Abstract
Background. Proteinuria may cause a worsening of accompanying renal disease or even lead to glomerulosclerosis. There is no data about the effect of carvedilol on patients with proteinuric (>0.5 g/day) glomerulonephritis. This study aimed to compare the effects of carvedilol with ramipril and losartan in patients with proteinuric glomerulonephritis. Methods. Twenty-one glomerulonephritis patients were followed for 12 months. Patients were divided into three groups. All patients were treated with losartan 50 mg once daily for two weeks. After two weeks (baseline), patients were given additional medications: 50 mg losartan, 5 mg ramipril, and 25 mg carvedilol were given additionally to the patients in groups 1, 2, and 3 respectively. Results. Baseline mean proteinuria values of patients in groups 1, 2 and 3 were 1.6 ± 1.1 g/day, 2.1 ± 1.3 g/day, and 1.4 ± 1.2 g/day, respectively. These values decreased to 0.5 ± 0.7 g/day, 0.6 ± 0.7 g/day, and 0.9 ± 0.9 g/day, respectively, at the end of the 12th month. These results were statistically significant only in group 1 (p = 0.04). The rational variation of proteinuria between the first and 12th month of losartan, ramipril, and carvedilol were −61%, −62%, and −27%, respectively. The decreases in blood pressures between baseline and the first, sixth, and twelfth-month measurements were significant in all groups. Conclusions. Thee results showed that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (AT1ras) provide marked decreases in proteinuria, making their use indisputable in patients with glomerulonephritis. Carvedilol was not found to be as effective as ACEIs and AT1ras in decreasing proteinuria and preserving renal function.
INTRODUCTION
Because proteinuria may cause the worsening of accompanying renal disease or even lead to glomerulosclerosis in both diabetics and non-diabetics, preventing proteinuria plays an important role in the treatment.Citation[1] Mild renal dysfunction, defined as a glomerular filtration rate (GFR) <60 mL/min/1.73 m2 and/or the presence of microalbuminuria >30 mg/day, is associated with a significantly increased risk of cardiovascular disease and death.Citation[2]
The activity of the renin angiotensin system (RAS) may contribute to the progression of renal disease as a result of its effect on arterial blood pressure and intraglomerular pressure. The RAS may also act via non-hemodynamic mechanisms, such as mesangial cell mitogenesis, and by influencing the balance of the accumulation and degradation of extracellular matrix in mesangial cells and interstitium, which may contribute to the development of glomerulosclerosis.Citation[3] The reduction of proteinuria that is toxic to glomeruli and the tubules is one of the main objectives of the therapy. Many drugs have been used for this purpose. The optimal treatment strategies include angiotensin-converting enzyme inhibitors (ACEI)Citation[3] for their antiproteinuric and renoprotective effects, angiotensin receptor blockers (AT1ra),Citation[4] and diet.
Prospective studies have shown that AT1ra significantly retards the progression to renal disease and improve cardiovascular events in patients with early or advanced renal disease.Citation[4–7] In patients with chronic nephropathy, ramipril reversed the tendency of glomerular filtration rate to decline with time in REIN (placebo-controlled trial of the effect of ramipril on the decline in glomerular filtration rate and the risk of terminal renal failure in proteinuric, non-diabetic nephropathy) study.Citation[8],Citation[9] Carvedilol exerts a unique hemodynamic effect through combined alpha and beta adrenoreceptor blockage.Citation[10] Studies have shown that carvedilol is an effective drug for the treatment of proteinuric patients with or without diabetes mellitus.Citation[10–12]
In the literature, studies about the effects of carvedilol generally included the proteinuric patients secondary to diabetes mellitus and/or hypertension.Citation[10–12] There is no sufficient data about the effects of carvedilol on patients with proteinuric (>0.5 g/day) glomerulonephritis. Thus, this losartan-based study aimed to compare the efficacy of ramipril, losartan, and carvedilol treatments in patients with proteinuric glomerulonephritis.
PATIENTS AND METHODS
The study was conducted in the outpatient clinics of Uludag University Faculty of Medicine Nephrology Departments between September 17, 2004, and December 1, 2005. Renal disease was confirmed with biopsy. Patients without systemic or urinary tract infections, pregnancy, hyperkalemia, hypersensitivity to drugs studied, active gastric ulcer, stage 2 or secondary hypertension, antihypertensive drug usage, recent myocardial infarction, uncontrolled angina, serious arrhythmias, severe peripheral vascular disease, obstructive pulmonary disease, serious liver disease or diabetes mellitus; or with a heart rate of <55 beats/min, a creatinine level of <2 mg/dL, >0.5 g/day proteinuria, or a C reactive protein level of <0.5 mg/dL without any clinical or laboratory activation criteria were included in the study. Written informed consent and the approval of the local ethic committee were taken.
Thirty-one glomerulonephritis patients were included in the study. The patients had stable proteinuria (i.e., proteinuria rates changed no more than 20% in the last three months prior to this study), and were not medicated with any other drugs except those for their primary renal disease treatment. Ten patients had no medication, two had a low-dose corticosteroid (5–10 mg/day, 4 ± 2.8 year), eight had a low-dose corticosteroid plus azathioprine (100 mg/day, 1.6 ± 0.6), six had a low-dose corticosteroid plus mycophenolate mophetile (1 g/day, 1.3 ± 0.5 year), three had a low-dose corticosteroid plus cyclosporine (125 mg/day, 1.5 ± 0.7 year), and two had only azatiopurin (100 mg/day). All patients received 1 g/kg/day protein and <6 g salt intake. Patient demographics and primary diseases of the study population are described in .
Table 1 Patient demographics and the distribution of primary renal diseases in study population
Patients were divided into three groups. All patients treated with losartan 50 mg once a day (Cozaar, Merck Sharp and Dohme, Northumberland, UK) for two weeks. After two weeks, patients in group 1 were given losartan 50 mg; patients in group 2 were given ramipril 5 mg once a day (Delix, Hoechst Marion Roussel, Frankfurt, Deuthschland); and patients in group 3 were given carvedilol 12.5 mg for the first two days following carvedilol 25 mg once a day (Dilatrent, Roche, Basel, Switzerland) in the remaining period. Six patients did not complete the follow-ups, two patients could not tolerate antihypertensive treatment, and in two patients (one in carvedilol group, one in ramipril group), immune-suppressive regimen were needed to be changed. The remaining 21 patients were followed for 12 months.
Systolic and diastolic blood pressures; body weights; serum urea, creatinine, albumin, sodium, and potassium levels; urinary sodium and protein levels; and creatinine clearance were checked just before losartan (i.e., −2 weeks) before the addition of second drug (baseline), and at the first, sixth, and twelfth months.
Urea, creatinine, sodium, and potassium levels were measured with Aeroset System Abbott (Abbott Laboratories, Diagnostic Division, North Chicago, Illinois, USA) with an enzymatic method. The creatinine clearance (CC) was calculated with Cockcroft and Gault formula (in females, the coefficient was 0.85):
The proteinuria levels were measured with the Esbach method. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice with two-minute intervals in a sitting position after 10 minutes of resting, using the patient's right arm with sphygmomanometer.
The data were assessed by using software program SPSS-13 for statistical analysis. Comparisons within each group and between groups were performed by using Wilcoxon and Friedman tests. All data were given as mean ± standard deviations. Rational variation was defined as follows:
All comparisons were between baseline and the first, sixth, and twelfth month values. A p value of <0.05 was assumed as statistically significant.
RESULTS
There was no difference between groups in respect to age, weight, body-mass index, blood pressure, proteinuria levels, creatinine clearances, serum creatinine levels, serum albumin levels, and clinical characteristics at −2 weeks and baseline.
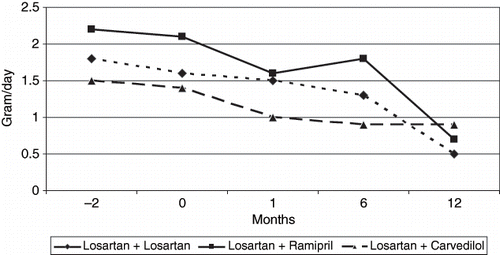
Basal mean proteinuria value of patients in group 1 (losartan 50 mg + losartan 50 mg) was 1.6 ± 1.1 g/day and decreased to 0.5 ± 0.7 g/day at the end of the twelfth month, a decrease that was statistically significant (p = 0.04). In group 2 (losartan 50 mg + ramipril 5 mg), mean basal proteinuria value was 2.1 ± 1.3 g/day and decreased to 0.6 ± 0.7 g/day at the end of the twelfth month, which was not statistically significant (p = 0.06). In group 3 (losartan 50 mg + carvedilol 25 mg), the mean basal proteinuria value was 1.4 ± 1.2 g/day and decreased to 0.9 ± 0.9 g/day, which was also not significant (p = 0.8). The rationale variation of proteinuria between the first and twelfth month of losartan, ramipril, and carvedilol were −61%, −62%, and −27%, respectively, and were not found to be statistically significant (see and ).
Table 2 The trends in laboratory values and blood pressures measured at baseline and at one, six, and twelve months
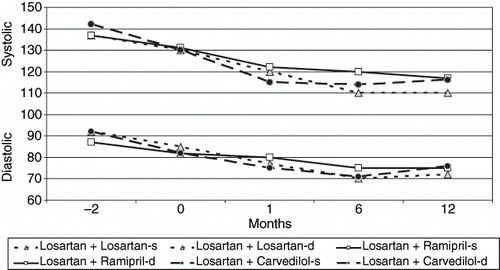
Differences between basal and the first, sixth, and twelfth month measurements of SBP indicated that the decreases in SBP were statistically significant in all groups. Differences between baseline and the first, sixth, and twelfth month measurements indicated that the decreases in DBP were statistically significant in the losartan group but not the ramipril group. In the carvedilol group, while the differences between the results of the basal and the first and sixth month measurements of DBP was statistically significant, no significant differences were found when compared with the results of the twelfth-month measurement (see and ). Heart rates showed no statistically significant differences between groups and within groups. There was no correlation between the decreases in blood pressure and proteinuria.
Increases in creatinine levels were found statistically significant at sixth month in the losartan group (p = 0.047) and at the twelfth month in carvedilol group (p = 0.038). There were no statistically significant differences in creatinine levels between groups. The amount of the decreases in CC values from baseline to the twelfth month was statistically significant only in carvedilol group (p = 0.043).
Sodium, potassium, C reactive protein, urinary sodium, and albumin levels did not display statistically significant differences within groups and between groups.
DISCUSSION
The results of the present study have shown that one year combined therapy with treatment doses of losartan + losartan, losartan + ramipril, and losartan + carvedilol reduced proteinuria by −61%, −62%, and −27% respectively. But it was statistically significant only in the losartan group (p < 0.05). The antiproteinuric effect was independent from blood pressure reductions, as blood pressure control in each group was virtually identical during the treatment periods.
Proteinuria is an independent risk factor for the progression of renal disease.Citation[13] Proteinuria control may be essential to prevent complications and morbidity. ACEIs are widely used for their antiproteinuric and renoprotective effects. In a prospective, randomized, double-blinded, multicenter trial, the GISEN group reported that ramipril safely reduced proteinuria and the rate of glomerular filtration rate (GFR) decline in patients with nondiabetic chronic nephropathies.Citation[5] In their follow-up study, the same group confirmed that ramipril reversed the tendency of GFR to decline over a long period of time.Citation[7] Clinical trials performed so far demonstrated that AT1ras could reduce proteinuria as ACE inhibitors. In one study, losartan reduced proteinuria in non-diabetic renal disease patients, achieving its maximum antiproteinuric effect after six weeks of treatment without changing GFR.Citation[14] In a previous study, it was also found that losartan decreased proteinuria in normotensive patients with primary glomerulopathy.Citation[4],Citation[5] Many studies have shown that a combination of these drugs could cause significantly greater antiproteinuric effect than either of these agents (i.e., ACEIs and AT1ras) as monotherapies.Citation[15–17] Also, in most studies, it was proven that ACEI, AT1ra, or a combination of these drugs significantly decreased proteinuria and had renoprotective effects in patients with diabetic proteinuria.Citation[3],Citation[6],Citation[18],Citation[19] AT1ra can reduce microalbuminuria in hypertensive renal transplant patients as well.Citation[20] The present study suggested that a doubled dose of AT1ra and AT1ra + ACEI combination reduced proteinuria by about 60%, an effect that was not significantly influenced by blood pressure levels.
There were no data in the literature about the proteinuric effect of carvedilol in patients with glomerulonephritis. Experimental studies showed that carvedilol can reduce focal sclerosis, lipid peroxidation, and serum urea and creatinine levels.Citation[21] Various hypotheses could be advanced to explain this effect of carvedilol. Unlike conventional beta blockers, carvedilol does not reduce glomerular filtration rate, effective renal plasma flow, blood urea nitrogen, or serum creatinine, and significantly decreases renal vascular resistance.Citation[22] Besides, carvedilol provides an improvement in erythroid elasticity, a decrease in platelet and erythrocyte aggregation, and a reduction in plasma viscosity.Citation[23] Carvedilol has antioxidant properties that can protect the glomerulus from the injury caused by free radicals and reduce glomerular cell proliferation.Citation[24]
In this study, while the drug was well-tolerated in losartan group, immune suppressive protocol of two patients in carvedilol and ramipril groups (one patient in each group) needed to be changed. No complication related to the study drugs was observed. Although previous studiesCitation[25],Citation[26] reported that carvedilol does not affect renal functions, there were no data about the effect of primary glomerular disease. An increase in serum creatinine and a decrease in CC levels were observed at the twelfth month in thie current study, but the results are needed to be confirmed by further controlled trials on larger series. Although the highest significant decrease in proteinuria would be expected to be in those with the highest amount of proteinuria, a 62% decrease, probably as a result of fewer patients (which was not significant), was observed in ramipril group, as they had the highest basal proteinuria. Also, while SBP was reduced in the ramipril group, there was no significant reduction in DBP. Some recent studies have reported that lowering blood pressure to <125/75 mmHg could not maintain a clear decrease in proteinuria in patients who had blood pressures of about 140/90 mmHg unless using an AT1ra.Citation[27] Similarly, in this study, a marked decrease in systolic and diastolic blood pressure levels was found in all groups but a significant decrease in proteinuria was observed only in the losartan group. Although there was no correlation between blood pressure levels and the decrease in proteinuria, the only significant decrease in proteinuria was achieved in the losartan group with the lowest blood pressure levels. Although it has been reported in previous studies that a significant decrease in proteinuria by carvedilol was seen in patients with diabetes mellitus and hypertension,Citation[10–12] in this study, no significant decrease was observed in proteinuria in patients with proteinuric glomerulonephritis.
The facts that the primary glomerular diseases were not homogenous among the study groups and the number of cases was small were main limitations of this study.
In conclusion, in patients having glomerulonephritis with mild/moderate proteinuria, higher doses of losartan and ramipril losartan have decreased proteinuria levels significantly more than the losartan/carvedilol combination. However, these changes should be interpreted with caution because of small number of patients. The efficiency and safety of ACEIs, AT1ras, and combination therapies in patients who are at high risk for the renal diseases would be better understood after getting the results of ONTARGET, the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial: a Large, Simple Randomized Trial of an Angiotensin II Receptor Antagonist (Telmisartan) and an ACE-Inhibitor (Ramipril) in Patients at High Risk for Cardiovascular Event study,Citation[28] which would be completed in the end of 2008. The evaluation of the effectiveness and safety of carvedilol in glomerulonephritis should be studied by multicenters on larger series of cases.
Notes
*The results presented in this paper have not been published previously in whole or partly, except in abstract form.
REFERENCES
- Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int. 1998; 53: 1209–1216
- Ruilope LM. Renin-angiotensin-aldosterone system blockade and renal protection: angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers?. Acta Diabetol. 2005; 42: 33–41
- Ravid M, Lang R, Rachmani R, Lishner M. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A seven-year follow-up study. Arch Intern Med. 1996; 156: 286–289
- Dilek K, Usta M, Ersoy A, et al. Long term effect of losartan on proteinuria and renal function in patient with renal amyloidosis. Scand J Urol Nephrol. 2003; 36: 443–446
- Usta M, Dilek K, Yavuz M, Ersoy A, Gullulu M, Yurtkuran M. Anti proteinuric effect of angiotension II receptor antagonist losartan in cases with glomerular lesions. Clin Nephrol. 2001; 55: 260–262
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345: 861–869
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345: 851–860
- GISEN. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet. 1997; 349: 1857–1863
- Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet. 1998; 352: 1252–1256
- Fassbinder W, Quarder O, Waltz A. Treatment with carvedilol is associated with a significant reduction in microalbuminuria: a multicentre randomised study. Int J Clin Pract. 1999; 53: 519–522
- Marchi F, Ciriello G. Efficacy of carvedilol in mild to moderate essential hypertension and effects on microalbuminuria: a multicenter, randomized, open-label, controlled study versus atenolol. Adv Ther. 1995; 12: 212–221
- Bakris GL, Fonseca V, Katholi RE, et al. Differential effects of beta-blockers on albuminuria in patients with type 2 diabetes. Hypertension. 2005; 46: 1309–1315
- Williams JD, Coles GA. Proteinuria—direct cause of renal morbidity?. Kidney Int. 1994; 45: 443–450
- Gansevoort RT, de Zeeuw D, de Jong PE. Is the antiproteinuric effect of ACE inhibition mediated by interference in the renin-angiotensin system?. Kidney Int. 1994; 45: 861–867
- Campbell R, Sangalli F, Perticucci E, et al. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 2003; 63: 1094–1103
- Tylicki L, Rutkowski P, Renke M, Rutkowski B. Renoprotective effect of small doses of losartan and enalapril in patients with primary glomerulonephritis. Short-term observation. Am J Nephrol. 2002; 22: 356–362
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003; 361: 117–124
- Segura J, Praga M, Campo C, Rodicio JL, Ruilope LM. Combination is better than monotherapy with ACE inhibitor or angiotensin receptor antagonist at recommended doses. J Renin Angiotensin Aldosterone Syst. 2003; 4: 43–47
- Barnett AH, Bain SC, Bouter P, et al. Diabetics Exposed to Telmisartan and Enalapril Study Group: Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004; 351: 1952–1961
- Ersoy A, Dilek K, Usta M, et al. Angiotensin-II receptor antagonist losartan reduces microalbuminuria in hypertensive renal transplant recipients. Clin Transplant. 2002; 16: 202–205
- Van den Branden C, Gabriels M, Vamecq J, Vanden Houte K, Verbeelen D. Carvedilol protects against glomerulosclerosis in rat remnant kidney without general changes in antioxidant enzyme status. A comparative study of two beta-blocking drugs, carvedilol and propanolol. Nephron. 1997; 77: 319–324
- Tomita K, Marumo F. Effect of long-term carvedilol therapy on renal function in essential hypertension. J Cardiovasc Pharmacol. 1992; 19: 97–101
- Nagakawa Y, Akedo Y, Kaku S, Orimo H. Effects of carvedilol on common carotid arterial flow, peripheral hemodynamics, and hemorheologic variables in hypertension. Eur J Clin Pharmacol. 1990; 38: 115–119
- Marchi F, Ciriello G. Efficacy of carvedilol in mild to moderate essential hypertension and effects on microalbuminuria: a multicenter, randomized, open-label, controlled study versus atenolol. Adv Ther. 1995; 12: 212–221
- Dupont AG, Van der Niepen P, Taeymans Y, et al. Effect of carvedilol on ambulatory blood pressure, renal hemodynamics, and cardiac function in essential hypertension. J Cardiovasc Pharmacol. 1987; 10: 130–136
- Brooks DP, Short BG, Cyronak MJ, et al. Comparison between carvedilol and captopril in rats with partial ablation-induced chronic renal failure. Br J Pharmacol. 1993; 109: 581–586
- PROCOPA Study Group. Dissociation between blood pressure reduction and fall in proteinuria in primary renal disease: a randomized double-blind trial. J Hypertens. 2002; 20: 729–737
- http://www.clinicaltrials.gov/ct/show/NCT00153101?order=1, ONTARGET Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial: A large, simple randomized trial of an angiotensin ii receptor antagonist (telmisartan) and an ace-inhibitor (ramipril) in patients at high risk for cardiovascular events