ).
Table 2. Risk of mortality and attributed cause of end-stage renal disease in patients receiving renal replacement therapy in Brazil between April 1997 and July 2000 (Cox proportional hazards model)
Figure 1. Risk ratio for mortality according to attributed cause of end-stage renal disease for patients undergoing renal replacement therapy in Brazil between April 1997 and July 2000 (Cox proportional hazards models). GN, idiopathic glomerulopathy; PKD, polycystic kidney disease; DM, diabetes mellitus; REF-HN, reference group—patients with ESRD due to hypertensive nephropathy; Adjusted, adjusted for gender, age, and previous time in RRT (before inclusion in registry).
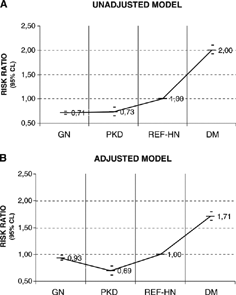
In the separate analysis of incident and prevalent cases, the risk ratio of death was lower in GN and PKD patients and higher in DM patients in the adjusted and unadjusted models that took REF HN patients as reference group, except for GN patients in adjusted model of prevalent cases ().
Table 3. Association between attributed cause of end-stage renal disease and death in incident and prevalent patients in Brazil between April 1997 and July 2000 (Cox proportional hazards model).
Discussion
This study confirms, in Brazilian patients, the consensus of a number of previous studies: that the diagnosis of DM as a cause of ESRD is associated with increased mortality in patients undergoing RRT. It also suggests that patients with diagnosis of PKD or idiopathic GN have a smaller risk of death than the chosen reference group comprised of patients who received the diagnosis of hypertensive nephrosclerosis.
The additional analyses that examined separately incident and prevalent cases showed similar results.
Few epidemiologic studies in developing countries have attempted to quantify risk of death in ESRD patients that may be attributed to the underlying nephropathy, although the question was addressed in developed countries' patients.Citation[7] This could be due, in part, to the difficulty of determining the cause of ESRD. This difficulty may occur even for patients with type 2 diabetes, considering their higher risk for nondiabetic nephropathy as compared with patents without diabetes.Citation[8] Reevaluation of patients diagnosed with HN suggests that a significant percentage did not satisfy established clinical criteria for that disease.Citation[9] Furthermore, there is evidence from other studies (one of which is a Brazilian study) that renal biopsy reveals important percentage of other primary nephropathies, even when preestablished diagnostic criteria of HN are used.Citation[10] Under notification of PKD diagnosis, however, is unlikely because the Brazilian Ministry of Health requires that all patients undergo renal ultrasound at the beginning of their RRT program.
The diagnosis of idiopathic GN was based on criteria outlined by the International Disease Codes (IDC 9th and 10th editions). Histologic diagnosis was found in only 22.5% of the patients with IDC of GN. Just as there are patients with the diagnosis of HN and contracted kidneys of unknown etiology (mainly patients with code related with contracted kidneys), there may also be patients with erroneous diagnosis of idiopathic GN. The comparison of these groups should be viewed with caution.
A study by Sesso et al., which evaluated the acceptance of RRT patients in the Brazilian state of São Paulo, suggested that patients with DM and GN were more frequently admitted to RRT than patients with other pathologies.Citation[11]
This study evaluated ESRD patients from 26 Brazilian states with huge economic differences. The analysis of the distribution of DM as an attributed cause or ESRD between the states showed percentages ranging from 3% to 24.4%. The different regions of the country showed differences in incidence (45/1,000,000 habitants of the North region to 129/1,000,000 habitants of the Southeast region) and prevalence (105/1,000,000 habitants of the North region to 416/1,000,000 habitants of the Southeast region), with the vast majority (73.2%) of the patients being from the South and Southeast regions. Analysis that included the region where the patient was submitted to RRT showed no impact on risk of death, even controlling for age, gender, time on RRT, and diabetes as an attributed cause of ESRD (unpublished observations). Our observations, however, do not permit us to conclude the actual reasons for the differences in incidence and prevalence by causes of ESRD. Health care-related issues (as different standards of care across Brazilian states) and the criteria that have been used for the diagnosis of the underlying disease are some of the potential explanations for the differences in attributed causes of ESRD that have been observed across regions.
The choice of the study groups was based in its homogeneity and the fact that they (apart from PKD patients) constitute the three biggest groups of ESRD patients.
Including unknown causes of ESRD (mainly contracted kidneys IDCs), reflux nephropathy, and chronic pyelonephritis, as was done in preliminary analysis, added no difference to the results because these groups had the same risk ratio as HN patients. The first of these groups (UK), although numerous, should include patients from the other groups (as diabetic, glomerulonephritis, and HN), and reflux nephropathy and chronic pyelonephritis groups had few patients.
Data from the U.S. Renal Data Systems (USRDS) show that patients with GNs have lower mortality rates (149.9 deaths per 1000 African American patient-years and 201.7 deaths per 1000 Caucasian patient-years) than patients with HN (189.5 deaths per 1000 African American patient-years and 343.5 deaths per 1000 Caucasian patient-years) and diabetes (259.9 deaths per 1000 African American patient-years and 402.5 deaths per 1000 Caucasian patient-years).Citation[12] The same trend was demonstrated in Latin American and Japanese patients.Citation[2&3] Similar differences were encountered in the group of patients comprising our study sample ().
Due to the nature of the data set used in our analysis, we were not able to control for the type of RRT received. All the patients who were receiving RRT in April 1997 and those that were subsequently admitted were followed until July 2000. Only 3% of the patients in this study had received transplants by April 1997. The annual rate of kidney transplants in Brazil varied between 11.2 per million per year in 1997 to 18.5 transplants per million per year in 2000, with approximately 9000 transplants over the 4 years included in our study period. The distribution in number of transplants per state and the rate of increase in number of transplants per year varies greatly between Brazilian states. The transplant rate varied between 0 in one state in 1997 to 35 transplants per million in another state in 2000.Citation[13]
The reduction in risk of mortality accompanying transplant has been shown to depend on the diagnosed cause of ESRD.Citation[12], Citation[14] Our study included only patients whose treatment was government paid and not those who received transplants through private services. As such, we are unable to assess the effect that the selection of patients for kidney transplant could have had on our results.
When we evaluated all 88,881 ESRD patients of this study, the age-specific mortality curve was bimodal “J-shaped” being highest for patients younger than 8 years old, and then increasing linearly with age in patients 8 years of age and older. Because we only studied patients older than 12 years in our final analysis, we included age as a continuous variable in our model.
Although some authors have demonstrated a positive association between mortality and prior duration of RRT, more recent studies of BrazilianCitation[15&16] and American patientsCitation[17] suggest that the risk of mortality is inversely related to the previous length of time in RRT (vintage). A more recent study of Brazilian patientsCitation[16] found that the risk of death in the unadjusted model declined substantially after the first year of RRT. Multiple logistic regression analysis of 1-year mortality in the same group of patients revealed that the length of time in RRT before inclusion in the registry and diagnosis of GN as the cause of ESRD were associated with decreased risk of mortality, whereas age, female gender, and diagnosis of DM as the cause of ESRD were associated with increased risk of mortality (unpublished data). Including previous time of RRT in our Cox proportional hazards model of primary analysis, as well as in the analysis of prevalent cases, corroborated that finding. The strength of the association of diagnosis with death rate in incident cases (without “vintage”) was reduced but persisted.
It is known that molecular alteration in animals with mutation in the PKD1 gene is associated with vascular fragility,Citation[18] and alterations have been found in arterial lesions of patients with PKD.Citation[19] Although these patients would be expected to have excess mortality, a more recent study demonstrated that patients in the United States with ESRD caused by PKD had significantly lower risk of death than nondiabetic patients in RRT.Citation[20] The risk of mortality associated with the diagnosed cause of ESRD appears to depend on the frequency and intensity of comorbidities, both of which differ between diabetic and nondiabetic patients. Analysis of several comorbidities that are associated with greater mortality in patients with ESRD has shown that diabetics and elderly patients have higher rates of coronary disease, peripheral vascular disease, and arterial hypertension than nondiabetics.Citation[21] The presence of inflammation and associated arteriosclerosis appears to be important in the general population,Citation[22] as well as in patients with ESRD,Citation[23] both beforeCitation[24] and afterCitation[25] they begin hemodialysis.
Studies investigating the association between inflammation and mortality of patients with ESRD have classified patients either as diabetic or nondiabetic. In one study, 34% of the nondiabetic patients had GN, and 14% had PKD.Citation[24] However, these studies did not evaluate the associations between these diagnoses, inflammation, and mortality due to arteriosclerosis-related diseases.Citation[24&25]
Our data show in Brazilian patients that the risk of mortality in RRT patients increases when their ESRD was caused by DM. What this article seems to suggest, however, is that other attributed causes of ESRD, in addition to DM, should be considered when studying the mortality risks of ESRD patients.
The high risk of death in patients with ESRD attributed to DM in Brazil could not be explained by the effects of age, gender, and time on ESRD. The lack of information on comorbidities in the data bank of the Brazilian Ministry of Health prevented a more detailed assessment of the factors that may contribute to the observed difference in the mortality risk by cause of ESRD. Currently, an advisory group is working with the Brazilian Ministry of Health to improve the data bank. The new data should provide information for the assessment of cause of death and the influence of comorbidities on differences between patients with different types of kidney diseases. We also hope that the new data will help identify clinical interventions and public health policies that may contribute to improve survival and quality of life of ESRD patients treated with RRT in Brazil.
Acknowledgment
Research support from Brazilian Ministry of Health.
References
- Herzog C A, Ma J Z, Collins J A. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998; 339:799–805. [PUBMED], [INFOTRIEVE], [CSA]
- Mazzuchi N, Schwedt E, Fernández J M, , et al. Latin American registry of dialysis and renal transplantation: 1993 annual dialysis data report. Nephrol Dial Transplant. 1997; 12(12):2521–2527. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Teraoka S, Toma H, Nihei H, , et al. Current status of renal replacement therapy in Japan. Am J Kidney Dis. 1995; 25(1):151–164. [PUBMED], [INFOTRIEVE], [CSA]
- Mailloux L U, Bellucci A G, Napolitano B, Mossey T, Wilkes B M, Bluestone P A. Survival estimates for 683 patients starting dialysis from 1970 through 1989: identification of risk factors for survival. Clin Nephrol. 1994; 42(2):127–135. [PUBMED], [INFOTRIEVE], [CSA]
- Harris S A, Brown E A. Patients surviving more than 10 years on hemodialysis. The natural history of the complications of treatment. Nephrol Dial Transplant. 1998; 13(5):1226–1233.. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Owen W F, Madore F, Brenner B M. An observational study of cardiovascular characteristics of long-term end-stage renal disease survivors. Am J Kidney Dis. 1996; 28(6):931–936. [PUBMED], [INFOTRIEVE], [CSA]
- Wolfe R A, Gaylin D S, Port F K, Held P J, Wood C L. Using USRDS mortality tables to compare local ESRD mortality rates to national rates. Kidney Int. 1992; 42:991–996. [PUBMED], [INFOTRIEVE], [CSA]
- Brancati F L, Whelton P K, Randall B L, Neaton J D, Stamler J, Klag M J. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA. 1997; 278(23):2069–2074. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zarif L, Covic A, Iyengar S, Sehgal A R, Sedor J R, Schelling J R. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant. 2000; 15:1801–1807. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Caetano E RSP, Zatz R, Saldanha L B, Praxedes J N. Hypertensive nephosclerosis as a relevant cause of chronic renal failure. Hypertension. 2001; 38:171–176. [PUBMED], [INFOTRIEVE], [CSA]
- Sesso R, Fernandes P F, Ancao M, , et al. Acceptance for chronic dialysis treatment: insufficient and unequal. Nephrol Dial Transplant. 1996; 11(6):982–986. [PUBMED], [INFOTRIEVE], [CSA]
- Wolfe R A, Ashby V B, Milford E L, , et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaver transplant. N Engl J Med. 1999; 341:1725–1730. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Associação Brasileira de Transplantes de Órgãos (ABTO). Registro Brasileiro de Transplantes, 2001, São Paulo, VII Ano, No 4.
- Port F K, Wolfe R A, Mauger E A, Berling D P, Jiang K. Comparison of survival probabilities for dialysis patients vs. cadaveric renal transplant recipients. JAMA. 1993; 270:1339–1343. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lopes A A, Batista P BP, Okechukwu C N, Costa F A, Nery M M. Does the mortality risk change with years on dialysis? The Brazilian experience [Abstract A1153 from ASN/ISN World Congress of Nephrology]. Am J Kidney Dis. 2001; 12:223A. [CSA]
- Lopes A A, Batista P BP, Costa F A, Nery M M. Número de anos em tratamento dialítico crônico e risco de morte em pacientes com e sem diabetes mellitus. Rev Assoc Med Bras. 2003; 49(3):266–269. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Okechukwu C N, Lopes A A, Stack A G, Feng S, Wolfe R A, Port F K. Impact of years of dialysis therapy on mortality risk and the characteristics of longer term dialysis survivors. Am J Kidney Dis. 2002; 39(3):533–538. [PUBMED], [INFOTRIEVE], [CSA]
- Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout M A. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci U S A. 2000; 97(4):1731–1736. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Griffin M D, Torres V E, Grande J P, Kumar R. Vascular expression of polycystin. J Am Soc Nephrol. 1997; 8(4):616–626. [PUBMED], [INFOTRIEVE], [CSA]
- Perrone R D, Ruthazer R, Terrin N C. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001; 38(4):777–784. [PUBMED], [INFOTRIEVE], [CSA]
- Prichard S S. Co morbidities and their impact on outcome in patients with end-stage renal disease. Kidney Int. 2000; 57(Suppl. 74):S100–S104. [CSA], [CROSSREF]
- Libby P, Ridker P M, Maseri A. Inflammation and atherosclerosis. Circulation. 2002; 105:1135–1143. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link?. Kidney Int. 2001; 59(2):407–414. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Stenvinkel P, Heimbürger O, Paultre F, , et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999; 55(5):1899–1911. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999; 55(2):648–658. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]