Abstract
Hypertonic glycerol injection is one of the most frequently used models of experimental acute renal failure. Late structural changes such as interstitial fibrosis in the renal cortex and tubular atrophy have been detected after severe acute tubular necrosis (ATN). The aim of this study was to investigate the expression of angiotensin II (AII) and endothelin during the evolution of the ATN induced by glycerol and their relationships with the late structural changes observed in the kidneys. Forty-nine male Wistar rats were injected with a 50% glycerol solution, 8 mL/kg, divided into equal amounts, each administered into one hind leg, and 18 with 0.15 M NaCl solution. Blood and urine samples were collected 1, 5, 30, and 60 days after the injections to quantify sodium and creatinine; the animals were killed and the kidneys removed for histologic and immunohistochemical studies. The results of the immunohistochemical studies were scored according to the extent of staining in the cortical tubulointerstitium. Glycerol-injected rats presented a transitory increase in plasma creatinine levels and in fractional sodium excretion. The immunohistochemical studies showed increased AII and endothelin staining in the renal cortex from rats killed 5 days after glycerol injection (p < 0.001) compared with control that persisted until day 60. The animals killed on days 30 and 60 also presented chronic lesions (fibrosis, tubular dilatation, and atrophy) in the renal cortex, despite the recovery of renal function. AII and endothelin may have contributed to the development of renal fibrosis in these rats.
Introduction
Renal function recovery post-acute tubular necrosis (ATN) is normally slow and may be incomplete, probably due to the loss of a certain number of nephrons.Citation[1] One of the most widely used model of myoglobinuric acute renal failure (ARF) and ATN is produced by intramuscular injection of hypertonic glycerol. The pathogenesis of ARF induced by glycerol may involve, among other causes, decreased renal blood flow, reactive oxygen metabolites, and myoglobin releasing from muscle damage.Citation[2-4] We observed in a previous study that rats treated with glycerol present on days 30 and 60 after the injection chronic lesions such as fibrosis, tubular dilatation, and atrophy in renal cortex.Citation[5] Late structural changes such as interstitial fibrosis in the renal cortex and tubular atrophy were also found after severe acute ischemiaCitation[6-8] and after gentamicin-induced acute renal injury.Citation[1] However, a long-term study with the evaluation of angiotensin II (AII) and endothelin expression, and their relationship with renal function and structure in rats with ATN induced by glycerol, has not been performed.
Increased renal production of endothelin and AII was observed in ATN.Citation[4], Citation[8&9] These polypeptides can provoke proliferation and modify the phenotypes of renal and extrarenal cells (macrophages).Citation[10-12] After activation, these cells transform into myofibroblasts, increase the production of extracellular matrix components (ECMs) and start to express α-SM-actin (α-smooth muscle actin), a protein that is normally expressed in renal cortex only by vascular smooth muscle cells. AII is a potent inducer of several fibrogenic cytokines, including transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF), and some of its effects are mediated by the expression of these factors.Citation[13&14] Endothelin can also stimulate fibroblast proliferation and production of ECM.Citation[9&10], Citation[15]
The aim of this study was to investigate the expression of AII and endothelin in the kidney during the evolution of ATN induced by glycerol, as well as their relationship with long-term histologic changes and renal function of these animals.
Materials and Methods
Animals and Experimental Protocols
Forty-nine male Wistar rats were injected with a 50% glycerol solution, 4 mL/kg applied IM to each hind leg and 18 with 0.15 M NaCl solution. Before glycerol injection on day 1, water was removed for 17 h. Twenty-four glycerol-injected animals died after injection. Blood and urine samples were collected from the surviving animals (25 rats) 1, 5, 30, and 60 days after the glycerol injection to quantify sodium and creatinine. The animals were killed 5, 30, and 60 days after the injections, the organs were perfused with PBS solution (0.15 M NaCl and 0.01 M sodium phosphate buffer, pH 7.4), and the kidneys removed for histologic and immunohistochemical studies.
Renal Function Studies
Plasma creatinine was measured by the Jaffé method and plasma, urine sodium (Na+), and potassium (K+) by flame photometry (Micronal, model 262, São Paulo, Brazil), and urine osmolality was determined by freezing point depression (Fiske OS Osmometer, Norwood, MS, USA). Glomerular filtration rate (GFR) was measured by inulin clearance in 8 control animals and in 8 rats killed on day 30 after glycerol injection. The animals were anesthetized with an IP injection of 50 mg/kg sodium thionembutal. After tracheostomy, the femoral artery and vein were cannulated to collect blood samples and to inject fluids, and the ureters were cannulated to collect urine. The animals received a priming insulin dose of 12 mg/100 g followed by a maintenance dose of 30 mg/100 g/h of insulin. After stabilization for about 60 min, urine was collected for a period of 60 min and blood was sampled at 30 and 60 min. Plasma and urine inulin was measured by Füehr method.Citation[16]
Light Microscopy
The kidneys from 10 control animals and 25 from rats killed 5, 30, and 60 days after glycerol injection were fixed in 4% paraformaldehyde, postfixed in Bouin's solution for 4 to 6 h, and processed for paraffin embedding. Four-micrometer histologic sections were stained with Masson's trichrome and examined under the light microscope.
Antibodies
Primary antibodies included 1) a rabbit polyclonal antiangiotensin II antibody (Peninsula Laboratories, Inc., San Carlos, CA, USA) and 2) a rabbit polyclonal antiendothelin antibody (Peninsula Laboratories, Inc.).
Immunohistochemical Studies
Ten control animals, 8 animals killed 5 days after glycerol injection, 8 animals killed 30 days after injection, and 9 animals killed 60 days after injection were used for this study. The rats were submitted to aortic perfusion with PBS (0.15 M NaCl and 0.01 phosphate buffer, pH 7.4) until the kidneys were blanched. The kidneys were then perfused with 4% paraformaldehyde, fixed in paraformaldehyde 4% for 2 h, postfixed in Bouin's solution for an additional 4 to 6 h, rinsed with 70% ethanol to eliminate picric acid, dehydrated through a graded alcohol series, embedded in paraffin, sectioned into 3-µm slices, deparaffinized, and subjected to immunohistochemical staining.Citation[17&18]
The sections were incubated overnight at 4°C with 1/200 antiangiotensin II or 1/500 antiendothelin polyclonal antibodies. The reaction product was detected with an avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA, USA). The color reaction was developed with 3,3′-diaminobenzidine (Sigma Chemical Co., St. Louis, MO, USA), and the material was counterstained with methylgreen, dehydrated, and mounted. Nonspecific protein binding was blocked by incubation with 20% goat serum in PBS for 20 min. Negative controls consisted of replacement of primary antibody with equivalent concentrations of normal rabbit IgG.
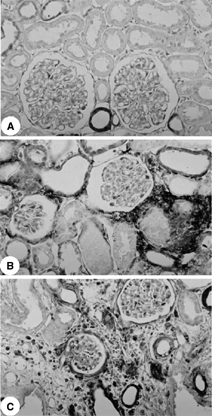
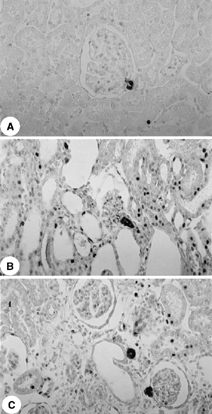
For evaluation of immunoperoxidase staining for endothelin, each tubulointerstitial grid field was graded semiquantitatively and the mean score per kidney was calculated.Citation[17] Each score reflected mainly changes in the extent, rather than the intensity, of staining and depended on the percentage of grid field showing positive staining: 0, absent or less than 5%; I, 5% to 25%; II, 25% to 50%; III, 50% to 75%, and IV, > 75%. To obtain mean numbers of infiltrating AII cells in the renal cortical tubulointerstitium, 30 grid fields measuring 0.245 mm2 each were evaluated, and mean counts per kidney were calculated.
Statistical Analysis
Data were submitted to analysis of variance with multiple comparisons by the Tukey test, with the level of significance set at p < 0.05.
Results
Renal Function
All glycerol-treated rats presented a transitory increase that peaked at day 1 after glycerol injection in plasma creatinine levels (1.84 ± 0.17 mg/dL) and in fractional excretion of sodium (9.22 ± 1.09%) and potassium (214 ± 36.4%) (p < 0.01) that returned to normal levels at day 30 (0.69 ± 0.05 mg/dL, 0.44 ± 0.05%, and 46.05 ± 2.84%, respectively). The GFR measured on day 30 after glycerol injection was 0.62 ± 0.05 mL/min/100 g body weight and did not differ from the control group (0.85 ± 0.09 mL/min/100 g). We also observed a transitory decrease in urine osmolality after glycerol injection (752 ± 77 mOsm/kg H2O) compared with control (2160 ± 215 mOsm/kg H2O).
Light Microscopy Studies
Light microscopy studies showed the following morphologic features characteristic of ATN in the renal cortex of rats on day 5 after glycerol injection: tubular cell necrosis, focal areas of denuded basement membrane, intraluminal casts, swelling and flattening of proximal tubular cells with brush border loss, diffuse interstitial edema, and interstitial inflammatory cell infiltrates. Glomerular morphology remained unchanged. However, in the animals killed on days 30 and 60 after glycerol injection, we found histologic alterations characteristic of chronic nephropathy such as interstitial fibrosis, tubular atrophy, and dilatation and inflammatory cell infiltrates.
Immunohistochemical Studies
The immunohistochemical studies showed increased AII and endothelin staining in the tubulointerstitium from the renal cortex of rats killed 5, 30, and 60 days after glycerol injection compared with control (p < 0.001), with a diffuse distribution on day 5 and a focal distribution primarily located in the damaged areas on days 30 and 60 ( and and ). The control rats presented angiotensin staining confined to the juxtaglomerular apparatus.
Table 1. Score for endothelin staining in cortical tubulointerstitium and number of AII-positive cells per grid field of renal cortex measuring 0.245 mm2 from control rats (C) and from rats killed 5 (G-5 days), 30 (G-30 days), and 60 days (G-60 days) after glycerol (G) injection
Discussion
Our data show that glycerol-injected rats presented transitory alterations in renal function and histologic changes in the renal cortex characteristic of ATN on day 5 after treatment. However, interstitial fibrosis, tubular atrophy, or dilatation was found in the renal cortex from the rats killed on days 30 and 60, despite the recovery of renal function. Probably the preserved areas from renal cortex of these animals were still sufficient to maintain renal function unchanged. Residual areas of interstitial fibrosis in renal cortex have been observed after ATN induced by nephrotoxic and ischemic injury.Citation[1], Citation[5-8]
Free radical releasing by the damaged tissues may induce the production of inflammatory substances, such as endothelin, angiotensin and chemotactic factors for macrophages and lymphocytes (MCP, osteopontin, RANTES).Citation[19] Macrophages are cells potentially able to release fibrogenic cytokines (TGF-β, PDGF, endothelin) and angiotensin, which can contribute to the progression of renal lesions to fibrosis and make renal function recovery more difficult.Citation[10], Citation[20] The renal fibrosis depends, among other factors, on the interaction between renal and extrarenal cells (macrophages) and cytokines. Cytokines such as TGF-β, PDGF, endothelin, and AII act on these cells (renal tubular cells, fibroblasts, macrophages) by inducing proliferation and modifying phenotypes. These cells start to express α-smooth muscle actin and increase the production of collagen and other matrix components.Citation[21] The accumulation of extracellular matrix proteins within the interstitial space lead to peritubular obliteration and hypoxia, which results in tubular atrophy, activation of macrophages, and increase of angiotensin and cytokines production.Citation[21] AII and free radicals are also able to induce the activation of nuclear factor-κB (NF-κB). Upon stimulation, NF-κB is released from an inhibitory subunit (IκB) and translocates into the nucleus, where it promotes the transcriptional activation of target genes, inducing the synthesis of inflammatory and fibrogenic substances (cytokines, growth factors, enzymes, chemotactic factors for macrophages and monocytes), which provoke damage to the kidneys.Citation[22]
The immunohistochemical studies showed an increased immunoreaction for AII and endothelin in the tubulointerstitial area from the renal cortex of glycerol-injected animals that persisted until days 30 and 60. Angiotensin II may be involved in the development of renal fibrosis in these glycerol-treated rats. Johnson et al. demonstrated that rats chronically infused with AII develop tubulointerstitial injury with tubular atrophy and dilatation, cast formation, interstitial monocyte infiltration, and interstitial fibrosis.Citation[12] This polypeptide is a potent inducer of TGF-β, and PDGF, and some of its effects are mediated by the expression of these factors.Citation[13&14] TGF-β has been considered to be one of the major fibrogenic cytokines. This polypeptide enhances the synthesis of matrix components and blocks matrix degradation, thus promoting ECM deposition.Citation[10], Citation[13], Citation[21], Citation[23] AII may also participate in the fibrogenesis because of its implication in the inflammatory process through the synthesis of chemotactic factors, such as monocyte chemoattractant protein-1 (MCP-1).Citation[24] In a previous study, we also found increased immunoreaction for α-SMA, TGF-β, and a larger number of ED1-positive cells (macrophages/monocytes) in the tubulointerstitial area from the renal cortex of glycerol-injected rats killed on days 5, 30, and 60.Citation[5] Myofibroblasts and macrophages can be contributing to the fibrosis observed in glycerol-injected rats by releasing of fibrogenic peptides such as TGF-β, interleukin I, endothelin, and AII, and, in addition, these cells can produce collagen and other ECM components.Citation[10] Pagtalunan et al. found that the reduction of angiotensin activity by treatment with enalapril prevents late secondary glomerular injury and reduces the proteinuria provoked by acute renal ischemia.Citation[8] However, this treatment did not modify the interstitial fibrosis observed during the recovery from ischemic injury.
The glycerol-injected animals also presented increased immunostaining for endothelin in renal cortex located primarily in interstitial and tubule cells. Increased endothelin production was observed in ATN of different causes.Citation[4],Citation[9] Endothelin can promote recruitment of monocytes/macrophages,Citation[25] and fibrosis by upregulating TGF-β expression, directly stimulating matrix synthesis, and decreasing collagenase activity.Citation[10&11] Due to its vasoconstrictive properties, renal production of endothelin may intensify the glycerol-induced ischemic damage.Citation[21] Endothelin antagonists and/or endothelin receptor blockers have been reported to preserve renal function and to decrease the severity of tubulointerstitial damage in experimental models of chronic cyclosporine nephrotoxicity, lupus nephritis, and 6-month postischemia.Citation[9], Citation[26&27] However, it was found that the blockade of both endothelin A and B receptors seems to be detrimental to long-term kidney function in ischemic acute renal failure.Citation[27] The animals treated with both endothelin receptors antagonists had, at 6 months postischemia, significantly lower creatinine clearances and higher proteinuria compared with ischemic group untreated.
In conclusion, taken together, these data show that the renal damage induced by glycerol can progress to fibrosis, despite the recovery of renal function, and suggest that AII and endothelin may contribute to this process.
Acknowledgments
The authors thank Erika Dellaiogono and Rubens Fernando de Melo for expert technical assistance. Research supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil. Roberto Silva Costa and Terezila Machado Coimbra are recipients of fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico, DF, Brazil.
References
- Cronin R E, Henrich W L. Toxic nephropathy. In: Brenner B M, ed. The Kidney.Philadelphia, Pa: WB Saunders, 2000:1563–1596.
- Abulezz S R, Walker P D, Shah S V. Role of glutathione in an animal model of myoglobinuric acute renal failure.Proc Natl Acad Sci U S A. 1991;88:9833–9837. [CSA]
- Zager R A, Durkhart K M, Conrad D S, Gmur D J. Iron, heme oxygenase, and glutathione: effects on myoglobinuric proximal tubular injury.Kidney Int. 1995;48:1624–1634. [PUBMED], [INFOTRIEVE], [CSA]
- Shimizu T, Kuroda T, Ikeda M, Hata S, Fujimoto M. Potential contribution of endothelin to renal abnormalities in glycerol-induced acute renal failure in rats.J. Pharmacol. Exp. Ther. 1998;286:977–983. [PUBMED], [INFOTRIEVE], [CSA]
- Soares T J, Costa R S, Volpini R A, Silva C G.A, Coimbra T M. Long-term evolution of the acute tubular necrosis (ATN) induced by glycerol: role of myofibroblasts and macrophages.Int. J. Exp. Pathol. 2002;83:165–172. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fox M. Progressive renal fibrosis following acute tubular necrosis. An experimental study.J. Urol. 1967;97:196–202. [PUBMED], [INFOTRIEVE], [CSA]
- Pagtalunan M E, Olson J L, Tilney N L, Meyer T W. Late consequences of acute ischemic injury to a solitary kidney.J Am Soc Nephrol. 1999;10:366–3783. [PUBMED], [INFOTRIEVE], [CSA]
- Pagtalunan M E, Olson J L, Meyer T W. Contribution of angiotensin II to late injury after acute ischemia.J Am Soc Nephrol. 2000;11:1278–1286. [PUBMED], [INFOTRIEVE], [CSA]
- Forbes J M, Leaker B, Hewitson T D, Becker G J, Jones C L. Macrophage and myofibroblast accumulation in ischemic acute renal failure is attenuated by endothelin receptor antagonists.Kidney Int. 1999;55:198–208. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Eddy A A. Molecular insights into renal interstitial fibrosis.J Am Soc Nephrol. 1996;7:2495–2508. [PUBMED], [INFOTRIEVE], [CSA]
- Xu S W, Denton C P, Dashwood M R, , et al. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1.J Invest Dermatol. 2001;116:417–425. [CSA], [CROSSREF]
- Johnson R J, Alpers C E, Yoshimura A, , et al. Renal injury from angiotensin II-mediated hypertension.Hypertension. 1992;19:464–474. [PUBMED], [INFOTRIEVE], [CSA]
- Sharma K, Ziyadeh F N. The emerging role of transforming growth factor β in kidney disease.Am J Physiol. 1994;266:F829–F842. [PUBMED], [INFOTRIEVE], [CSA]
- Border W A, Noble N A. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis.Hypertension. 1998;31(suppl):181–188. [PUBMED], [INFOTRIEVE], [CSA]
- Bruzzi I, Corna D, Zoja C, , et al. Time course and localization of endothelin-1 gene expression in a model of renal disease progression.Am. J. Pathol. 1997;151:1241–1247. [PUBMED], [INFOTRIEVE], [CSA]
- Füehr Y, Kaczmarszk Y, Kruttgen G D. Eine einfache colorimetrische Methode zur Inulin Bestimmung für Nieren Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern.Klin. Wochenschr. 1955;33:729–730. [CSA], [CROSSREF]
- Coimbra T M, Janssen U, Gröne H J, , et al. Early events leading to renal injury in obese Zucker (fatty) rats type II diabetes.Kidney Int. 2000;57:167–182. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Baroni E A, Costa R S, Silva C GA, Coimbra T M. Heparin treatment reduces glomerular injury in rats with adriamycin-induced nephropathy but does not modify tubulointerstitial damage or the renal production of transforming growth factor-beta.Nephron. 2000;84:248–257. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Schllöndorff D. The role of chemokines in the initiation and progression of renal disease.Kidney Int. 1995;47(Suppl. 49 S44–S47. [CSA]
- Kliem V, Johnson R J, Alpers C E, , et al. Mechanism involved in the pathogenesis of tubulointerstitial fibrosis in 5/6 nephrectomized rats.Kidney Int. 1996;49:666–678. [PUBMED], [INFOTRIEVE], [CSA]
- Eddy A A. Molecular basis of renal fibrosis.Pediatr Nephrol. 2000;15:290–301. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Guijarro C, Egido J. Transcription factor κB (NF-κB) and renal disease.Kidney Int. 2001;59:415–424. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Coimbra T M, Wiggins R C, Noh J W, Merritt S, Phan S. Transforming growth factor-β production in anti-glomerular basement membrane in the rabbit.Am J Pathol. 1991;138:223–234. [PUBMED], [INFOTRIEVE], [CSA]
- Mezzano S A, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis.Hypertension. 2001;38:635–638. [PUBMED], [INFOTRIEVE], [CSA]
- Achmad T H, Rao G S. Chemotaxis of human blood monocytes toward endothelin-1 and the influence of calcium channel blockers.Biochem Biophys Res Commun. 1992;189:994–1000. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Benigni A. Endothelin antagonists in renal disease.Kidney Int. 2000;57:1778–1794. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Forbes J M, Hewitson T D, Becker G J, Jones C L. Simultaneous blockade of endothelin A and B receptors in ischemic acute renal failure is detrimental to long-term kidney function.Kidney Int. 2001;59:333–1341. [CSA], [CROSSREF]