Abstract
The description of the association between the use of amphotericin-B (amB) and the development of systemic arterial hypertension was only anecdotal so far. We describe the case of a 19-year-old female patient who had acute lymphoblastic leukemia and developed prolonged neutropenia after reinduction chemotherapy. Candida parapsilosis was isolated from blood cultures, and amB was started. Sustained severe arterial hypertension developed shortly after amB administration and continued for several hours after the infusion. Aldosterone, blood urea nitrogen, and creatinine levels were normal. After clinical improvement, amB was replaced by fluconazole, and blood pressure normalized. Severe hypertension may be an adverse event associated with AmB treatment that requires intensive treatment.
INTRODUCTION
Amphotericin B (amB) is still the mainstay of therapy for invasive fungal infections, especially in patients with hematologic malignancies. Chills, acute renal failure, fever/chills, thrombophlebitis, nausea, vomiting, bronchospasm, and hypotension are frequently described side effects related to amB infusion.[Citation[1]] Renal toxicity is common leading to hypokalemia, hypomagnesemia, acidosis, and increased creatinine levels. Lipid preparations that carry fewer side effects, especially less nephrotoxicity, are also available.[Citation[2],Citation[3]]
Hypertension has also been described during AmB infusion in some patients, and it usually subsides after infusion is stopped.[Citation[3]] To our knowledge, there have been only three reports of amB-induced sustained hypertension in previously normotensive patients. We describe one case in which this association clearly occurred.
The patient was 7 years old when she presented with fatigue and gingival bleeding. Early pre-B acute lymphoblastic leukemia (ALL) was diagnosed, and she was successfully treated according to childhood ALL BFM 83 protocol for 2 years. After 10 years off treatment, she presented with lower limb pain and fatigue. Early pre-B ALL was again diagnosed (late relapse), and she was treated according to the adult ALL BFM 5/93 protocol.
After 7 days of induction chemotherapy, she was having daily fever and blood cultures yielded positive for Candida parapsilosis. She had a central venous catheter, which was promptly removed. Conventional AmB was initiated (0.7 mg/kg/d), but the patient remained febrile. Imipenem and vancomycin were added, and AmB dose increased to 1.0 mg/kg/d. Imipenem and vancomycin were withdrawn after 10 days because there was no bacterial isolation in blood cultures.
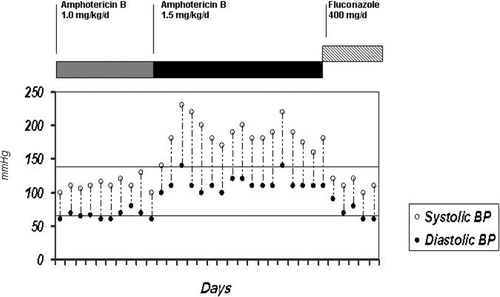
Fourteen days after amB dose was increased, hepatosplenic candidiasis was diagnosed, and AmB dose was increased to 1.5 mg/kg/d.
The day after, the patient's blood pressure (BP) rose from 110/70 mmHg to 180/110 mmHg, 30 min after the infusion was started. She remained hypertensive from this day on. The maximum measured BP level was 230/140 mmHg (mean = 190 mmHg) (). She was no longer taking other antibiotics or any medication possibly associated to hypertension development, and neither was under excessive fluid intake at that time. Daily oral antihypertensive drugs (captopril 25 mg tid and nifedipine 20 mg bid) were needed to control BP.
After 44 days with AmB and still presenting isolated fever spikes, amB was replaced by fluconazole (400 mg/day). BP normalized and remained normal. The patient remained well and afebrile until 20 days later, when refractory ALL and fulminate hepatitis led to a fatal outcome.
DISCUSSION
AmB use is limited by its relatively toxic profile. Development or worsening of hypertension is an uncommon side effect. With increased AmB use, it has lately been described in different settings.
Severe hypertension associated with the use of AmB preparation has been previously reported in seven cases. In only one case, this adverse event was associated with a lipid-containing preparation, which usually causes fewer infusion-related reactions and side effects, especially nephrotoxicity.[Citation[4]]
Of the seven cases of AmB-associated hypertension, four had preexisting well-controlled hypertension.[Citation[4–7]] In the other three reported cases,[Citation[6],Citation[8],Citation[9]] as in the present case, there was no past history of systemic hypertension. Among them, mean arterial pressure ranged from 123 to 200 mmHg. gives a brief summary of these cases.
Table 1 Clinical features of three cases previously reported in the literature of amB-associated hypertension without past history of hypertension and the present case
In most patients, hypertensive reaction developed early during infusion, within the first hour,[Citation[4–7]] as it was in our case. This was the first case described of AmB-induced hypertension in a patient suffering from hematologic malignancy, and that makes this adverse event even more concerning, considering the prolonged severe thrombocytopenia presented by this patient.
We strongly believe that in our case hypertension was related to amB because of the temporal association and the ceasing of hypertension after its withdrawal. The mechanism responsible for hypertension is unknown and seems to be an idiosyncratic reaction to amB infusion. Other possible explanations are vasoconstricting properties of the drug as demonstrated in a rat model,[Citation[10]] and the administration of intravenous 0.9% NaCl solution prior to amB infusion.[Citation[6]]
So far, given the limited available data, the best approach to manage amB-associated hypertension remains to be determined. Katz and Cohn pretreated their patient with oral nifedipine 10 mg, controlling hypertension and allowing amB to be continued without hypertension,[Citation[11]] but the efficacy of this measure was not confirmed by others.[Citation[5]]
In recent years, the options of effective drugs for the systemic treatment of invasive fungal infections have expanded. In the empirical setting, large randomized studies support the use of caspofungin and voriconazole in first-line therapy. These new antifungal agents may be a safe option in hypertensive patients or in patients who develop hypertension with AmB.[Citation[11]]
We conclude that, in addition to the known adverse drug reactions, severe hypertension may also associated with AmB treatment of systemic fungal infections. Identifying hypertension as a potentially serious side effect of amB is important in patients with hematologic malignancies, considering its broad use in this population.
REFERENCES
- Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis 1990; 12(2)308–329
- Garnacho-Montero J, Ortiz-Leyba C, Garcia Garmendia JL, Jimenez F. Life-threatening adverse event after amphotericin B lipid complex treatment in a patient treated previously with amphotericin B deoxycholate. Clin Infect Dis 1998; 26(4)1016
- Walsh TJ, Hiemenz JW, Seibel NL, et al. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis 1998; 26(6)1383–1396
- Rowles DM, Fraser SL. Amphotericin B lipid complex (ABLC)-associated hypertension: case report and review. Clin Infect Dis 1999; 29(6)1564–1565, [CROSSREF]
- Ferreira E, Perreault MM. Hypertension exacerbated by amphotericin B administration. Ann Pharmacother 1997; 31(11)1407–1408
- Le Y, Rana KZ, Dudley MN. Amphotericin B-associated hypertension. Ann Pharmacother 1996; 30(7-8)765–767
- Omizo MK, Bryant RE, Loveless MO. Amphotericin B‐induced malignant hypertension. Clin Infect Dis 1993; 17(4)817–818
- Katz BZ, Cohn RA. Amphotericin B and hypertension. Pediatr Infect Dis J 1994; 13(9)839–840
- Dukes CS, Perfect JR. Amphotericin B-induced malignant hypertensive episodes. J Infect Dis 1990; 161(3)588
- Sawaya BP, Weihprecht H, Campbell WR, et al. Direct vasoconstriction as possible cause for amphotericin B-induced nephrotoxicity in rats. J Clin Invest 1991; 87(6)2097–2107
- Potter M. Strategies for managing systemic fungal infection and the place of itraconazole. J Antimicrob Chemother 2005; 56(suppl 1)i49–i54, [CROSSREF]