Abstract
Background. Renal oncocytoma has been repeatedly reported in Western countries, but only a few cases have been reported in Eastern countries. This study aims to review the clinical course of renal oncocytoma in an Eastern country such as Taiwan. Materials and Methods. Sixteen cases of renal oncocytoma seen between 1987 and 2002 at Chang Gung Memorial Hospital, Taipei, Taiwan, were studied. Results. Preoperatively, all patients were diagnosed to have renal cell carcinoma, following various radiologic studies. Perioperatively, frozen sections of three patients indicated renal oncocytoma in two and renal cell carcinoma in one. Renal oncocytoma has marked similarities to renal cell carcinoma, according to various radiologic, cytologic, and pathological investigations, so an accurate diagnosis is difficult to achieve, either preoperatively or perioperatively. Therefore, rather than being treated with partial nephrectomy, all patients were treated aggressively with unilateral radical nephrectomy. Postoperatively, all 16 patients were followed up, from 12 to 189 months, with a mean of 58.7 months. Notably, all patients survived with no evidence of tumor recurrence. Conclusions. The experience in Taiwan is generally that renal oncocytoma behaves benignly, as reported in other areas. The excellent prognosis associated with this tumor appears to indicate that partial nephrectomy may suffice for removing the tumor, while sparing other unaffected renal parenchyma.
INTRODUCTION
Renal oncocytoma, a benign tumor of renal tubular origin, was first described by Zippel in 1942.[Citation[1]] This tumor has subsequently been identified in the thyroid, parathyroid, salivary, and adrenal glands. In 1976, Klein and Valensi suggested that renal oncocytoma was a distinct clinicopathological entity because then renal oncocytoma has been diagnosed more frequently.[Citation[2]]
Macroscopically, renal oncocytoma is typified by its well-circumscribed, frequently encapsulated appearance, its uniform tan color, and a lack of evidence of necrosis or hemorrhage. On cross-sectioning, a central satellite scar is often observed.[Citation[3]] Microscopically, renal oncocytoma is characterized by substantially eosinophilic epithelial cells called oncocytes, with protuberant mitochondria-rich cytoplasm arranged in a solid trabecular or tubular pattern. Other cellular organelles are sparse. Mitotic activity is absent, and nuclear pleomorphism is rare.[Citation[3]]
A literature review reveals that renal oncocytoma has been repeatedly reported in Western countries,[Citation[3–14]] but only a few cases have been reported in Eastern countries.[Citation[15],Citation[16]] Whether the disease is either genetically[Citation[17]] or environmentally determined to affect Western populations predominantly, or it is just underdiagnosed and thus underreported in Eastern countries, is unknown. Accordingly, clinicians from Eastern countries may be unfamiliar with this disease entity, and may be further confused by the considerable similarities between renal oncocytoma and renal cell carcinoma observed in various radiologic studies, and even similarities of tumor histopathology. Therefore, an accurate preoperative diagnosis may not be easily achieved.
The recent widespread use of ultrasound, computed tomography, and magnetic resonance imaging has increased the number of incidentally detected solid renal masses. However, renal oncocytoma is often indistinguishable from renal cell carcinoma by common diagnostic radiologic modalities. To complicate the situation, renal oncocytoma also shares some histologic features with renal cell carcinoma, which in fact can make the establishment of a final pathology difficult. Because renal oncocytoma is believed to be a benign entity, attempts have been made to diagnose it preoperatively. The excellent prognosis associated with this tumor appears to indicate that partial nephrectomy[Citation[7],Citation[14],Citation[18]] may suffice for removing the tumor while sparing other unaffected renal parenchyma. However, this requires an accurate preoperative differentiation of renal oncocytoma from renal cell carcinoma. Renal cell carcinoma is the most lethal of the common urologic malignancies, with approximately 40% of patients eventually dying of cancer progression. Approximately one-third of patients present with metastatic disease and up to 50% treated for localized disease have a recurrence.[Citation[19]]
This study aims to review the clinical course of renal oncocytoma in an Eastern country such as Taiwan. Furthermore, we attempt to identify any preoperative studies that might conclusively distinguish a renal oncocytoma from a renal cell carcinoma, thus enabling conservative partial nephrectomy in future practice.
MATERIALS AND METHODS
Between 1987 and 2002, 16 Taiwanese patients under the impression of renal cell carcinoma underwent unilateral radical nephrectomy at Chang Gung Memorial Hospital, Taipei, Taiwan. However, all were diagnosed to have renal oncocytoma after pathological examinations of the resected specimens. The charts, the radiologic films, and the pathology sections available in each case were carefully reviewed by the authors to determine how these tumors had misled clinicians from an accurate preoperative diagnosis. Furthermore, the postoperative conditions were obtained from primary care physicians.
RESULTS
summarizes the clinical course of the 16 patients studied herein. There were eight men and eight women. Patients ranged in age from 47 to 79, with a mean of 54.5. The tumors varied in size from 3 to 15 cm, with a mean of 6.6 cm. Ten tumors were in right kidneys, six in left kidneys, and none was found bilaterally.
Table 1 Clinical course of 16 patients with renal oncocytoma
Many of these tumors, 6 out of 16 (or 37.5%) were discovered incidentally during diagnostic evaluation for complaints unrelated to the kidney or to renal neoplasm. In addition, three patients (or 18.8%) reported abdominal pain, four (25%) reported flank pain, two (12.5%) reported gross hematuria, and one patient (6.2%) reported recent-onset hypertension. Neither weight loss nor tumor fever was found in any patient.
No patient had a family history of renal neoplasm or other hereditary disease, such as von Hippel-Lindau disease or tuberous sclerosis, known to be associated with the development of renal neoplasm. Eight of the 16 patients (or 50%) had no other illness; two patients had diabetes mellitus; one had hypertension; one had hypertension, gout, and chronic renal insufficiency; one had gout; one had a huge liver cyst (13 cm in diameter, segment 4); one had rectal cancer (upper rectum, 5 cm in length); and finally, one had concomitant renal angiomyolipoma (1 cm in diameter, in the kidney in which the renal oncocytoma was present), congestive heart failure, and hypertension.
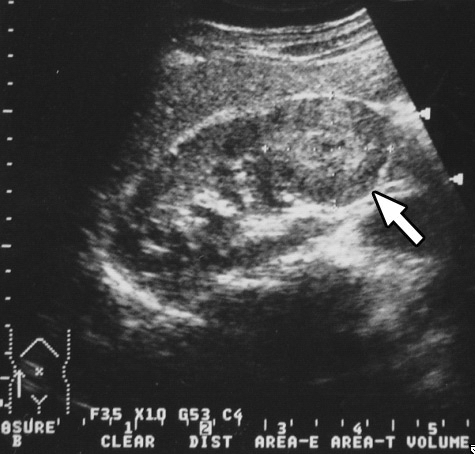
Using various radiologic means, all patients were examined thoroughly before operation. In this regard, all cases were studied primarily with ultrasound. Computed tomography was performed in 14 patients, magnetic resonance imaging in 3 patients, intravenous pyelography in 2 patients, and angiography in 3 patients. (ultrasound of case 16) depicts a 4-cm hypoechoic lesion in the lower pole of the right kidney. (intravenous pyelography of case 13) depicts a huge soft-tissue mass in the upper pole of the right kidney, with stretching of calyces. (computed tomography of case 2) depicts an 11cm exophytic mass in the upper pole of the right kidney. (magnetic resonance imaging of case 3) depicts a 5-cm protruding mass in the inferolateral aspect of the left kidney.
Figure 1. Ultrasound of case 16. A 4-cm hypoechoic mass (arrow) in the lower pole of the right kidney.
Preoperatively, all patients were diagnosed to have renal cell carcinoma. Perioperatively, frozen sections in three cases revealed renal oncocytoma in two and renal cell carcinoma in one. Accordingly, the surgeons were uncertain about the accuracy of the frozen section. Because the surgeons had the impression of renal cell carcinoma, they performed only a unilateral nephrectomy in the operating theater. Hence, 10 patients received right radical nephrectomy, and 6 patients received left radical nephrectomy. Case 11 underwent, in addition to left radical nephrectomy, fenestration of a huge liver cyst at the same time. Similarly, case 12 underwent low abdominal resection of rectal cancer 10 days following nephrectomy. (hematoxylin and eosin stain of case 7) shows that the tumor consists of quite uniform and nonanaplastic tumor cells with abundant eosinophilic cytoplasm, growing in a nesting fashion. No tumor hemorrhage or necrosis is observed. Moreover, no capsular invasion or renal vein thrombosis is observed. Furthermore, the of quite uniform and nonanaplastic tumor cells with abundant eosinophilic cytoplasm, growing in a nesting fashion. No tumor hemorrhage or necrosis is observed. Moreover, no capsular invasion or renal vein thrombosis is observed. Furthermore, the of quite uniform and nonanaplastic tumor cells with abundant eosinophilic cytoplasm, growing in a nesting fashion. No tumor hemorrhage or necrosis is observed. Moreover, no capsular invasion or renal vein thrombosis is observed. Furthermore, the tumor cells are negative for Hale's colloidal iron staining.
Figure 2. Intravenous pyelography of case 13. A huge soft-tissue mass (arrow) in the upper pole of the right kidney with stretching of calyces.
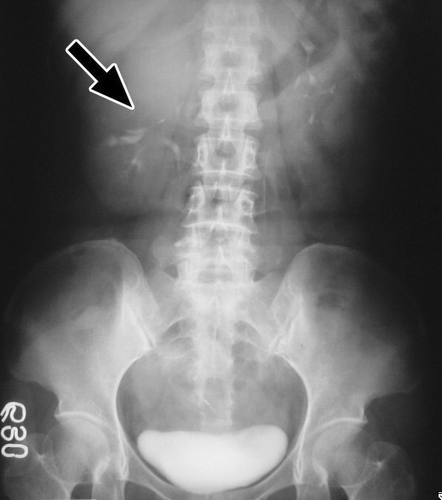
Figure 3. Computed tomography of case 2. An 11-cm exophytic mass (arrow) in the upper pole of the right kidney.
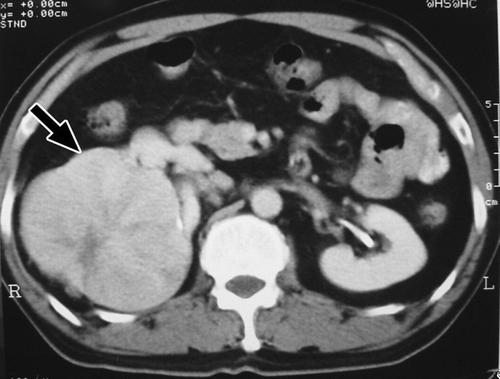
Figure 4. Magnetic resonance imaging of case 3. A 5-cm protruding mass (arrow) in the inferolateral aspect of the left kidney.
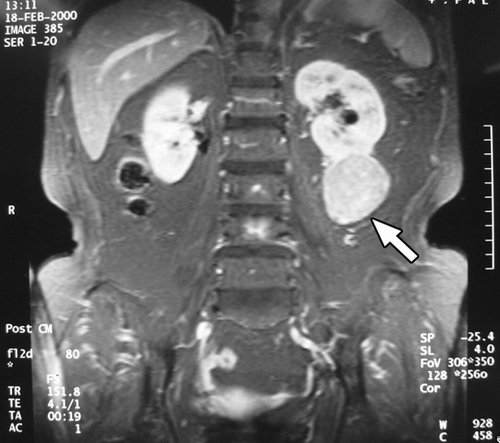
Figure 5. Hematoxylin and eosin stain of case 7. The tumor consists of quite uniform and nonanaplastic tumor cells with abundant eosinophilic cytoplasm that grows in a nesting fashion. The nuclei are generally round with smooth nuclear contours and prominent nucleoli. Magnifications: upper panel ×200, middle panel ×400, and lower panel ×1000.
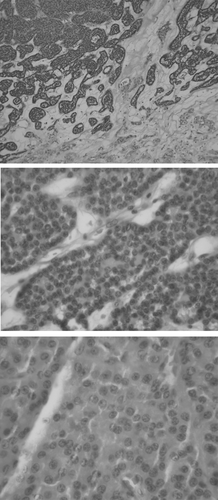
Postoperatively, all 16 patients were followed from 12 to 189 months, with a mean of 58.7 months. Notably, all patients survived with no evidence of tumor recurrence. Case 9 was an elderly patient who had been diagnosed to have gout, chronic renal failure, and hypertension before the operation. After right radical nephrectomy, he developed end-stage renal disease necessitating long-term hemodialysis in 18 months. Case 12 showed no recurrence of rectal cancer on follow-up.
DISCUSSION
The presented data indicated that renal oncocytoma in Taiwan is a disease of the late middle age, with a mean patient age at diagnosis of 54.5. No gender preponderance is evidence. The mean size of the tumor is 6.6 cm. There are no bilateral synchronous tumors. Most of the patients were presented to us following an incidental discovery of a solid renal mass. Finally, none has a traceable family history of renal neoplasm.
Case 15 is of particular interest: The patient had concurrent renal angiomyolipoma and oncocytoma in the left kidney, which were removed altogether during left radical nephrectomy. Renal angiomyolipoma and oncocytoma are uncommon neoplasms, and their simultaneous presence in the same kidney is very rare.[Citation[20]] The mechanisms that cause a kidney to have concurrent angiomyolipoma and oncytoma are at present unclear.
Duchene et al. commented on the proportion of benign renal tumor by reviewing records of all 173 patients (with 186 renal tumors) treated between November 1999 and July 2002.[Citation[21]] They found that, of the 186 tumors, 48% were discovered incidentally. For masses 4 cm or less, the percentage of incidentally discovered tumors increased to 58%. The pathological evaluation demonstrated malignancy in 160 (86%) and benignity in 26 (14%) overall. For tumors 4 cm or less, 18 (20%) of 90 were benign; for tumors between 4 and 7 cm, 8 (17%) of 47 were benign. No tumors greater than 7 cm were benign.
The experience in Taiwan is generally that renal oncocytoma behaves benignly, as reported elsewhere. The 16 patients studied herein presented no evidence of tumor recurrence on follow-up. In view of the excellent prognosis, partial nephrectomy seems to be the ideal treatment for these patients. However, an accurate preoperative or perioperative diagnosis is a prerequisite for conservatively treating this neoplasm. Unfortunately, such an accurate diagnosis was not available preoperatively for the 16 patients studied herein. Consequently, they were aggressively treated with unilateral radical nephrectomy in the operating theater.
Many attempts have been made to diagnose renal oncocytoma preoperatively using various modalities. To this end, much attention has been focused on various imaging techniques (e.g., intravenous pyelography, angiography, ultrasound, computed tomography, magnetic resonance imaging) and cytopathological techniques (e.g., preoperative aspiration biopsy, perioperative frozen section).
Although formerly the standard diagnostic method, intravenous pyelography now serves more of a supportive function in evaluating and screening solid renal parenchymal masses because ultrasound, computed tomography, and magnetic resonance imaging can also be used. In particular, intravenous pyelography is of little help in differentiating renal oncocytoma from other solid renal parenchymal masses.
Angiography, although now rarely performed, has been the most specific type of diagnostic examination for renal oncocytoma. Angiographic features of renal oncocytoma include the spoke-wheel appearance of tumor arterioles, the lucent rim of the capsule and a homogeneous capillary blush.[Citation[22],Citation[23]] A definite preoperative diagnosis, however, cannot normally be made because some typical renal cell carcinomas display similar features, and some renal oncocytomas may not have the classic angiographic appearance.[Citation23]
Computed tomography and ultrasound may suggest the correct preoperative diagnosis of those renal oncocytomas that contain a central scar or in those renal cell carcinomas, demonstrating hemorrhage, necrosis, or parenchymal or perirenal invasion.[Citation[24],Citation[25]] A small (<5.5 cm), well-marginated homogeneous mass is approximately equally likely to be either a renal cell carcinoma or a renal oncocytoma.[Citation[24],Citation[25]] Computed tomography and ultrasound often fail to differentiate between smaller renal cell carcinoma and renal oncocytoma because of the absence of imagable tumor necrosis.[Citation[24],Citation[25]]
The most consistent finding of magnetic resonance imaging of renal oncocytoma is the low signal intensity associated with the renal cortex on T1-weighted spin echo sequences. However, 27% of the cases did not exhibit this low intensity and were actually isointense in relation to the renal cortex.[Citation[26]] The tumors are usually hyperintense on T2-weighted spin echo images,[Citation[26],Citation[27]] but isointensity and low intensity have also been attained.[Citation[26]] A low-intensity rim and central stellate areas of hypointensity can occasionally be detected on T1- and T2-weight images, and can be differentiated from the necrosis common to renal cell carcinoma, which appears as areas of reduced spinal intensity on T1-weighted images and as areas of increased signal intensity on T2-weighted images.[Citation[26]] Unfortunately, a direct comparison of various renal tumors, using magnetic resonance imaging, revealed that these tumors could not be distinguished.[Citation[28]]
Preoperative aspiration biopsy and perioperative frozen sections have been used successfully in some cases. Alanen et al. correctly diagnosed a renal oncocytoma in two of seven cases using fine-needle aspiration biopsy,[Citation[29]] and Nguyen et al. was successful in two of three cases.[Citation[30]] However, a definite diagnosis still depends on thorough sampling of the tumor mass because of the cytologic similarity between renal oncocytoma, the well-differentiated granular renal cell carcinoma, and some renal cell carcinomas having areas that closely resemble renal oncocytoma.
The unreliable clinical history, and the similarities between the overlapping radiologic or cytopathological pictures of renal cell carcinoma and oncocytoma, explain why various, currently used imaging methods have not been able to replace more invasive means (e.g., nephrectomy) of diagnosing renal oncocytoma. Correspondingly, these similarities may also explain why an accurate preoperative diagnosis is difficult to achieve in the series of cases reported herein.
REFERENCES
- Zippel L. Zur Kenntnis der Onkocyten. Virchow Arch Pathol Anat 1942; 308: 360–382
- Klein MJ, Valensi QJ. Proximal tubular adenomas of kidney with so-called oncocytic features. A clinicopathological study of 13 cases of a rarely reported neoplasm. Cancer 1976; 38: 906–914, [PUBMED], [INFOTRIEVE]
- Morra MN, Das S. Renal oncocytoma: a review of histogenesis, histopathology, diagnosis and treatment. J Urol 1993; 150: 295–302, [PUBMED], [INFOTRIEVE]
- Lieber MM, Tomera KM, Farrow GM. Renal oncocytoma. J Urol 1981; 125: 481–485, [PUBMED], [INFOTRIEVE]
- Toth T. Renal oncocytoma. Int J Urol Nephrol 1985; 17: 119–125, [CROSSREF]
- Engel U, Horn T, Nielsen O, Olsen JH. Renal oncocytoma. Acta Path Microbiol Immunol Scand 1987; 95: 107–111
- Frydenberg M, Eckstein RP, Saalfield JAAH, Breslin FHD, Alexander JH. Renal oncocytoma—an Australian experience. Br J Urol 1991; 67: 352–357, [PUBMED], [INFOTRIEVE]
- Licht MR, Novick AC, Tubbs RR, Klein EA, Levin HS, Streem SB. Renal oncocytoma: clinical and biological correlates. J Urol 1993; 150: 1380–1383, [PUBMED], [INFOTRIEVE]
- Dutkiewicz S. Renal oncocytoma. A rare entity in Poland. Int Urol Nephrol 1994; 26: 395–398, [PUBMED], [INFOTRIEVE]
- Rostating L, Escourrou G, Mazerolles C, Durand D, Lloveras JJ, Suc JM. Renal oncocytoma in transplant patients: report of 2 cases. Nephron 1994; 68: 375–377
- Perez-Ordonez B, Hamed G, Campbell S, et al. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Surg Pathol 1997; 21: 871–883, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Tryggvason G, Jonsson E, Guojonsson H, Einarsson O, Luoviksson M, Nikulasson S. Bilateral multifocal renal oncocytoma: a case report and literature review. Scand J Urol Nephrol 2000; 35: 150–152
- Tickoo SK, Reuter VE, Amin MB, et al. Renal oncocytosis. Am J Surg Pathol 1999; 23: 1094–1101, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Chao DH, Zisman A, Pantuck AJ, Freedland SJ, Said JW, Belldegrun AS. Changing concept in the management of renal oncocytoma. Urology 2002; 59: 635–642, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Makita Y, Inenaga T, Kinjo M, Komatsu K, Onoyama K, Fujishima M. Renal oncocytoma developed in a long-term hemodialysis patient. Nephron 1991; 57: 355–357, [PUBMED], [INFOTRIEVE]
- Peng CWB, Yip SKH, Tan PH, Ng LG. Bilateral synchronous renal oncocytoma. Ann Acad Med Singapore 2001; 30: 642–645, [PUBMED], [INFOTRIEVE]
- Weirich G, Glenn G, Junker K, et al. Familial renal oncocytoma: clinicopathological study of 5 families. J Urol 1998; 160: 335–340, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Steinbach F, Stockle M, Muller SC, et al. Conservative surgery of renal cell tumors in 140 patients: 21 years of experience. J Urol 1992; 148: 24–29, [PUBMED], [INFOTRIEVE]
- Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep 2005; 6: 7–18, [PUBMED], [INFOTRIEVE]
- Siracusano S, Zanon M, D'Aloia G, Plaino F, Trombetta C, Bussani R. Rare association of renal angiomyolipoma and oncocytoma. Urology 1998; 51: 837–839, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Duchene DA, Lotan Y, Cadeddu JA, Sagalowsky AI, Koeneman KS. Histopathology of surgically managed renal tumors: analysis of a contemporary series. Urology 2003; 62: 827–830, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Weiner SN, Bernstein RG. Renal oncocytoma. Angiographic features of two cases. Radiology 1977; 125: 633–635, [PUBMED], [INFOTRIEVE]
- Ambos MA, Bosniak MA, Valensi QJ, Madayag MA, Leufleur RS. Angiographic patterns on renal oncocytomas. Radiology 1978; 129: 615–622, [PUBMED], [INFOTRIEVE]
- Levine E, Huntrakoon M. Computer tomography of renal oncocytoma. Am J Roentgenol 1983; 141: 741–745
- Quinn MJ, Hartman DS, Friedman AC, et al. Renal oncocytoma: new observations. Radiology 1984; 153: 49–53, [PUBMED], [INFOTRIEVE]
- Harmon WJ, King BF, Lieber MM. Renal oncocytoma: magnetic resonance imaging characteristics. J Urol 1996; 155: 863–867, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Remark RR, Berquist TH, Lieber MM, Charboneau JW, Hartman GW. Magnetic resonance imaging of renal oncocytoma. Urology 1988; 31: 176–179, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Pretorius ES, Siegelman ES, Ramchandani P, Cangiano T, Banner MP. Renal neoplasms amenable to partial nephrectomy: MR imaging. Radiology 1999; 212: 28–34, [PUBMED], [INFOTRIEVE]
- Alanen KA, Tyrkko JE, Nurmi MJ. Aspiration biopsy cytology of renal oncocytoma. Acta Cytol 1985; 29: 859–862, [PUBMED], [INFOTRIEVE]
- Nguyen GK, Amy RW, Tsang S. Fine needle aspiration biopsy of renal oncocytoma. Acta Cytol 1985; 29: 33–36, [PUBMED], [INFOTRIEVE]
