Abstract
Amikacin is a commonly used antibacterial drug that can cause significant nephrotoxic effects in both humans and experimental animals. It has been reported that one mechanism of the toxic effects of aminoglycoside antibiotics are the result of oxidative reactions. The aim of this study is to examine the effects of N-acetylcysteine, a thiol-containing antioxidant, on renal function (serum creatinine) and morphology (renal tubular damage) in mice subjected to amikacin-induced nephrotoxicity. A total of 32 mice were equally divided into four groups that were injected with either saline, amikacin (1.2g/kg intraperitoneally), N-acetylcysteine (150mg/kg intraperitoneally for three days) plus amikacin (1.2 g/kg intraperitoneally on the third day as a single dose), or N-acetylcysteine (150mg/kg intraperitoneally). Amikacin administration led to granulovacuolar tubular degeneration in light microscopic examination and myeloid bodies, mitochondrial electron-dense material deposition, and mitochondrial swelling in the proximal tubule epithelium in the electron microscopic evaluation. N-acetylcysteine administration before amikacin injection caused significant decreases in myeloid body and mitochondrial swelling and granulovacuolar tubular degeneration formation. Serum creatinine levels did not change as a result of any treatment. The results show that N-acetylcysteine has a protective effect on nephrotoxicity induced by amikacin. Higher doses of amikacin should be tried to observe biochemical effects.
INTRODUCTION
Aminoglycoside antibiotics, including amikacin (AK), are commonly used for the treatment of severe gram-negative bacterial infections.Citation[1] Despite their beneficial effects, aminoglycosides have considerable side effects. The clinical use of aminoglycosides may be limited by the development of nephrotoxicity. The nephrotoxic side effects of aminoglycoside antibiotics have been documented in numerous species of experimental animals.Citation[2] One mechanism of this toxicity is believed to be the generation reactive oxygen radical species (ROS).Citation[3] ROS are important mediators of tissue damage, especially in renal ischemia-reperfusion injury.Citation[4]
N-acetyl-L-cysteine (NAC), a thiol-containing antioxidant, is an attractive drug because it has few side effects and there is considerable experience of its use in critically ill patients. NAC has antioxidant properties and as a sulfhydryl donor may contribute to the regeneration of endothelium-derived relaxing factors and glutathione.Citation[5],Citation[6]
The present study examined the protective effect of N-acetylcysteine against amikacin-mediated nephropathy based on both biochemical and morphological parameters.
MATERIALS AND METHOD
Thirty-two Balb/c type mice weighing 25–30 g were kept in a temperature (21 ± 2ºC)- and humidity (60 ± 5%)-controlled room in which a 12:12 h light: dark cycle was maintained. The mice were distributed equally into four groups:
injected with saline intraperitoneally (i.p.) for three days (control group),
injected with single dose of 1.2 g/kg amikacin sulfate i.p.Citation[3] (Amikozit flk 100mg, Eczacibasi, Turkey) (AK Group),
injected with 150mg/kg NAC i.p.Citation[7] for three days (Asist flk 300mg, Hüsnü Arsan, Turkey) (NAC Group), and
injected with 150mg/kg NAC for three days; on the third day, 30 minutes later, a single dose of 1.2g/kg amikacin administrated i.p.Citation[7] (AK + NAC Group).
Mice were anesthetized with ketamine and xylazine (90mg/kg, 9mg/kg i.p., respectively) 24 hours after amikacin injection.Citation[8] After two random specimens from each group were taken for electron microscopy examination, the kidneys were sectioned and placed in formaldehyde solution for routine histopathological examination by light microscopy. Trunk blood was extracted to determine the serum levels of creatinine (Cr) using the Roche/Hitachi modular autoanalyzer (Roche diagnostics Corporation Indianapolis, Indiana, USA).
All experiments in this study were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by the Committee on Animal Research at Karadeniz Technical University.
Histological Analysis
For light microscopic evaluation, portions of each kidney were fixed in 10% neutral phosphate-buffered formalin solution. Following dehydration in an ascending series of ethanol (70, 80, 96, 100%), tissue samples were cleared in xylene and embedded in paraffin. Tissue sections of 6mm were stained with hematoxylin-eosin (H-E) and examined using a light microscope (Olympus BX-50). Eight coded slides from each group were examined by an observer blinded to treatment.
The light microscopic examination of the kidney sections was done according to Houghton et al.Citation[9] The changes were graded as follows:
0 = normal,
1 = areas of focal granulovacuolar epithelial cell degeneration and granular debris in the tubular lumina with or without evidence of desquamation in small foci (<1% of total tubule population involved by desquamation),
2 = tubular epithelial necrosis and desquamation easily seen but involving less than half of the cortical tubules,
3 = more than half of the proximal tubules showing necrosis and desquamation, but intact tubules are easily identified,
4 = complete or almost complete proximal tubular necrosis.
Tissues for electron microscopy were fixed with ice-cold 2% glutaraldehyde in phosphate buffer (pH 7.4) at 4ºC overnight and postfixed with 1% osmium tetroxide in phosphate buffer at 4ºC for 2 h, then dehydrated in graded ethanol series (15, 35, 70, 95, and 100) for 10 min in each step and embedded in an Epon mixture. Ultrathin sections were cut with a glass knife, stained with a lead citrate-uranyl acetate solution, and observed under electron microscopy.
Statistical Analysis
Statistical differences between creatinine levels of groups (all values were reported as mean ± SEM) were computed by Kruskal-Wallis test, and light microscopic scores of the groups were compared by the Fisher's exact test. The findings were considered statistically significant if p < 0.05.
RESULTS
Serum levels of creatinine were not statistically different between the groups (see ). Amikacin administration at this dose (1.2 g/kg) did not produce a significant increase in serum creatinine. Light microscopic appearance of the proximal tubular injury was graded by the criteria defined by Houghton et al.Citation[9] In order to support the light microscopic findings, two kidney specimens were selected randomly from each group and processed for examination by electron microscopy. During the experiment, one mouse died in the AK + NAC group.
Table 1 The creatinine levels of groups (mean ± SEM)
The light microscopic findings were illustrated in . Morphological damage changed from none (control and NAC groups) to moderate (AK and AK + NAC groups). Grade 1 histological changes (focal areas of granulovacuolar tubular degeneration) in six mice were detected in the AK group (see ), whereas grade 1 histological changes in three mice were detected in the AK + NAC group. Amikacin at this dose (1.2 g/kg) did not produce severe tubule epithelium necrosis and desquamation. Statistically toxicity grading was significantly more in AK group than control group (p = 0.007). Histological grading was not statistically different between AK + NAC and control groups (p = 0.077).
Table 2 Histopathological grading obtained from the light microscopic analysis of the kidney sections
Figure 1. Light microscopic examination of focal areas of granulovacuolar degeneration of proximal tubule epithelium in AK group (HE × 200).
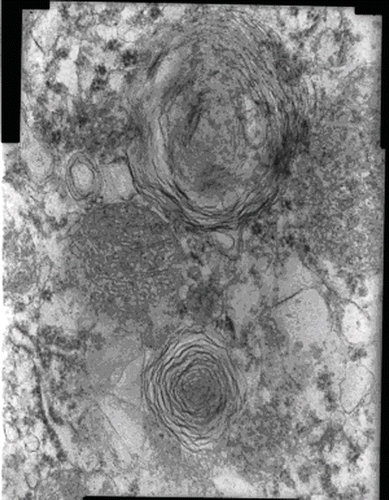
Electron microscopic changes were apparent between the AK and the other groups. Myeloid bodies, mitochondrial electron-dense material deposition, and mitochondrial swelling were detected in the AK group (see ). Brush border microvilli were intact. In the AK + NAC group, there were focal mitochondrial electron-dense material deposition and fewer myeloid bodies compared to the AK group. Electron microscopic findings were completely normal in NAC and control group.
DISCUSSION
NAC, a potent antioxidant that scavenges a wide variety of oxygen-derived free-radicals, may prevent amikacin-induced nephropathy both by improving renal hemodynamics and avoiding direct oxidative damage.Citation[10–12] In vitro, NAC dose-dependently increases the synthesis of cellular glutathione in umbilical endothelial cells depleted of their glutathione by incubation in a sulfur-amino acid-free medium.Citation[13]
Aminoglycoside antibiotics are commonly used to treat gram-negative bacterial infections.Citation[1] However they can be associated with renal and ototoxicity, hypothesized to follow oxidative injury.Citation[14–16] Reactive oxygen metabolites are reported to be involved in gentamicin-induced nephrotoxicity in rats demonstrated by increases in renal cortical lipoperoxidation and peroxide generation.Citation[17–19] Thus, reactive oxygen radicals play a role in the pathophysiology of aminoglycoside-induced nephrotoxicity.
Because reactive oxygen metabolite formation is one of the main causal factors related to amikacin-induced renal injury, the potent antioxidant NAC was used in the current study. The effects of NAC on amikacin-induced changes in creatinine were deduced, and morphological changes in the kidney were also examined using light and electron microscopy. Creatinine did not change as a result of any treatment. However, the administration of amikacin to mice induced granulovacuolar tubular degeneration in light microscopic examination. These findings correlated well with electron microscopic examinations that revealed the presence of myeloid bodies and mitochondrial swelling. Taken together with the normal creatinine level, these data confirmed that amikacin at this dose produced toxicity only detected by morphological examinations. N-acetylcysteine administration to amikacin-treated mice reduces granulovacuolar tubular degeneration, myeloid body formation, mitochondrial electron-dense material deposition, and mitochondrial swelling in tubule epithelium.
There is no study in the literature showing the effects of NAC on amikacin-induced nephropathy. However, Mazzon et al. confirmed that N-acetylcysteine could exert a potent protective effect on nephrotoxicity associated with gentamicin treatment.Citation[20] They confirmed previous observations that nephrotoxicity of gentamicin is mediated, at least in part, by the additive production of free radicals.Citation[21]
The present study found that NAC had protective effects on morphology in amikacin-induced nephropathy in Balb/c mice. However, higher levels of amikacin dosage might be needed to observe this effect biochemically. The protective effects of NAC may lie in the ability of this compound to reduce oxyradical-related oxidant processes by either directly interfering with the oxidants or upregulating antioxidant systems such as superoxide dismutase or enhancing the catalytic activity of glutathione peroxidase.Citation[22],Citation[23]
ACKNOWLEDGMENTS
The authors are indebted to Dr. E. Yenilmez for his experiences in electron microscopic examination and to Dr. M. Erturk for his generous presentation of laboratory facilities.
REFERENCES
- Begg EJ, Barclay ML. Aminoglycosides—50 years on. Br J Clin Pharmacol 1995; 39: 597–603
- Klein J, Koren G, McLeod SM. Comparison methods for prediction of nephrotoxicity during development. Dev Pharmacol 1992; 19: 80–89
- Parlakpinar H, Koç M, Polat A, et al. Protective effect of aminoguanidine against nephrotoxicity induced by amikacin in rats. Urol Res 2004; 32: 278–282
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 1984; 74: 1156–1164
- Auroma OI, Halliwell B, Hoey BM. The antioxidant action of n-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. J Free Radicals Biol Med 1989; 6: 593–597
- Harrison PM, Wendon YA, Gimson AES. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Eng J Med 1991; 324: 1852–1857
- Sener G, Sehirli AO, Dulger GA. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J Pineal Res 2003; 35: 61–68
- Gava AL, Peotta VA, Cabral AM, Meyrelles SS, Vasquez EC. Decreased baroreflex sensitivity in isoproterenol-treated mice with cardiac hypertrophy. Auton Neurosci 2004; 114: 47–54
- Houghton DC, Plamp III CE, Defehr JM, Bennett WM, Porter G, Gilbert D. Gentamicin and tobramycin nephrotoxicity: a morphologic and functional comparison in the rat. Am J Pathol 1978; 93: 137–151
- Dimari J, Megyesi J, Udvarhelyi N, et al. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol 1997; 272: 292–298
- Tariq M, Morais C, Sobki A, et al. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant 1999; 14: 923–929
- Andrews NP, Prasad A, Quyymi AA. N-acetylcysteine improves coronary and peripheral vascular function. J Am Coll Cardiol 2001; 37: 117–123
- Cotgrease I, Moldeus P, Schuppe I. The metabolism of N‐acetylcysteine by human endothelial cells. Biochem Pharmacol 1991; 42: 13–16
- Walker PD, Bari Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail 1999; 21: 433–442
- Bates DE, Beaumont SJ, Baylis BW. Ototoxicity induced by gentamicin and furosemide. Ann Pharmacother 2002; 36: 446–451
- Low W, Dazert S, Baird A, Ryan AF. Basic fibroblast growth factor (FGF-2) protects rat cochlear hair cells in organotypical culture from aminoglycoside injury. J Cell Physiol 1996; 167: 443–450
- Walker PD, Shah SV. Reactive oxygen metabolites in endotoxin-induced acute renal failure in rats. Kidney Int 1990; 38: 1125–1132
- Guidet B, Shah SV. Enhanced in vivo H2O2 generation by rat kidney in glycerol-induced renal failure. Am J Physiol 1989; 257: F440–F445
- Guidet BR, Shah SV. In vivo generation of hydrogen peroxide by rat kidney cortex and glomeruli. Am J Physiol 1989; 256: F158–F164
- Mazzon E, Britti D, Sarro AD, Caputi AP, Cuzzocrea S. Effect of N-acetylcysteine on gentamicin-mediated nephropathy in rats. Eur J Phar 2001; 424: 75–83
- Nakajima T, Hishida A, Kato A. Mechanism for protective effects of free radical scavengers on gentamicin-mediated nephropathy in rats. Am J Physiol 1994; 266: 425–431
- Galley HF, Howdle PD, Walker BE. The effect of intravenous antioxidants in patients with septic shock. Free Radical Biol Med 1997; 23: 768–774
- Schillier HJ, Reilly PM, Bulkley GB. Antioxidant therapy. Crit Care Med 1993; 21: 92–102