Abstract
This study focuses on glutathione (GSH) level in red blood cells, as well as on glutathione peroxidases (GSH-Px) activities in red blood cells and in plasma of chronic renal failure (CRF) patients following renal transplantation. We want to focus our main attention on plasma GSH-Px, the selenoenzyme that is synthesized primarily in the kidney. In CRF patients, activity of this enzyme is significantly reduced, and the reduction decreases with the progress of the disease, reaching in the end-stage 20% to 30% of the activity of healthy patients. We have shown that following renal transplantation the activity of plasma GSH-Px is restored very rapidly, and 2 weeks after surgery it reached the value of the control group. Red blood cell GSH level is significantly higher in CRF patients, and following transplantation, no significant changes were observed. Red blood cell GSH-Px activity before transplantation was the same as in healthy patients and did not change significantly after surgery.
Introduction
We have read the paper written by De Vega et al.Citation[1] with great interest. It addresses the issue concerning the measurements of glutathione (GSH) concentration and GSH-related enzymes in blood of patients before and shortly after kidney transplantation. The authors studied the concentrations of reduced and oxidized glutathione in whole blood and the activities of glutathione peroxidase (GSH-Px), glutathione reductase, and glutathione-S transferase. The activities of all three enzymes were measured in red blood cells and plasma of 10 patients before and 2, 7, and 14 days after renal transplantation. The results were compared with the values found in 20 healthy subjects. Unfortunately, the authors did not present the history of patients recruited for renal transplantation and did not comment on the kind of transplanted kidneys (cadaveric or related donors). We assume the patients were in the end stage of chronic renal failure (CRF).
We want like to focus our attention on GSH concentration and GSH-Px activity in red blood cells and plasma. The authorsCitation[1] have shown that the GSH concentration in patients before surgery was 3.5 times lower than in healthy patients; its significant decrease 2 days after surgery was followed by increase; however, after 14 days it did not reach the values observed in the control group. The red cell GSH-Px activity in patients before surgery was lower by 60% as compared with healthy subjects and did not change significantly during a 2-week period after transplantation. Plasma GSH-Px activity in patients before surgery was by 44% lower than in controls, and only small, insignificant changes were observed during a 2-week period after surgery.
Patients and Methods
We have studied GSH concentration in red cells and GSH-Px activity in red cells and plasma of 17 patients (6 males and 11 females, ages 30–67 years; mean, 47.3 ± 12.0 years) in the end stage of CRF, before and after cadaveric kidney transplantation. Blood samples were collected into heparinized Vacutainer tubes just before the surgery and 3, 7, 14, and 30 days after transplantation. Part of the blood was centrifuged, and plasma was harvested. The red cells were washed in an excess of chilled 0.9% saline solution and hemolyzed by two cycles of freezing and thawing. GSH concentration was assayed by the colorimetric method of BeutlerCitation[2] with 5,5′-dithiobis (2-nitro benzoic acid); the results were expressed as mmol/L red blood cells. GSH-Px activities in red cell hemolysates and plasma were measured spectrophotometrically at 25°C, using the method of Paglia and ValentineCitation[3] with tert-butyl hydroperoxide as a substrate; the results were expressed as U/g Hb or U/L plasma. The results obtained in patients were compared with 15 healthy subjects (8 males and 7 females, ages 44–54 years, mean, 48.1 ± 3.1 years).
Results
The data of our study are summarized in . The results show that prior to transplantation, GSH concentration in red cells of CRF patients was significantly (p< 0.0001) higher as compared with the control group. During the first month after surgery, small differences were noted between mean GSH levels, but they did not differ significantly from the initial value. In the subsequent period, a slight albeit significant decrease was observed.
Table 1. GSH concentration and GSH-Px activity in red blood cells and plasma in patients before and after kidney transplantation and in healthy patients
We did not find any difference in red cell GSH-Px activities between CRF patients before transplantation and the control group. No changes were noted during a 30-day postsurgery period. Before kidney transplantation, plasma GSH-Px activity in CRF patients was by 41% lower (p< 0.0001) than in controls. However, following the surgery, a rapid increase in its activity was observed; 3 days after transplantation, plasma GSH-Px activity exceeded the initial value by 50% (p< 0.0001) and increased significantly thereafter, reaching the initial value 2 weeks after surgery.
Discussion
In patients with end stage of CRF, the antioxidant capacity was strongly reduced and the reduction was evidenced in blood components by several parameters.Citation[4-8] Among small molecular compounds, the peptide GSH plays an important role. As the most abundant antioxidant inside the cells, GSH can directly scavenge free radicals or act as a cosubstrate in the GSH-Px-catalyzed reduction of H2O2 and lipid hydroperoxides, the constituents of cell membrane.Citation[9&10]
A higher level of GSH in red cells of uremic patients found in our study could play a protective role due to hyperproduction of reactive oxygen species (ROS) in those patients.Citation[11] De Vega et al.Citation[1] found a significant decrease in GSH concentration 2 days after renal transplantation followed by its increase within 2 weeks. In our study, we did not observe any differences in GSH concentration within a month after surgery. Other authorsCitation[12&13] studied the GSH concentration in red cells of patients with transplants received more than 1 year earlier and did not find any changes as compared with the pretransplantation values.
Studies of GSH-Px activity in red cells of CRF patients reported significantly lower,Citation[4], Citation[7], Citation[14-18] significantly higher,Citation[5], Citation[19&20] and lower or higher albeit insignificantCitation[21&22] activity as compared with healthy controls. In our pretransplantation patients, this activity was the same as in the control group, whereas De Vega et al.Citation[1] noted extremely low activity of this enzyme in their patients. At the moment, we have no explanation of the disparate results. Observations similar to those of De Vega et al.,Citation[1] no changes in red cell GSH-Px activity, have been made in our investigations during a postsurgery period. The absence of changes in the activity of this enzyme shortly after surgery is not surprising because the GSH-Px synthesis occurs during erythropoiesis, and the life span of red cells is about 100 to 120 days.
Other authors did not observe any changes in red cell GSH-Px activity in female patients 4 and 10 years after surgery.Citation[13] In kidney transplant recipients, supplemented with selenium for 3 months after surgery, red cell GSH-Px activity increased significantly, but after cessation of selenium supply, it returned to the values observed in the control group.Citation[18]
All authors who studied plasma GSH-Px activity in CRF patients have demonstrated its significantly lower values as compared with healthy controls. The degree of the reduction of the activity depends on the stage of the disease. Several groups of researchers have shown that the higher the stage of the disease, the lower the activity of plasma GSH-Px.Citation[5], Citation[21], Citation[23] This may be explained by the fact that in humans the kidney is the main site of the plasma GSH-Px synthesis.Citation[24] This enzyme is then secreted to the circulation, and the decreased activity in plasma most likely results from the enzyme impaired synthesis in the kidney; thus, very quick synthesis of this enzyme after renal transplantation is not surprising. However, very little information on plasma GSH-Px activity in patients shortly after kidney transplantation is available. Within et al.Citation[25] demonstrated that in 16 patients with renal diseases who received kidney transplants from related donors, plasma GSH-Px activity increased very rapidly, and 3 days after transplantation, it was almost twice as high as that found in the pretransplant plasma samples (). On day 21 after transplantation, the averaged plasma GSH-Px activity was within a normal range, 2.8 times higher as compared with the initial value. In another study, in 6 patients with cadaveric kidney transplants, plasma GSH-Px activity also increased rapidly, and 9.8 days after surgery, reached the values observed in the control group. After 3 weeks, it exceeded the values of healthy patients, and after a lapse of a longer period of time since the kidney transplantation, plasma GSH-Px activity decreased to a range similar to that of normal individuals.
Figure 1. Plasma GSH-Px activities in pre- and postkidney transplant patients presented by different authors. Means ± standard deviation (SD) for plasma GSH-Px activity as the percentage of control plasma GSH-Px activities are given: our study (▴), studies carried out by Within et al.: cadaveric recipients (▪); related donor recipients (×); and study carried out by De Vega et al. (♦).
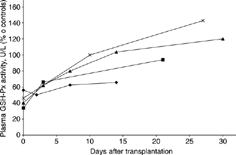
The results of our study are in agreement with the data presented by Within et al.Citation[25] that has also shown a rapid increase in plasma GSH-Px in transplant recipients. The results in both groups support an assumption that the transplanted kidney immediately takes on its function in the recipient body. Somewhat unexpected results were presented by De Vega et al.Citation[1] They have shown that during a 2-week period after surgery, only small, insignificant changes occurred in the plasma of transplant recipient. The authors speculate that the decrease in the enzyme activity, 48 hours after transplantation, may be caused by the attack of malondialdehyde (MDA) or by a decreased level of selenium. Such an explanation seems to be rather unlikely because the same authors showed in the same study that 48 h after surgery, the red cell MDA concentration was lower, although insignificantly, than that before transplantation.Citation[26] Moreover, we had measured MDA concentration in red cells and plasma (data not shown) and did not find any difference within a month after transplantation. In our opinion, neither selenium deficiency can be regarded as a causative factor. It has already been evidenced that in the end stage of CRF, selenium supplementation does not give rise to plasma GSH-Px activity.Citation[27&28] Thus, the question why changes in plasma GSH-Px activity do not occur after renal transplantation as shown by De Vega et al.Citation[1] needs to be further investigated.
References
- De Vega L, Fernandez R P, Martin Mateo M C, Bustamante J B, Herrero A M, Munguira E B. Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail. 2002; 24:421–432. [PUBMED], [INFOTRIEVE], [CSA]
- Beutler E. A manual of biochemical methods. In: Beutler E, ed. Red Cell Metabolism. Orlando, Fla: Grune & Stratton, 1984:131–134.
- Paglia D E, Valentine W N. Studies on quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169. [PUBMED], [INFOTRIEVE], [CSA]
- Bulucu F, Vural A, Aydin A, Sayal A. Oxidative stress status in adults with nephrotic syndrome. Clin Nephrol. 2000; 53:169–173. [PUBMED], [INFOTRIEVE], [CSA]
- Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, , et al. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med. 1996; 21:845–853. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Annuk M, Fellstrom B, Akerblom O, Zilmer K, Vihalemm T, Zilmer M. Oxidative stress markers in pre-uremic patients. Clin Nephrol. 2001; 56:308–314. [PUBMED], [INFOTRIEVE], [CSA]
- Martin-Mateo M C, Sanchez-Portugal M, Iglesias S, de Paula A, Bustamante J. Oxidative stress in chronic renal failure. Ren Fail. 1999; 21:155–167. [PUBMED], [INFOTRIEVE], [CSA]
- Loughrey C M, Young I S, Lightbody J H, McMaster D, McNamee P T, Trimble E R. Oxidative stress in haemodialysis. Q J Med. 1994; 87:679–683. [CSA]
- Forman H J, Liu R-M, Tian L. Glutathione cycling in oxidative stress. In: Clerch L B, Massaro D J, eds. Oxygen, Gene Expression and Cellular Function. New York, NY: Marcel Dekker, 1997:99–121.
- Valencia E, Marin A, Hardy G. Circadian rhythmically of whole-blood glutathione in healthy subjects. Nutrition. 2001; 17:731–733. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zachee P, Ferrant A, Daelemans R, Goossens W, Boogaerts M A, Lins R L. Reduced glutathione for the treatment of anemia during hemodialysis: a preliminary communication. Nephron. 1995; 71:343–349. [PUBMED], [INFOTRIEVE], [CSA]
- Cristol J.-P, Bosc J.-Y, Badiou S, , et al. Erythropoietin and oxidative stress in haemodialysis: beneficial effect of vitamin E supplementation. Nephrol Dial Transplant. 1997; 12:2312–2317. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Juskowa J, Paczek L, Laskowska-Klita T, Rancewicz Z, Gajewska J, Jedynak-Oldakowska U. Selected parameters of antioxidant capacity in renal allograft recipients (in Polish). Pol Arch Med Wewn. 2001; 105:19–27. [PUBMED], [INFOTRIEVE], [CSA]
- Pasaoglu H, Muhtaroglu S, Gunes M, Utas C. The role of oxidative state of glutathione and glutathione-related enzymes in anemia of hemodialysis patients. Clin Biochem. 1996; 29:567–572. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Richard M J, Arnaud J, Jurkovitz C, , et al. Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure. Nephron. 1991; 57:10–15. [PUBMED], [INFOTRIEVE], [CSA]
- Zachara B A, Trafikowska U, Adamowicz A, Nartowicz E, Manitius J. Selenium, glutathione peroxidases, and some other antioxidant parameters in blood of patients with chronic renal failure. J Trace Elem Med Biol. 2001; 15:161–166. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zachara B A, Adamowicz A, Trafikowska U, Trafikowska A, Manitius J, Nartowicz E. Selenium and glutathione levels, and glutathione peroxidase activities in blood components of uremic patients on hemodialysis supplemented with selenium and treated with erythropoietin. J Trace Elem Med Biol. 2001; 15:201–208. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hussein O, Rosenblat M, Refael G, Aviram M. Dietary selenium increases cellular glutathione peroxidase activity and reduces the enhanced susceptibility to lipid peroxidation of plasma and low density lipoprotein in kidney transplant recipients. Transplantation. 1997; 63:679–685. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mimic-Oka J, Simic T, Ekmescic V, Dragicevic P. Erythrocyte glutathione peroxidase and superoxide dismutase activities in different stages of chronic renal failure. Clin Nephrol. 1995; 44:44–48. [PUBMED], [INFOTRIEVE], [CSA]
- Zima T, Stipek S, Crkovska J, , et al. Antioxidant enzymes—superoxide dismutase and glutathione peroxidase—in haemodialyzed patients. Blood Purif. 1996; 14:257–264. [PUBMED], [INFOTRIEVE], [CSA]
- Yoshimura S, Suemizu H, Nomoto Y, , et al. Plasma glutathione peroxidase deficiency caused by renal dysfunction. Nephron. 1996; 73:207–211. [PUBMED], [INFOTRIEVE], [CSA]
- Sommerburg O, Grune T, Ehrich J H.H, Siems W G. Adaptation of glutathione peroxidase activity to oxidative stress occurs in children but not in adult patients with end-stage renal failure undergoing hemodialysis. Clin Nephrol. 2002; 58(Suppl. 1):S31–S36. [PUBMED], [INFOTRIEVE], [CSA]
- Zachara B A, Salak A, Koterska D, Manitius J, Wasowicz W. Selenium and glutathione peroxidases in blood of patients with different stages of chronic renal failure. J Trace Elem Med Biol. 2004; 17:291–299. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Avissar N, Ornt D B, Yagil Y, , et al. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol. 1994; 266(Cell Physiol. 35):C367–C375. [PUBMED], [INFOTRIEVE], [CSA]
- Whitin J C, Tham D M, Bhamre S, , et al. Plasma glutathione peroxidase and its relationship to renal proximal tubule function. Mol Genet Metab. 1998; 65:238–245., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fernandez R P, Martin Mateo M C, De Vega L, Bustamante J B, Herrero M, Munguira E B. Antioxidant enzyme determination and a study of lipid peroxidation in renal transplantation. Ren Fail. 2002; 24:353–359. [CSA], [CROSSREF]
- Zachara B A, Adamowicz A, Trafikowska U, Pilecki A, Manitius J. Decreased plasma glutathione peroxidase activity in uremic patients. Nephron. 2000; 84:278–279. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zachara B A, Koterska D, Manitius J, , et al. Selenium supplementation on plasma glutathione peroxidase activity in patients with end-stage chronic renal failure. Biol Trace Elem Res. 2004; 97:15–30. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]