Abstract
Background. To investigate the safety, feasibility, efficacy, and long-term patency rate of manual declotting under duplex ultrasound (US) guidance followed by percutaneous transluminal angioplasty (PTA) in thrombosed native arteriovenous fistulas (AVFs). Methods. Of 87 consecutive thrombosed AVFs evaluated by duplex US, 22 patients with 25 recently thrombotic events in 22 AVFs were suitable for manual declotting. PTA was performed following successful declotting, and long-term patency was assessed. Results. The procedure success rate of manual declotting was 80% (20 of 25), and a residual stenosis of 74 ± 9% was identified by duplex US after declotting. PTA reduced the diameter stenosis to 25 ± 6% and increased the lumen diameter from 1.33 ± 0.85 mm to 4.62 ± 0.98 mm. Neither embolic nor bleeding complications were noted during the procedure. The average procedure time and the fluoroscopy time were 28.4 ± 9.9 and 7.2 ± 4.1 minutes, respectively. Primary patency rates at 1, 2, and 3 years were 47%, 35%, and 28%; assisted primary patency rates at 1, 2, and 3 years were 71%, 63%, and 63%; and secondary patency rates at 1, 2, and 3 years were 76%, 71%, and 63%, respectively, during a maximum follow-up period of 42 months. Conclusion. The combination of duplex US-guided manual declotting and angioplasty of underlying stenosis is a safe and feasible method to treat recently thrombosed native AVFs in selected patients. It simplifies the interventional procedure, reduces cost and radiation exposure time, and extends life span of dialysis fistula with acceptable long-term patency rate.
Introduction
Functioning vascular access is essential to achieving long-term survival and optimal quality of life for hemodialysis patients. Vascular access-related complications are the most common cause of hospitalization in these patients, contributing significantly to overall mortality.Citation[1-3] Early detection of failing dialysis access followed by adjunctive percutaneous transluminal angioplasty (PTA) could restore dialysis fistula function and then reduce the risk of fistula thrombosis.Citation[4] Color flow Doppler ultrasound (US) is widely used for surveillance and detection of vascular access dysfunction with a good sensitivity.Citation[5] Moreover, this technique has good correlation in diagnosing anatomic stenosis with fistulography.Citation[6] Duplex US has been used as a guide for managing failing vascular access during PTA;Citation[7] however, the role of duplex US in the management of thrombosed native arteriovenous fistula (AVF) is unknown. Many interventional radiologists restored the function of thrombosed polytetrafluoroethylene (PTFE) grafts under fluoroscopic guidance, either using pharmacologic or percutaneous methods. The reported success rate was 75% to 94%, and procedure time ranged from 90 to 151 min.Citation[8-10] There are few reports in the literature regarding percutaneous interventions for salvaging thrombosed native AVFs because of technical difficulties and unfavorable outcome.Citation[11-13] Thus, this study is designed to evaluate the safety, feasibility, efficacy, and long-term patency of thrombosed native AVFs using a combination method of duplex-guided manual declotting followed by adjunctive PTA.
Materials and Methods
From June 2000 to October 2003, 87 consecutive thrombosed native AVF in 83 patients referred from the dialysis unit received duplex US examinations in this institution. An ATL HDI 3500 (Advanced Technology Laboratories, Bothell, WA, USA) or an Acuson 128X (Acuson Corporation, Symrna, GA, USA) machine was done. Using a 5- to 12-MHz liner transducer in the longitudinal and traverse planes, the feeding artery, arterial anastomosis, and entire venous limb, extending as far as the subclavian vein, were sonographically interrogated. The thrombus length, maximal lumen diameter of the thrombosed vessel, and size of main outflow drainage vein were also measured. Manual declotting was performed if patient fulfilled the following inclusion criteria: 1) time from onset of thrombus to intervention less than 72 h; 2) length of thrombus less than 10 cm; 3) maximal lumen diameter of thrombosed vessel less than 10 mm; 4) no thrombus within 1 mm of the arterial anastomosis; 5) adequate vessel size (>5 mm) of the main drainage vein; 6) age of fistula at least 2 months; and 7) absence of active infection of dialysis fistula. The technique of manual declotting was described as follows: the arterial anastomosis site was slightly compressed to prevent the thrombus from entering the feeding artery. Repeated manual compressions were applied over the venous limb to declot and macerate thrombus, and then the dissolved thrombus was headed toward the central vein or other collateral branches. Duplex US was performed immediately after each manual declotting to evaluate the blood flow, measure the severity of underlying stenosis, and identify the appropriate puncture site for subsequent PTA (). Patients with residual stenosis more than 50% or minimal lumen diameter less than 3 mm (determined from both the direct gray scale measurement of the residual flow lumen and the color Doppler flow imaging) were considered candidates for subsequent PTA ( and ).
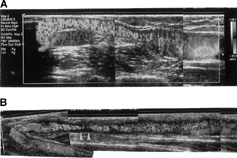
Figure 1. (A) A 37-year-old male patient received color flow Doppler ultrasound showing the presence of long segmental thrombus (arrowheads) in the basilic vein without detection of blood flow. (B) After manual declotting, blood flow reappeared in the basilic vein (arrows) unmasked the underlying stenosis.
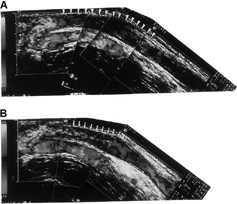
Figure 2. (A) Retrograde fistulography in same patient revealed a discrete stenosis at the basilic vein (arrowheads). (B) The stenosis was dilated by balloon angioplasty with improvement of blood flow.
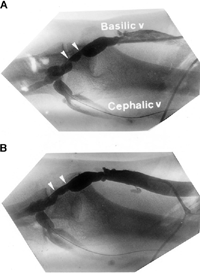
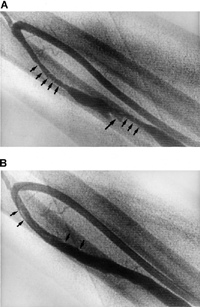
Figure 3. (A) A 59-year-old female patient underwent duplex-guided manual declotting. Subsequent fistulography revealed two segmental stenoses in the cephalic vein of a wrist fistula (arrows) with some residual thrombus at the aneurysmal dilatation site (arrowhead). (B) Following angioplasty, these stenoses were resolved, thrombus was macerated by balloon catheter, and a few intimal dissections were left without interrupting blood flow (arrows).
Fistulography and PTA were performed with a Philips Angio Unit (OCI or Integris BH 5000, Philips, Eindhoven, The Netherlands). A Terumo guidewire (Radiofocus, Tokyo, Japan) was introduced into the access using a retrograde venous approach, and a 6F sheath with a side arm was advanced to the venous limb. Ivoversol (Optiray, Mallinckrodt Canada, Inc., Pointe-Claire, Quebec, Canada) was injected via the sheath to visualize the extent of stenosis and the arterial anastomosis while applying manual occlusion of the venous outflow. Moreover, anteroposterior, left anterior oblique, or right anterior oblique views were taken to identify the most severe stenotic site. Before balloon catheter insertion, each patient was administered 3000 to 5000 IU heparin and midazolam hydrochloride (Versed; Roche, Nutley, NJ, USA) for sedation. The stenosed segment was then dilated by high-pressure (14–24 atmospheres) balloon inflation (Wanda TM, Boston Scientific, MK, USA, Blue-Max, Meditech/Boston Scientific, Watertown, MA, USA). Residual thrombus identified by fistulogram was removed via balloon maceration and catheter clot aspiration. The disappearance of significant stenosis was considered as end points. Symptoms (chest pain and dyspnea), vital signs, peripheral oxygenation, and electrocardiogram were continuously monitored during the procedure. Total procedure time and fluoroscopy time were also recorded. No anticoagulation therapy was administrated before and after the procedure.
Angiographic Analysis
A contrast-filled inflation balloon with nominal pressure was used as a reference for calibration. Moreover, the reference vessel diameter, the minimal lumen diameter, the diameter stenosis, and the length of stenotic segment were measured using the automated edge detection method or a digital caliber before and after PTA.
Definitions
Clinical success was defined as the resumption of normal dialysis for at least three sessions following intervention. Meanwhile, procedural success was defined as restoration of flow and residual diameter stenosis of less than 50% for any significant underlying stenosis. Furthermore, primary patency was the period that fistula remained patent without surgical or percutaneous intervention. The assisted primary patency period began when any intervention was used to maintain patency of the fistula before thrombosis. Secondary patency was the interval following intervention until the fistula was surgically declotted, revised, and abandoned; renal transplantation was performed; or the patient was lost to follow-up. Procedure time was measured from the first manual manipulation to the final angiogram. Major complications included procedure-related death, pulmonary or arterial embolization, vessel rupture requiring surgical repair, and massive bleeding requiring blood transfusion. Intimal dissections without interfering blood flow and small ecchymosis or hematoma over the puncture site were considered minor complications.
Follow-Up
All patients were followed up at a hemodialysis center, and patient status was updated between clinic visits by telephone interview. Periodic duplex US was performed to evaluate the anatomy and hemodynamic status of the fistula (). Finally, the dates and types of any subsequent intervention were recorded, along with that of fistula failure.
Figure 4. (A) Two months after the initial procedure, follow-up duplex ultrasound (US) disclosed functioning dialysis fistula without significant stenosis or turbulence flow causing aliasing. (B) Restenosis developed 9 months after the initial procedure and caused turbulence on duplex US (arrowheads). This lesion was successfully dilated by balloon angioplasty.
Statistical Analysis
All continuous data were expressed as mean ± SD, and categorical data were presented as numbers and percentages. The STATA 8.0 software package was used for the statistical analysis. Kaplan-Meier survival analysis was used to calculate the primary patency rates, the assisted primary patency rates, and the secondary patency rates following intervention.
Results
Study Population and Baseline Demographics
Of 87 consecutive thrombosed native AVFs evaluated by duplex US, 62 were not suitable for manual declotting: 47 with chronic occluded main drainage vein, 6 with huge thrombus inside the venous limb, 3 with thrombus formation more than 3 days, 3 with thrombus length over 15 cm, 2 with active infection in dialysis fistulas, and 1 with thrombus involving the feeding artery. A total of 22 patients, 10 male and 12 female, with a mean age 55 ± 14 years old were enrolled in this study. There were 15 dialysis fistulas in the wrist and 7 in the elbow, with a mean fistula age of 32.2 ± 39.1 months. Ten patients had previous intervention for fistula dysfunction: 6 with PTA, 3 undergoing surgical embolectomy, and 1 receiving stent implantation in the axillary vein. The average time from onset of thrombus formation to intervention was 28 ± 17 hours (12 episodes within 24 h and 13 episodes between 24 and 72 h). The baseline characteristics of patients receiving manual declotting are listed in .
Table 1. Patient demographics
Lesion Characteristics of Duplex and Angiographic Measurements
Manual declotting was undergone in 25 thrombosed AVFs, with a mean thrombus length of 4.0 ± 1.7 cm (1.8 ∼ 7.2 cm) and a mean maximal lumen diameter of thrombosed vessel of 6.2 ± 1.2 cm (4.3 ∼ 8.7 cm) by duplex US (). Two fistulas with a high echogenic adherent thrombus and 2 with soft thrombus cannot be declotted manually. The other one restored the flow initially, but rapid thrombus propagation to the whole drainage vein caused puncture failure during subsequent PTA. Three failed fistulas underwent surgical embolectomy and revision. Two fistulas with soft thrombus restored the blood flow after dilatation of stenosis by balloon angioplasty. The procedure success rate was 80% (20 of 25). Two to five manual manipulations were required to declot the thrombus before restoration of the blood flow. Duplex US revealed a mean diameter stenosis of 74 ± 7% and a mean minimal lumen diameter of 1.75 ± 0.50 mm following declotting. The underlying stenosis identified by fistulography had a mean diameter stenosis of 75 ± 13%, a mean minimal lumen diameter of 1.33 ± 0.85 mm, and a mean stenotic length of 2.9 ± 1.5 cm (1.0–5.9 cm). PTA reduced the diameter stenosis to 25 ± 6% and increased the minimal lumen diameter to 4.62 ± 0.98 mm. The average procedure time was 28.4 ± 9.9 min (14.2–41.9 min), and the average fluoroscopy time was 7.2 ± 4.1 min (2.2–15.6 min). No patients suffered major complications such as procedure-related deaths, pulmonary or arterial embolization, vessel rupture, and bleeding complications. Two fistulas (10%; 2 of 20) had small intimal dissection after high-pressure balloon dilatation but no influence on blood flow.
Table 2. Lesion characteristics of duplex and angiographic measurements
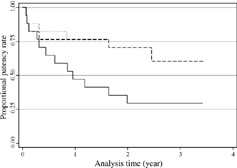
Technically successful cases were followed up periodically, with a maximum follow-up of 42 months (mean 24.4 ± 12.7 months). Six fistulas were abandoned owing to recurrent thrombosis; 5 patients underwent surgical clot removal or revision after referral for surgery by the dialysis center, and 1 patient suffered recurrent thrombotic events at 2, 4, and 11 months after the initial procedure, which was successfully treated by repeat manual declotting and PTA. However, the final intervention only kept the fistula patent for 3 days because marked intimal hyperplasia caused early recurrence of thrombus formation. This patient underwent surgical revision with interposition of new PTFE graft. Repeat PTAs were performed in nine fistulas because restenosis caused fistula dysfunction. Five fistulas remained patent following the initial procedure without further intervention. Fistula patency rates by Kaplan-Meier analysis revealed that the primary patency rates at 6, 12, 24, and 36 months were 65%, 47%, 35%, and 28%; the assisted primary patency rates at 6, 12, 24, and 36 months were 76%, 71%, 63%, and 63%; and the secondary patency rates at 6, 12, 24, and 36 months were 82%, 76%, 71%, and 63% ().
Discussion
This study showed manual declotting under duplex guidance is a simple, effective, and safe thrombolysis for selected patients with recent thrombosed native AVFs. The morphologic and functional characteristics of AVF determined by duplex US after declotting significantly reduced the radiation exposure time and the cost of percutaneous intervention. Vascular access failure secondary to thrombosis was a major cause of access function loss in hemodialysis patients. The major cause of thrombosis was progressive stenosis induced by turbulent flow just beyond the arteriovenous anastomosis, and the stenosis distal to anastomosis was often related to repeated puncturing with an arterial hemodialysis needle.Citation[13] American Dialysis Outcome Quality (DOQI) guidelines recommend early detection and dilatation of hemodynamically significant fistula stenosis,Citation[4] both to prolong fistula patency and to avoid the need to use a temporary hemodialysis catheter.
Duplex US was a noninvasive method for evaluating failing fistula and had a good correlation with fistulography, as reported by Merit et al.Citation[6] They found that the percentage stenosis measured by Doppler US was linearly related to the percentage stenosis measured by fistulography. The result in our study was similar (74% vs. 75%). Bacchini et al. reported the role and effectiveness of color flow Doppler US in dealing with the stenotic dialysis fistula.Citation[7] However, the role of duplex US in the management of thrombosed native AVFs was not reported previously. This study showed that the duplex US can easily estimate the thrombus burden and the anatomic structure of dialysis fistula prior to the attempted procedure, and disclose the stenosis and the blood flow during manipulation, as well as the appropriate puncture site for subsequent PTA.
Several mechanical or pharmacomechanical methods have been applied to treat thrombosed dialysis access.Citation[8-19] These methods include pulse-spray thrombolysis with urokinase supplementation and balloon maceration of residual clot,Citation[8&9] crossed catheter pharmacomechanical thrombolysis,Citation[10] and mechanical thrombectomy using thrombectomy catheter and clot aspiration.Citation[14&15] These methods have reported technical success rates of 75% ∼ 92%, but experiences were limited to PTFE grafts. Percutaneous interventions of thrombosed native AVFs were more difficult than those performed on PTFE grafts and had unfavorable results. Previous studies used hydrolyser catheter, hydrodynamic thrombectomy, and thromboaspiration to treat thrombosed native AVFs with a procedure success rate of 81% to 84%, a primary patency rate of 50% to 74% at 6 months. However, these techniques required multiple punctures and were time consuming, with a procedure time of 90 to 151 min.Citation[11], Citation[16&17] Zaleski et al. used angioplasty and bolus urokinase infusion to restore function of 17 thrombosed native fistulas, and achieved a procedure success rate of 82%, primary patency rate of 71%, and secondary patency rate of 100% at 6 months. However, the average procedure time was 1.7 h, and two or three crossed or uncrossed catheters were required. In addition, dilators should be left for 3 to 4 h to prevent bleeding from the puncture site.Citation[12] In this study, most recent thrombus can be fragmented by manual first and few residual thrombus was more easily treated by balloon maceration and clot aspiration. Those could explain our study had a shorter procedure and radiation exposure time and less numbers of catheter use as compared with previous reports. The primary and secondary patency rates at 6 and 12 months were 65% and 88%, and 47% and 76%, respectively, which was comparable to previous studies. The safety of manual declotting remained a concern, and thus patients with huge thrombus burden and occluded drainage vein were excluded in this study. Precautions were taken, such as compression of the arterial anastomosis site during manual declotting to prevent the clot from being pushed into the feeding artery. Maceration of clots by repeated compressions and heparin administration during the procedure reduced the risk of pulmonary embolism. Previous studies dealing with the issue of pulmonary embolism following declotting of PTFE grafts reported 35% ∼ 59% perfusion defects on ventilation-perfusion scan,Citation[20&21] but most patients were clinically silent. Therefore, emboli from pharmacomechanical or mechanical thrombolysis may be small or may have undergone spontaneous lysis before affecting the lungs.Citation[22] Although this study was free from embolic complications, silent pulmonary embolism may still ensue following manual declotting. In conclusion, manual declotting guided by duplex US for thrombosed dialysis fistula is a safe and feasible method for selective patients. The combination of duplex US and PTA can simplify percutaneous procedures, reduce costs and radiation exposure time, and also extend the life span of dialysis fistula while maintaining acceptable long-term patency.
Study Limitations
The technique described in this study has some limitations. First, only 29% thrombosed native fistula were suitable for this procedure because patients with huge thrombus burden, occluded, or inadequate size main drainage vein were not good candidates for this intervention, which may be better managed by surgical embolectomy or creation of new AVF. Second, manual declotting may be ineffective in thrombotic events lasting over 3 days, organized, or very soft thrombus. Third, ventilation-perfusion scan was not routinely performed, and the incidence of silent pulmonary embolism after manual declotting was unknown.
References
- Woods J D, Turenne M N, Strawderman R L, , et al. Vascular access survival among incident hemodialysis patients in the United States. Am J Kidney Dis. 1997;30(1):50–57. [PUBMED], [INFOTRIEVE], [CSA]
- Schwab S J. Vascular access for hemodialysis. Kidney Int. 1999;55(5):2078–2090. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Porile J L, Richter M L. Preservation of vascular access. J Am Soc Nephrol. 1993;4(4):997–1003. [PUBMED], [INFOTRIEVE], [CSA]
- National Kidney Foundation. DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. 1997;30 (4)(Suppl. 3):S150–S191., [CSA]
- Older R A, Gizienski T A, Wilkowski M J, , et al. Hemodialysis access stenosis: early detection with color Doppler US. Radiology. 1998;207(1):161–164. [PUBMED], [INFOTRIEVE], [CSA]
- Gadallah M F, Paulson W D, Vickers B, , et al. Accuracy of Doppler ultrasound in diagnosing anatomic stenosis of hemodialysis arteriovenous access as compared with fistulography. Am J Kidney Dis. 1998;32(2):273–277. [PUBMED], [INFOTRIEVE], [CSA]
- Bacchini G, Gappello A, Milia V L, , et al. Color Doppler ultrasonography imaging to guide transluminal angioplasty of venous stenosis. Kidney Int. 2000;58(4):1810–1813. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Valji K, Bookstein J J, Roberts A C, , et al. Pharmacomechanical thrombolysis and angioplasty in the management of clotted hemodialysis grafts: early and late clinical results. Radiology. 1991;178(1):243–247. [PUBMED], [INFOTRIEVE], [CSA]
- Beathard G A, Welch B R, Maidment H J. Mechanical thrombolysis for the treatment of thrombosed hemodialysis access grafts. Radiology. 1996;200(3):711–716. [PUBMED], [INFOTRIEVE], [CSA]
- Poulain F, Raynoud A, Bourquelot P, , et al. Local thrombolysis and thromboaspiration in the treatment of acutely thrombosed arteriovenous hemodialysis fistulas. Cardiovasc Interv Radiol. 1991;14(2):98–101. [CSA]
- Turmel-Rodrigues L, Sapoval M, Pengloan J, , et al. Manual thromboaspiration and dilatation of thrombosed dialysis access: mid-term results of a simple concept. J Vasc Interv Radiol. 1997;8(5):813–824. [PUBMED], [INFOTRIEVE], [CSA]
- Zaleski G X, Funaki B, Kenney S, , et al. Angioplasty and bolus urokinase infusion for the restoration of function in thrombosed Brescia-Cimino dialysis fistula. J Vasc Interv Radiol. 1999;10(2):129–136. [PUBMED], [INFOTRIEVE], [CSA]
- Turmel-Rodrigues L, Pengloan J, Baudin S, , et al. Treatment of stenosis and thrombosis in hemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant. 2000;15(12):2029–2036. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Trerotola S O, Lund G B, Scheel P J, Jr, , et al. Thrombosed hemodialysis access grafts: percutaneous mechanical declotting without urokinase. Radiology. 1994;191(3):721–726. [PUBMED], [INFOTRIEVE], [CSA]
- Sharafuddin M, Kadir S, Joshi S, , et al. Percutaneous balloon-assisted aspiration thrombectomy of clotted hemodialysis access grafts. J Vasc Interv Radiol. 1996;7(2):177–183. [PUBMED], [INFOTRIEVE], [CSA]
- Overbosch E H, Pattynama P M, Aorts H J, , et al. Occluded hemodialysis shunts: Dutch multicenter experience with the hydrolyser catheter. Radiology. 1996;201(2):485–488. [PUBMED], [INFOTRIEVE], [CSA]
- Vorwerk D, Sohn M, Schurmann K, , et al. Hydrodynamic thrombectomy of hemodialysis fistulas: first clinical results. J Vasc Interv Radiol. 1994;5(6):813–821. [PUBMED], [INFOTRIEVE], [CSA]
- Cohen M AH, Kumpe D A, Durham J D, , et al. Improved treatment of thrombosed hemodialysis access sites with thrombolysis and angioplasty. Kidney Int. 1994;46(5):1375–1380. [PUBMED], [INFOTRIEVE], [CSA]
- Schilling J J, Eiser A R, Slifkin R F, , et al. The role of thrombolysis in hemodialysis access occlusion. Am J Kidney Dis. 1987;10(2):92–97. [PUBMED], [INFOTRIEVE], [CSA]
- Swan T L, Smyth S H, Ruffenach S J, , et al. Pulmonary embolism following hemodialysis access thrombolysis/thrombectomy. J Vasc Interv Radiol. 1995;6(5):683–686. [PUBMED], [INFOTRIEVE], [CSA]
- Smits Van Rijk P P, Van Isselt J W, Mali W PTM, , et al. Pulmonary embolism after thrombolysis of hemodialysis access grafts. J Am Soc Nephrol. 1997;8(9):1458–1461. [CSA]
- Petronis J D, Regan F, Briefel G, , et al. Ventilation-perfusion scintigraphic evaluation of pulmonary clot burden after percutaneous thrombolysis of clotted hemodialysis access grafts. Am J Kidney Dis. 1999;34(2):207–211. [PUBMED], [INFOTRIEVE], [CSA]