Abstract
This study was designed to determine the possible protective effect of mibefradil on renal ischemia/reperfusion (I/R) injury. Unilaterally nephrectomized Sprague-Dawley rats were subjected to 60 min of left renal ischemia followed by 45 min of reperfusion. Group 1 were sham-operated animals; group 2, I/R/untreated animals; and group III, I/R/mibefradil-treated animals. A 99mTc-DTPA scan was taken to measure kidney perfusion, glomerular filtration rate (GFR) and the time elapsed from isotope injection to the maximum of the curve. Serum creatinine, blood urea nitrogen (BUN), kidney malondialdehyde (MDA) level were determined as well as examining the kidneys histologically. Treatment of rats with mibefradil produced a significant reduction in the serum levels of creatinine and urea nitrogen. T-max-sec (renal perfusion) was significantly lower in group 2 than in groups 1 and 3. The GFR was markedly greater in group 3 than in the group 2. The Tmax-min was significantly greater in group 2 than in group 3. Mibefradil treatment significantly decreased the MDA levels. The histopathologic score was significantly less in the group 3 rats compared with group 2 rats. Kidneys of group 2 rats showed tubular cell swelling, cellular vacuolization, pyknotic nuclei, medullary congestion, and moderate to severe necrosis. Treatment with mibefradil preserved the normal morphology of the kidney and shows normal glomeruli and slight edema of the tubular cells. These findings suggest that mibefradil reduces the renal dysfunction associated with I/R of the kidney.
Introduction
Renal ischemia observed in a variety of clinical situation, such as cardiac arrest with recovery, arterial occlusion, organ transplantation, and heminephrectomy is a common cause of renal cell death, renal failure, delayed graft functionCitation[1&2] and renal graft rejection.Citation[3&4] Thus, kidney injury caused by ischemia/reperfusion (I/R) is a major focus for both fundamental and clinical research. The acute renal failure (ARF) observed after ischemia is characterized by decreased glomerular filtration rate, tubular necrosis, and increased renal vascular resistance.Citation[5] The formation of reactive oxygen species (ROS) may be related to oxidative damage to the nucleic acids, proteins, and lipids.Citation[6&7] A number of processes include disturbances of cell calcium metabolism, disruption of the generation of free radicals, activation of phospholipases with production of toxic lipid metabolites, and loss of cell volume and monovalent cation homeostasis have been implicated in the pathogenesis of oxygen deprivation-induced cell injury.Citation[8]
Mibefradil is a calcium antagonist (CA) that belongs to a new structural class of benzimidazole-substituted tetraline derivatives.Citation[9] Unlike traditional CAs, mibefradil binds to a unique receptor siteCitation[10] and blocks both T- and L-type calcium channels with a high selectivity for the T-type calcium channels.Citation[11&12] Administration of mibefradil was shown to reduce afferent and efferent arteriolar resistances in spontaneously hypertensive rats,Citation[13] increase renal blood flow in vivo in dogs,Citation[14] and block the ANG II-induced efferent arteriolar constriction observed in the isolated, perfused hydronephrotic rat kidney.Citation[15] We are unaware of any previous studies using mibefradil in I/R injury of the kidney. Then, this study was designed to determine the possible protective effect of mibefradil during I/R injury of the kidney, by determining biochemical parameters and histologic examination.
Materials and Methods
Thirty male Sprague-Dawley rats weighing 200 to 220 g were used in the study. All the experimental protocols were performed according to the guidelines for the ethical treatment of experimentation animals.
Animals and Experimental Protocol
The rats were housed individually in cages and were allowed free access to standard rat chow and water before and after the experiments. The animal rooms were windowless with temperature (22 ± 2°C) and lighting controls. The animals were fasted overnight before the experiments but were given free access to water. They were anesthetized by 100 mg/kg ketamine and 20 mg/kg xylazine body weight, IP. The right femoral vein was cannulated for administration of drugs and saline. The animals were randomly separated into three groups, each containing 10 rats.
Sham-control group (G1, n = 10): For determining the basal values of histologic parameters, we performed a sham operation. The abdomen was dissected, the right kidney was harvested, and then the left renal pedicle exposed. However, renal clamps were not applied.
I/R/untreated group (G2, n = 10): Rats were subjected to the surgical procedures described as follows and underwent left renal ischemia for 60 min, followed by reperfusion for 45 min.
I/R/mibefradil-treated group (G3, n = 10): The same surgical procedure as in group 2 was performed. Calcium channel antagonist, mibefradil (0.1 µM/kg, IV), was administered at the starting time of reperfusion.
Ischemic Reperfusion Model
The abdomen was dissected under anesthesia, the right kidney was harvested, and then the left renal artery and vein were clamped with a hemostasis clip for 60 min. The clip was subsequently removed to permit reperfusion. The abdomen was closed during I/R. Blood samples (4–5 mL) were collected at 2 h after reperfusion from the abdominal aorta. Plasma was separated by centrifugation (3000 RPM for 10 min at room temperature). The serum samples were stored at 280°C for the measurement of blood urea nitrogen (BUN) and creatinine. The rats were sacrificed at 2 h after reperfusion, and the kidney tissue was harvested. Sham-operated control rats underwent the same surgical procedure, including dissection of the renal pedicle. However, renal clamps were not applied.
At the end of the reperfusion, a 99mTc-DTPA diuretic scan was taken to measure kidney perfusion (Tmax-sec), GFR (mL/min), and the time elapsed from isotope injection to the maximum of the curve (Tmax-min).
Renal Scintigraphy
Scintigraphic images were obtained with a large field-of-view gamma camera (GCE-601 digital gamma camera, Toshiba, Japan), equipped with a low-energy, parallel hole collimator (140-keV peak, 20% energy window). Renal scintigrams were taken in the posterior projection. A dose of 37 MBq 99mTc-DTPA (Amersham, UK) was injected rapidly through the femoral vein catheter. Data were stored in two time series; one of 64 frames at 5 s/frame and a second series of 80 frames at 5 s/frame. Static images were also obtained simultaneously for a total of 2 min. The isotope syringe was measured before and after the injection to calculate values of GFR for the left kidney. Regions of aorta and the left kidney, as well as individual regions for background subtraction, were outlined. From these regions, one curve representing the time variation of activity in the blood pool and two curves representing the background-subtracted renogram were extracted, and the Tmax-sec, Tmax-min, and GFR (mL/min) of the left kidney calculated.
Blood Biochemistry
BUN and creatinine were measured as indicators of disorders of glomeruli and endothelial cell. The activities of BUN and creatinine in plasma were determined by standard autoanalyzer methods on an Abbot Aeroset (USA).
Histopathologic Evaluation
The extracted kidneys were divided into two pieces. One piece was fixed in 10% buffered formalin and embedded in paraffin. Sections were cut at 5 µm, and coded kidney specimens were stained with hematoxylin and eosin and examined in blinded fashion. Histologic changes were evaluated by quantitative measurement of tubulointerstitial injury, which was assessed by counting the number of necrotic and apoptotic cells, loss of tubular brush border, tubular dilatation, cast formation, and neutrophil infiltration. The scoring was 0 = none, 1 = 10%, 2 = 11% to 25%, 3 = 26% to 45%, 4 = 46% to 75%, and 5 = 76%.
Biochemical Analyses
The other piece was washed in ice-cold 0.9% saline solution, weighted and stored at − 70°C. Homogenates of the tissues were prepared as 1.0 g/10 mL in 250 mM sucrose, 1 mM EDTA, 1 mM-dl-dithiothreitol, and 15 mM Tris HCl (pH 7.4), using an all-glass Potter Elvehjem homogenizer (Selecta, Barcelona, Spain). Each homogenate was centrifuged for 20 min at 800 g. The resulting supernatant fraction was used to determine enzyme activities. The protein concentrations of the supernatant were determined by the method described by Bradford.Citation[16]
Malondialdehyde Determination
Renal MDA levels were determined by Wasowiczs's method,Citation[17] based on the reaction of MDA with thiobarbituric acid at 95°C to 100°C. Fluorescence intensity was measured in the upper n-butanol phase by a fluorescence spectrophotometry (Hitachi, Model F-4010) adjusted exitation at 525 nm and emission at 547 nm. Arbitrary values obtained were compared with a series of standard solutions (1,1,3,3 tetramethoxypropane). Results were expressed as nanomole per gram wet testes tissue (nmol/g wet tissue).
Protein Assays
The protein content of homogenates was determined according to the procedure of Lowry et al.Citation[18]
Statistical Analysis
Data were entered and analyzed on an IBM-compatible personal computer using SPSS version 9.0. All values were expressed as mean ± SD. The significance of the data obtained was evaluated by using analysis of variance (ANOVA). Differences between means were analyzed by using the post-ANOVA (Tukey's b) test. P values of less than 0.05 were considered significant.
Results
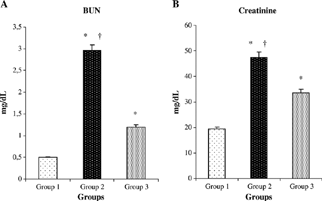
Rats that underwent renal I/R/untreated exhibited significant increase in serum concentrations of creatinine and BUN as compared with sham-operated animals, suggesting a significant degree of glomerular dysfunction mediated by renal I/R. Treatment of rats with mibefradil produced a significant reduction in the serum levels of creatinine and BUN compared with renal I/R groups (p < 0.0001, p < 0.0001) ( and ).
Figure 1. Effect of mibefradil on serum creatinine (A) and blood urea nitrogen (BUN) (B) in rats exposed to renal I/R. Values expressed as mean ± SEM. *p < 0.0001 as compared with group 1. †p < 0.0001 as compared with group 3.
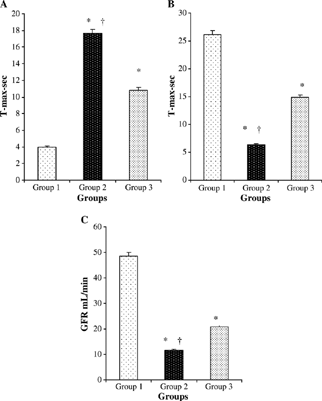
Tmax-sec (renal perfusion) was significantly lower in group 2 than in groups 1 and 3. (p < 0.0001, p < 0.0001, respectively). The GFR was markedly greater in group 3 than in group 2. There were significant differences in Tmax-min among the control group and other experimental groups (all P < 0.0001). The Tmax-min was significantly greater in group 2 than in group 3 (p < 0.0001) (–).
Figure 2. Comparative Tmax-min (A), Tmax-sec, (B) and GFR (mL/min) (C) measurements at the groups. Values are mean ± SEM. *p < 0.0001 compared with group 1. †p < 0.0001 compared with group 3.
The MDA content in control group (5.2 ± 0.7 nmol/g) was elevated by I/R injury (18 ± 1.3 nmol/g, p < 0.001). Mibefradil treatment significantly decreased the MDA levels (6.7 ± 0.6 nmol/g) in the I/R/untreated group (p < 0.0001) ().
Figure 3. Comparative MDA (A) and histologic score (B) measurements at the groups. *p < 0.05 compared with group 1. †p < 0.05 compared with group 3. Values are mean ± SEM. MDA, malondialdehyde.
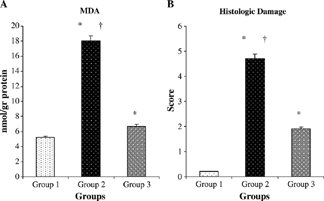
The histopathologic score was found as 0.2 ± 0.1, 4.7 ± 0.5, and 1.9 ± 0.3 in groups 1, 2, and 3, respectively. The histopathologic score tended to be more in groups 2 and 3 compared with that in the sham-control group rats (p < 0.0001 and p < 0.0001, respectively). However, the histopathologic score was significantly less in the group 3 compared with the group 2 rats (p < 0.0001) ().
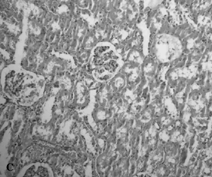
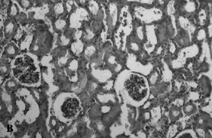
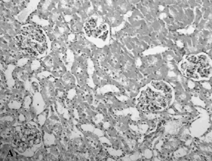
The sham-operated group did not show any morphologic changes (). In contrast, kidneys of untreated ischemia rats showed tubular cell swelling, cellular vacuolization, pyknotic nuclei, medullary congestion, and moderate to severe necrosis (). Treatment with mibefradil preserved the normal morphology of the kidney () and shows normal glomeruli and slight edema of the tubular cells.
Figure 4. Hematoxylin- and eosin-stained sections of rat kidneys. (A) Kidney section of sham-operated rat. (B) Kidney section of the rat exposed to renal I/R. Rats showed tubular cell swelling, cellular vacuolization, pyknotic nuclei, medullary congestion, and moderate to severe necrosis. (C) Kidney section of mibefradil + renal I/R-treated rat showing normal morphology.
Discussion
It is now well recognized that renal ischemia initiates a complex and interrelated sequence of events, resulting in the injury and death of renal cells and reperfusion that although essential for the survival of ischemic tissue, causes additional damage.Citation[19-21] Collectively, I/R of the kidney contributes to the renal dysfunction and injury associated with ischemic acute renal failureCitation[21&22] involving both apoptotic and necrotic renal cell death pathways.Citation[23] Increased creatinine levels, tubular cell necrosis, and kidney leukocyte infiltration are early findings after renal I/R injury.Citation[24] It has been suggested that calcium antagonists have specific actions in the renal microvasculature by dilating preglomerular vessels,Citation[25] therefore increasing glomerular blood flow, as for example demonstrated in isolated perfused kidneys.Citation[26] More recently, increased attention has been focused on T-type Ca2 + channels and their possible physiologic and pathophysiologic roles. Mibefradil used in this study is a structurally novel calcium channel blocker from a new class of benzimidazole-substituted tetraline derivatives.Citation[27&28] In addition, this T-type channel is highly expressed in the kidney.Citation[29] Mibefradil, acting on these channels possibly present on both afferent and efferent arterioles, could lead to a hemodynamic balance without an increase in intraglomerular pressure and proteinuria.Citation[30] In the pathogenesis of I/R injury, intracellular Ca2 + overload,Citation[31] and particularly, intramitochondrial overloadCitation[32&33] play an important role. Calcium overload results from a diminished or reversed function of many ionic pumps and exchangers pumping Ca2 + ions out of the cell or exchanging them for sodium, hydrogen, and other ions.Citation[33] Supplementation of mibefradil was demonstrated to reduce afferent and efferent arteriolar resistances in hypertensive rats,Citation[13] increase renal blood flow,Citation[14] and block the ANG II-induced efferent arteriolar constriction observed in the isolated, perfused hydronephrotic rat kidney.Citation[15]
In this study, we demonstrate that I/R of the rat kidney results in significant renal dysfunction and injury, together with evidence of increased oxidative stress. We show here for the first time that administration of mibefradil produces a significant reduction of the renal dysfunction and injury caused by I/R of the rat kidney. Treatment with mibefradil preserved the normal morphology of the kidney and shows normal glomeruli and slight edema of the tubular cells. In addition, the increase in BUN and creatinine levels was more significant in group 2, but a decrease in BUN and serum creatinine in group 3, showed that the mibefradil had a positive effect on the functional capacity of kidneys damaged by I/R. The present rats in group 2 showed a lower elimination rate of the isotope with increased Tmax-min, but there was an improvement in the renal isotope elimination rate in group 3. These results may be due to mibefradil-induced decreases in afferent and efferent arteriolar resistances, as shown in previous studies.Citation[13-15], Citation[30]
Lipid peroxidation, mediated by free oxygen radicals, is believed to be an important cause of destruction and damage to cell membranes because polyunsaturated fatty acids of the cellular membranes are degraded by this process with consequent disruption of membrane integrity. Membrane peroxidation can lead to changes in membrane fluidity and permeability and also to enhanced rates of protein degradation, and these will eventually lead to cell lysis.Citation[34] MDA levels have been widely used a marker of lipid peroxidation products and lipid peroxidation damage in tissue, cells, and body fluids in both clinical and experimental studies.Citation[35-37] We studied the effect of mibefradil on LPO, which was measured in terms of MDA. Mibefradil reversed the increase in MDA levels to a considerable extent, thereby confirming its antioxidant role in I/R. Thus, mibefradil could be protective against I/R-induced oxidative organ injury by preserving the cellular integrity.
In conclusion, results of this study show that mibefradil has protective effects on I/R-induced renal injury. The impaired renal functions, increased MDA levels, and histopathologic changes are reversed by mibefradil management. Overall, these results demonstrate that this therapeutic strategy may be useful against ischemic acute renal failure.
References
- Almond P S, Matas A J, Gillingham K, , et al Predictors of chronic rejection in renal transplant recipients. Transplant. Proc. 1993;25(1 Pt 2):936. [PUBMED], [INFOTRIEVE], [CSA]
- Pirsch J D, Gange S, D'Alessandro A M, , et al Determinants of graft survival after renal transplantation. Transplantation. 1996;61(11):1581-1586. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cole E, Naimark D, Aprile M, , et al An analysis of predictors of long-term cadaveric renal allograft survival. Clin. Transplant. 1995;9(4):282-288. [PUBMED], [INFOTRIEVE], [CSA]
- Carpenter C B.Long-term failure of renal transplants: adding insult to injury. Kidney Int. 1995;50(suppl):S40-S44. [CSA]
- Conesa E L, Valero F, Nadal J C, , et al N-acetyl-l-cysteine improves renal medullary hypoperfusion in acute renal failure. Am. J. Physiol., Regul. Integr. Comp Physiol. 2001;281(3):R730-R737. [CSA]
- Goode H F, Webster N R, Howdle P D, , et al Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology. 1994;19(2):354-359. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Carini R, Albano E Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125(5):1480-1491. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bulkley G B.Free radical mediated reperfusion injury: a selective review. Br. J. Cancer. 1987;55: 66-73. [CSA]
- Osterrieder W, Hick M In vitro pharmacologic profile of Ro 40-5967, a novel Ca21 channel blocker with potent vasodilatory but weak inotropic action. J. Cardiovasc. Pharmacol. 1989;13(5):754-759. [PUBMED], [INFOTRIEVE], [CSA]
- Rutledge A, Triggle D J.The binding interactions of Ro 40-5967 at the L-type Ca2 + channel in cardiac tissue. Eur. J. Pharmacol. 1995;280(2):155-158. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mehrke G, Zong X G, Flockerzi V, Hofmann F The Ca2 + channel blocker Ro 40-5967 blocks differently T-type and L-type Ca2 + channels. J. Pharmacol. Exp. Ther. 1994;271(3):1483-1488. [PUBMED], [INFOTRIEVE], [CSA]
- Mishra S K, Hermsmeyer K Selective inhibition of T type Ca2 + channels by Ro 40-5967. Circ. Res. 1994;75(1):144-148. [PUBMED], [INFOTRIEVE], [CSA]
- Honda M, Hayashi K, Matsuda H, , et al Divergent renal vasodilator action of L- and T-type calcium antagonists in vivo. J. Hypertens. 2001;19(11):2031-2037. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nakamura Y, Ono H, Frohlich E D.Differential effects of T- and L-type calcium antagonists on glomerular dynamics in spontaneously hypertensive rats. Hypertension. 1999;34(2):273-278. [PUBMED], [INFOTRIEVE], [CSA]
- Ozawa Y, Hayashi K, Nagahama T, Fujiwara K, Saruta T Effect of T-type selective calcium antagonist on renal microcirculation: studies in the isolated perfused hydronephrotic kidney. Hypertension. 2001;38(3):343-347. [PUBMED], [INFOTRIEVE], [CSA]
- Bradford M M.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(7):248-254. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wasowicz W, Neve J, Peretz A Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storge. Clin. Chem. 1993;39(12):2522-2526. [PUBMED], [INFOTRIEVE], [CSA]
- Lowry O H, Rosebrough N J, Faar A L, Randall R J.Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193(1):265-275. [PUBMED], [INFOTRIEVE], [CSA]
- Molitoris B A.The potential role of ischemia in renal disease progression. Kidney Int. 1992;36(suppl):S21-S25. [CSA]
- Paller M S.The cell biology of reperfusion injury in the kidney. J. Investig. Med. 1994;42(4):632-639. [PUBMED], [INFOTRIEVE], [CSA]
- Weight S C, Bell P R, Nicholson M L.Renal ischaemia-reperfusion injury. Br. J. Surg. 1996;83(2):162-170. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Thadhani R, Pascual M, Bonventre J V.Acute renal failure. N. Engl. J. Med. 1996;334(22):1448-1460. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Padanilam B J.Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am. J. Physiol. 2003;284(4):F608-F627. [CSA]
- Heinzelmann M, Mercer-Jones M A, Passmore J C.Neutrophil and renal failure. Am. J. Kidney Dis. 1999;34(2):384-399. [PUBMED], [INFOTRIEVE], [CSA]
- Loutzenhiser R D, Epstein M Calcium antagonists and the kidney. Am. J. Hypertens. 1989(2):154S-161S. [CSA]
- Kimura K, Tojo A, Hirata Y, Hayakawa H, Goto A, Omata M Effects of a calcium antagonist and an angiotensin II receptor antagonist on rat renal arterioles. Blood Press. 1994;5(suppl):71-74. [CSA]
- Osterrieder W, Holck M In vitro pharmacological profile of Ro 40-5967, a novel Ca2 + channel blocker with potent vasodilator but weak inotropic action. J. Cardiovasc. Pharmacol. 1989;13(5):754-759. [PUBMED], [INFOTRIEVE], [CSA]
- Avdonin P V, Buhler F R, Tkachuk V A.Ca2 + -agonistic effect of a T-type Ca-channel blocker mibefradil (Ro 40-5967). Membr. Cell Biol. 2000;13(5):645-655. [PUBMED], [INFOTRIEVE], [CSA]
- Cribbs L L, Lee J H, Yang J, , et al Cloning and characterization of α1H from human heart, a member of the T-type Ca2 + channel gene family. Circ. Res. 1998;83(1):103-109. [PUBMED], [INFOTRIEVE], [CSA]
- Hackenthal E, Paul M, Ganten D, Taugner R Morphology, physiology and molecular biology of renin secretion. Physiol. Rev. 1990;70(4):1067-1116. [PUBMED], [INFOTRIEVE], [CSA]
- Opie L H.Reperfusion injury and its pharmacological modification. Circulation. 1989;80(4):1049-1061. [PUBMED], [INFOTRIEVE], [CSA]
- Miller T W, Tormey J M.Subcellular Ca2 + pools of ischaemic and reperfused myocardium characterised by electron probe. Cardiovasc. Res. 1995;29(1):85-94. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Halestrap A P, Kerr P M, Javadov S, Woodfield K Y.Elucidating the molecular mechanism of permeability transition pore and its role in reperfusion injury of the heart. Biochim. Biophys. Acta. 1998;1366(1–2):79-94. [PUBMED], [INFOTRIEVE], [CSA]
- Garcia J J, Reiter R J, Guerrero J M, , et al Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 1997;408(3):297-300. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Trimarchi H, Mongitore M R, Baglione P, , et al N-acetylcisteine reduces malondialdehyde levels in chronic hemodialysis patients—a pilot study. Clin. Nephrol. 2003;59(6):441-446. [PUBMED], [INFOTRIEVE], [CSA]
- Freudenthaler S M, Schreeb K H, Wiese A, Pilz J, Gleiter C H.Influence of controlled hypoxia and radical scavenging agents on erythropoietin and malondialdehyde concentration in humans. Acta Physiol. Scand. 2002;174(3):231-235. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Agarwal R Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am. J. Physiol. 2003;284(4):F863-F869. [CSA]