Abstract
Background. The association of cigarette smoking with albuminuria has been reported but not examined in a representative U.S. population. No study has evaluated the association between serum cotinine (a biological marker for tobacco exposure) and kidney damage. Methods. A cross-sectional analysis was conducted among 15,719 adult participants of the third National Health and Nutrition Examination Survey to assess the association between smoking exposure and kidney damage. Smoking was assessed by self-reported lifetime cigarette use and serum cotinine. Kidney damage was assessed by urine albumin-to-creatinine ratio (ACR), with albuminuria defined as ACR of ≥17 μg/mg in males and ≥25 μg/mg in females. Results. The analysis included 13,121 with normal albumin (mean ACR 6.3 μg/mg) and 2,414 with albuminuria (mean ACR 143 μg/mg); hypertension was prevalent in 27% and 59%, respectively. Former smoking was similar between groups (21%), while current smoking was more common in persons with albuminuria (26%) compared to normal ACR (21%). Adjusted for other risk factors, among hypertensives, current smokers were 1.85 (95% CI: 1.29, 2.64) times more likely to have albuminuria than never smokers. Current smokers with a ≥40 pack-year history were at highest risk for albuminuria. Among non-smoking hypertensives, those exposed to passive smoke (highest versus lowest quartile of serum cotinine) were 1.41 (95% CI: 1.04, 1.90) times more likely to have albuminuria. Former smoking with cessation of ≥1 year among hypertensives was not associated with albuminuria. Among non-hypertensives, smoking and albuminuria were not consistently associated. Conclusion. Current and passive smoking, but not former smoking, were associated with the presence of albuminuria in the general U.S. population with hypertension, indicating a benefit to the kidney from smoking cessation.
INTRODUCTION
Although a decline in cigarette smoking has been observed in recent years, nearly one-quarter of all adult Americans, approximately 45.8 million persons, are current smokers.Citation[1] The adverse health effects of cigarette smoking, most prominently cardiovascular and lung diseases, have been widely reported. However, relatively fewer studies have been devoted to kidney disease outcomes. While the association between cigarette smoking and end-stage renal disease (ESRD) has been inconsistently observed in population studies,Citation[2–4] several studies have reported that cigarette smoking is related to urine albumin excretionCitation[5–7] as well as elevated serum creatinine or decreased glomerular filtration rate (GFR).Citation[8],Citation[9] A recent longitudinal cohort analysis using the Framingham Heart Study by Fox et al.Citation[10] examined predictors for new onset kidney disease defined by an estimated GFR at or below the sex-specific 5th percentile over a mean of 18.5 years of follow-up. Authors found that participants who smoked in the year prior to examination were 42% more likely to develop new onset kidney disease than those who did not smoke.
The primary purpose of this study was to evaluate the cross-sectional association between lifetime smoking duration and dose with albuminuria in the adult population examined in the third National Health and Nutrition Examination Survey (NHANES III). Serum cotinine is one of the primary metabolites in nicotine and is a widely accepted biological measure of not only cigarette smoking but also exposure to environmental tobacco smoke (such as passive smoking). To our knowledge, this study is the first to examine both self-reported cigarette smoking and serum cotinine in relation to kidney disease. Information collected as part of NHANES III provided the opportunity to address this association in a probability sample representative of the U.S. population.
MATERIALS AND METHODS
Study Participants
The NHANES III was designed to assess the health and nutritional status of persons aged 2 months and older in the U.S., between 1988 and 1994.Citation[11] Details of the NHANES III study participants and methods have been previously published.Citation[12] Briefly, the study used a stratified, multistage cluster sampling design to obtain a representative sample of the U.S. non-institutionalized, civilian population. Over-sampling of the very young, elderly, non-Hispanic blacks and Mexican-Americans was done in order to improve the precision of estimates in these subgroups. Of the 19,618 NHANES III participants older than 18 years of age, 912 were excluded due to missing information on albuminuria and 184 were excluded due to missing information on other covariates. In addition, 264 pregnant women, 159 persons with overt renal insufficiency (serum creatinine ≥ 1.8 mg/dL [> 159 μmol/L] in women and ≥ 2.0 [177 μmol/L] in men), and 698 persons of race other than non-Hispanic white, non-Hispanic black, or Mexican-American were excluded, leaving 15,535 participants for the current analyses.
Measurement of Albuminuria and Kidney Damage
The physical exam included the collection of a random urine specimen and measurement of serum creatinine. Urine was evaluated for albumin and creatinine by solid-phase fluorescent immunoassay with external quality control provided by the Centers for Disease Control and Prevention.Citation[13] The ratio of urine albumin (μg/mL) to urine creatinine (mg/dL) (ACR) concentration was used to estimate total daily albumin excretion. Albuminuria was defined as a urinary ACR of 17 μg/mg or higher in males and 25 μg/mg or higher in females. Macroalbuminuria was defined as urinary ACR greater than or equal to 250 μg/mg in males and greater than or equal to 355 μg/mg in females.Citation[14] Serum creatinine was analyzed using the modified Jaffe reaction.Citation[13] To evaluate kidney function, estimated GFR was calculated using the abbreviated Modification in Diet and Renal Disease (MDRD) Study formula based on serum creatinine, age, gender, and race.Citation[15],Citation[16] The serum creatinine values from NHANES III were calibrated to the values obtained at the laboratory in which the glomerular filtration rate equation was derived.Citation[17] Categories of kidney function were defined using GFR (mL/min per 1.73 m2) less than 60, 60 to 90, and greater than or equal to 90, to represent moderate to severe, mildly decreased, and normal kidney function, respectively.Citation[18]
Measurement of Smoking
The amount of cigarettes smoked, years of smoking, and duration of smoking cessation were recorded as part of the NHANES III questionnaire.Citation[12] Lifetime smoking was estimated in pack-years, defined as the number of years a full pack of cigarettes (20 cigarettes) was smoked per day. Smoking status was categorized as current (smoking > 1 cumulative pack years up until 12 months prior to examination), former (quit smoking and sustained cessation for at least 12 continuous months prior to examination), or non-smokers (never smoked or smoked ≤ 1 cumulative pack-year). Serum cotinine level was measured using high-performance liquid chromatography atmospheric-pressure chemical ionization tandem mass spectrometry. Persons with elevated serum cotinine, defined as greater than or equal to 10 ng/mL, were considered exposed to environmental tobacco smoke.
Measurement and Definition of Covariates
Seated blood pressure was measured three times during the home interview using a mercury sphygmomanometer.Citation[12] Systolic and diastolic blood pressures were estimated as the average of all available blood pressure readings. Hypertension was defined as a mean systolic blood pressure greater than or equal to 140 mm Hg, diastolic blood pressure greater than or equal to 90 mm Hg, ever having been diagnosed as having high blood pressure by a physician on two or more occasions, or by reported use of antihypertensive agents during the month prior to their study visit. Diabetes was defined as those who had ever been told by a physician that they had diabetes or sugar diabetes (excluding those only told they were diabetic during pregnancy).
Statistical Methods
The mean or proportion of each characteristic was calculated by albuminuria status. Tests of differences by albuminuria status were done using chi-square tests for categorical measures and t-tests or analysis of variance for continuous variables. Logistic regression models were used to estimate age, race, and gender-adjusted as well as multivariable adjusted odds ratios of albuminuria compared to normal albumin excretion associated with cigarette smoking. Generalized logit regression models were used to evaluate the multivariable association of smoking with estimated GFR at specific, pre-defined cut-points of GFR.Citation[18] Multivariable models were adjusted for variables known to be associated with kidney outcomes including age, gender, race, education, body mass index, diabetes, blood pressure, use of anti-hypertension medication, HDL cholesterol, and alcohol intake.
Multiplicative interaction terms of current and former smoking and serum cotinine with gender, race, education level, diabetic status, and hypertensive status were evaluated using logistic regression models. Interaction terms with p values less than 0.10 were considered important stratification measures. A significant interaction was evident between former smoking and hypertension (p = 0.039); therefore, all models were stratified by hypertensive status. All other interaction terms with current and former smoking as well as cotinine were non-significant, with p values greater than 0.10.
The statistical package SUDAAN (release 8.0.1)Citation[19] was used to account for weights and adjustments for multi‐stage sampling, sample selection probabilities, non-response, and undercounts of the U.S. population estimates by gender, race, and age.
RESULTS
A total of 15,535 adults were included in our analysis. Normal albumin was observed in 13,121 participants (mean ACR 6.3 μg/mg). Albuminuria was detected among 2,414 (15.5%, mean ACR 143 μg/mg) participants overall; 28% of hypertensives and 8.5% of non-hypertensives. As expected, there were differences in demographic and clinical measures by albuminuria status (see ). Among non-hypertensive participants, those with albuminuria were more likely to be older, female, less educated, and diabetic compared to participants with normal albuminuria. In this same group, systolic blood pressure was higher while serum creatinine estimated GFR were similar in subjects with albuminuria compared to subjects with normal albuminuria (see ). Among hypertensive participants, those with albuminuria were more likely to be older, non-Hispanic black, diabetic, and be in the lowest GFR category (30–59 mL/min/1.73m2) than those without albuminuria. Also, mean serum creatinine was higher and estimated GFR lower among hypertensives with albuminuria compared to those with normal albumin. The comparison of other characteristics among hypertensives between persons with albuminuria and normal albumin were similar to what was observed in non-hypertensives.
Table 1 Characteristics of NHANES III participants by level of albuminuria and hypertension status
shows self-reported smoking characteristics and levels of serum cotinine adjusted for age, sex, race, education, and diabetes. Among all participants as well as by hypertensive status, those with albuminuria were more likely to be current smokers and have smoked for a greater number of years and pack-years compared to those with normal albumin (among current and former smokers). Non-hypertensive former smokers with albuminuria were more likely to have smoked for greater than or equal to 60 pack-years and less likely to have smoked less than 20 pack years.
Table 2 Tobacco exposure by level of albuminuria and hypertension status among NHANES III participantsFootnote**
The odds ratios of albuminuria associated with smoking status, pack-years of smoking, and level of serum cotinine are shown by hypertension status in . Among hypertensives, the risk of current smoking was associated with a 1.85 increased odds of albuminuria (95% CI: 1.29, 2.64) in multivariable adjusted models. Among hypertensive current and former smokers, cotinine in the lower and upper 50th percentile above elevated cotinine (10 ng/mL) demonstrated a 1.43 (95% CI: 1.0, 2.04) and 1.61 (95% CI: 1.15, 2.27) excess risk of albuminuria, respectively, compared to those with levels less than 10 ng/mL. No similar associations were observed among non-hypertensives.
Table 3 Multivariable adjusted odds ratios (and 95% confidence intervals) of albuminuria associated with measures cigarette smoking exposures by hypertensive status
To explore if there were a specific level of cigarette smoking where an association with albuminuria was evident among current and former smokers by hypertension status, pack-years were categorized into 20 pack-year groups (< 20, 20–39, 40–59 and > 60 pack-years). As shown in , among hypertensives, there were greater associations between albuminuria and current smokers who smoked greater than 40 pack-years but no associations among former smokers. No dose response trend for pack-years of smoking was demonstrated among hypertensive participants. Among non-hypertensives, there were greater associations between albuminuria and current and former smoking for greater than 40 pack-years, although only the association with 60 or more pack-years among former smokers was statistically significant. In former non-hypertensive smokers, a dose response relationship emerged between pack years of smoking and albuminuria.
Figure 1. Adjusted odds ratios and 95% confidence intervals for albuminuria by pack-years of cigarette smoking and hypertension status compared to non-smokers. Odds ratios for all current and former smokers were estimated using non-smokers as the reference group (odds ratio = 1.0), as represented by the dashed (---) line. Odds ratios were adjusted for gender, race, age, education, body mass index, diabetes, systolic blood pressure, use of antihypertensive medication, alcohol intake in the last 12 months, and HDL cholesterol.
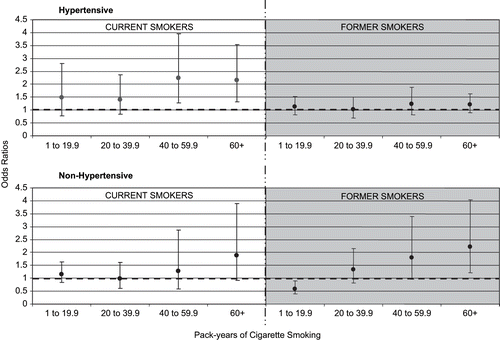
The duration of smoking cessation was explored among former smokers to see if longer duration of smoking cessation attenuated the association of pack-years and albuminuria (15 years was used as the cut point for smoking cessation because it represents the median number years of cessation reported by former smokers; see ). There were no significant associations between duration of smoking and smoking cessation with albuminuria.
Serum cotinine was categorized into quartiles to assess the effect of passive smoking among non-smokers on the risk of albuminuria. Hypertensive non-smokers in the highest quartile of serum cotinine demonstrated a 1.41 (95% CI: 1.04, 1.90) increased risk of albuminuria compared to those in the lowest quartile. In contrast, non-hypertensive non-smokers showed no significant association with albuminuria at any level of serum cotinine.
All associations of smoking and serum cotinine with albuminuria were similar when subjects with macroalbuminuria were excluded (data not shown).
Moderate chronic kidney disease (estimated GFR 30–59 mL/min/1.73m2) was also evaluated as an outcome in this population. Among hypertensives, the adjusted odds ratio of chronic kidney disease associated with current smoking was 1.36 (95% CI: 0.91, 2.02) and the risk associated with former smoking was 1.47 (95% CI: 1.05, 2.07). Smoking status was not associated with an increased risk of chronic kidney disease among non-hypertensives. Among hypertensive and non-hypertensive smokers (including current and former), serum cotinine was not associated with risk of chronic kidney disease.
DISCUSSION
This study demonstrates a strong association between current smoking and albuminuria among hypertensive persons, independent of other risk factors. The association between current self-reported smoking and albuminuria was validated by serum cotinine, which was associated with an increased risk of albuminuria in hypertensives. A relationship between 40 or more pack-years of smoking with albuminuria was observed in current hypertensive smokers. Among non-hypertensives, a relationship was observed between 60 or more pack-years and albuminuria. Even more striking was the observed association between high levels of serum cotinine and albuminuria among those who never smoked. This study provides strong, detailed, and consistent support for the association between smoking exposure and early kidney damage among persons with hypertension in the general U.S. population.
The findings from the current study are consistent with several other cross-sectional studies that have evaluated the association between smoking and kidney damage.Citation[5],Citation[7–9],Citation[20],Citation[21] These studies observed an association between increased smoking and several markers of kidney impairment, including decreased kidney plasma flow,Citation[9] elevated serum creatinine,Citation[8] decreased and/or elevated glomerular filtration rate,Citation[7],Citation[9] and the presence of proteinuria Citation[20],Citation[21] or albuminuria.Citation[5],Citation[7] Numerous studies have also consistently found an association between smoking and diabetic kidney disease.Citation[22] However, irrespective of diabetic status, persistent albuminuria is almost always due to intrinsic kidney disease Citation[23] and is associated with higher risk for cardiovascular morbidity and mortality.Citation[24] The extent to which microalbuminuria mediates some of the increased risk of cardiovascular disease due to smoking needs further study.
Multivariable estimates of relative risks for current smoking were similar across general population studies with statistically significant increased risk estimates ranging between 1.50 and 2.00.Citation[3],Citation[5],Citation[10],Citation[21] The only study that evaluated a dose-response effect found increased risks among those who smoked greater than 20 cigarettes per day as compared to those who smoked 20 or less per day.Citation[7] This was also the only other study to distinguish the risk of microalbuminuria among former smokers with an age- and sex-adjusted increased odds ratio of 1.27 (95% CI: 1.04 to 1.54, p < 0.05) observed.Citation[7] However, no information was provided across pack-years of smoking for former smokers or a biological marker of smoking exposure, such as cotinine.
Many epidemiologic studies have pointed to an effect of passive smoking on coronary heart disease (CHD).Citation[25–30] In a meta-analysis of CHD risk from passive smoking,Citation[25] there was a 25% overall increased risk of CHD in non-smokers exposed to passive smoke compared to non-smokers not exposed to passive smoke. No study to our knowledge has examined the effect of passive smoke on kidney disease. By examining levels of serum cotinine among non-current smokers, it was found that among hypertensives, relatively high levels of exposure to passive smoke resulted in a 41% increased prevalence of albuminuria. Although those with high cotinine values (>71 ng/mL) could represent the inclusion of some current smokers who classified themselves as never or former smokers, the bias from misclassification due to self-reported smoking status is usually considered insignificant.Citation[31],Citation[32]
Although there was an increased risk of chronic kidney disease among both current and former smokers, only the impact of former smoking was statistically significant. Bias from differential survival among both current smokers and hypertensives may influence cross-sectional studies of these factors and chronic kidney disease. The ratio of smoker to non-smoker mortality peaks at age 45 in developed countries.Citation[33] Differential death from cardiovascular causes including hypertension is also well known. Furthermore, chronic kidney disease is most often observed beyond middle age. Therefore, the influence of current smoking on chronic kidney disease requires further evaluation in longitudinal and not cross-sectional studies.Citation[10]
Several potential mechanisms for the role of smoking in the development of albuminuria have been hypothesized. The effects of smoking on the kidney are likely to be mediated through a combination of effects, including alterations in kidney hemodynamics and blood pressure and damage to the microvasculature within the kidney. Evidence suggests a similarity between atherosclerotic lesions and glomerulosclerosis.Citation[34–38] It has been hypothesized that the atherogenic effects of cigarette smoking extend to the microvasculature of the kidney, affecting both renal tubular and glomerular structures.Citation[39],Citation[40] Autopsy studies have supported this theory and have shown an association between a history of smoking and thickening of renal arterioles.Citation[41–44]
Blood cadmium concentrations in smokers are about four times higher than in nonsmokers,Citation[45] and cadmium has also been implicated in the development of kidney disease. Cadmium is an environmental pollutant with nephrotoxic properties that are absorbed into the body by, among other sources, cigarette smoking, and has a slow excretion rate due to renal reabsorption.Citation[46] The effects of cadmium nephrotoxicity may include proteinuria, calciuria, glycosuria, and tubular necrosis. Also, because of an extensive half-life (30 years), chronic low level exposures to cadmium are also detrimental and may be associated with end-stage renal disease, early onset diabetic kidney disease, and abnormal blood pressure regulation.Citation[45]
Our analysis showed that the association between smoking and albuminuria was mediated by hypertension, which may indicate that smoking promotes the progression of kidney disease among hypertensives. It is possible that the vascular injury already present among hypertensive individuals potentiates the physiological damage of smoking on the kidney microvasculature, resulting in albuminuria or the initiation of kidney damage. Once this damage has occurred, the development of chronic kidney disease due to continued exposure to smoking or a myriad of other promoting factors is likely to progress.
Our study had several important strengths. First, because of the NHANES III sampling methods and weights, our results can be generalized to U.S. adults. Second, the large sample size included in our analyses allowed us to detect any small but important associations between smoking and kidney disease. Even small associations with a common exposure such as smoking can contribute to substantial absolute numbers of persons affected. Third, using available data, we analyzed dose and duration of both self-reported current and former smoking as well as cotinine, a biological marker of smoking exposure for smokers and non-smokers.
Despite its strengths, several limitations of the current study warrant mention. As with any cross-sectional study design, inferences are limited in terms of establishing a causal role of smoking in the development of albuminuria, and large, prospective studies are needed. Also, the use of a single random urine sample for evaluation of albuminuria is not ideal. Although albuminuria is almost always due to intrinsic kidney disease, false positive evaluations can occur due to heavy exercise, fever, and extreme emotional stress,Citation[23] but these conditions are likely to be rare. Fortunately, false negative evaluations are also rare; therefore, cases are unlikely to be defined as non-cases in the current study, and any bias resulting from misclassification will likely underestimate the association of smoking and albuminuria. It is also important to note that diabetes as diagnosed by fasting glucose ( ≥ 126 mg/dL) was determined in only approximately half of the NHANES III participants. There was no evidence of different associations between smoking and albuminuria by diabetic status in the current study.
In summary, this study provides evidence that cigarette smoking is associated with the presence of albuminuria among hypertensive adults in the general U.S. population. Associations with albuminuria among current smokers are higher than among former smokers, but the association is still evident among former smokers with a high pack-year history of smoking. Ultimately, the continued emphasis on smoking prevention and cessation may prove to have an impact on the incidence of albuminuria in the general population and subsequently contribute to the reduction of morbidities associated with albuminuria.
ACKNOWLEDGMENTS
A portion of this manuscript was published in abstract form: Hogan SL, Colindres RE, Cai J, Coresh J. Association of smoking with albuminuria in a cross-sectional probability sample of U.S. adults. J Am Soc Nephrol. 2001;12:209A.
REFERENCES
- Trosclair A, Husten C, Pederson L, Dhillon I. Cigarette smoking among adults—United States, 2000. MMWR Morb Mortal Wkly Rep. 2002; 51: 642–645
- Whelton PK, Randall B, Neaton J, Stamler J, Brancati FL, Klag M. Cigarette smoking and ESRD incidence in men screened for MRFIT. J Am Soc Nephrol. 1995; 6: 408A
- Vupputuri S, Sandler DP. Lifestyle risk factors and chronic kidney disease. Ann Epidemiol. 2003; 13: 712–720
- Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. 2003; 14: 479–487
- Cirillo M, Senigalliesi L, Laurenzi M, Alfieri R, Stamler J, Stamler R, Panarelli W, De Santo NG. Microalbuminuria in nondiabetic adults: relation of blood pressure, body mass index, plasma cholesterol levels, and smoking: The Gubbio Population Study. Arch Intern Med. 1998; 158: 1933–1939
- Goetz FC, Jacobs DR, Chavers B, Roel J, Yelle M, Sprafka JM. Risk factors for kidney damage in the adult population of Wadena, Minnesota. A prospective study. Am J Epidemiol. 1997; 145: 91–102
- Pinto-Sietsma SJ, Mulder J, Janssen WM, Hillege HL, de Zeeuw D, De Jong PE. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med. 2000; 133: 585–591
- Culleton BF, Larson MG, Evans JC, Wilson PW, Barrett BJ, Parfrey PS, Levy D. Prevalence and correlates of elevated serum creatinine levels: the Framingham Heart Study. Arch Intern Med. 1999; 159: 1785–1790
- Gambaro G, Verlato F, Budakovic A, Casara D, Saladini G, Del P, Bertaglia G, Masiero M, Checchetto S, Baggio B. Renal impairment in chronic cigarette smokers. J Am Soc Nephrol. 1998; 9: 562–567
- Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004; 291: 844–850
- Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Health. Stat 2. 1992; 113: 21–35
- Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1, No. 32. Programs and Collection Procedures. Department of Health and Human Services Publication No. 94-1308. 1–416, 1994
- Gunter EW, McQuillan G. Quality control in planning and operating the laboratory component for the Third National Health and Nutrition Examination Survey. J Nutr. 1990; 120(Suppl. 11)1451–1454
- Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996; 7: 930–937
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130: 461–470
- Levey AS, Green T, Kusek JW, Beck GJ, MDRD Study Group. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000; 11: 155A
- Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002; 39: 920–929
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002; 39: S1–246
- Shah BV, Barnwell BG, Bieler GS. SUDAAN User's Manual. Release 7.5, Research Triangle Institute, Research Triangle Park, NC 1997
- Ramirez SB, Saleh A, Nandakumar M, Burford S, Lau TWL, Hsu SIH, Owen WF, McClellan W. Smoking is a risk factor for proteinuria as detected by screening in a large cohort of young adult Asians. J Am Soc Nephrol. 1999; 10: 87A
- Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, Atkins RC. Smoking is associated with renal impairment and proteinuria in the normal population: the AusDiab kidney study. Australian Diabetes, Obesity and Lifestyle Study. Am J Kidney Dis. 2002; 40: 704–712
- Ritz E, Ogata H, Orth SR. Smoking: a factor promoting onset and progression of diabetic nephropathy. Diabetes Metab. 2000; 26(Suppl. 4)54–63
- Lafayette RA, Perrone D, Levey AS. Laboratory evaluation of renal function. Diseases of the Kidney6th, RW Schrier, CW Gottschalk. Little, Brown and Company, Boston 1997; 307
- Mlacak B, Jaksic Z, Vuletic S. Albuminuria, cardiovascular morbidity and mortality in diabetic and non-diabetic subjects in a rural general practice. Fam Pract. 1999; 16: 580–585
- He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease—a meta-analysis of epidemiologic studies. N Engl J Med. 1999; 340: 920–926
- He J, Whelton PK. Passive cigarette smoking increases risk of coronary heart disease. Eur Heart J. 1999; 20: 1764–1765
- Garland C, Barrett-Connor E, Suarez L, Criqui MH, Wingard DL. Effects of passive smoking on ischemic heart disease mortality of nonsmokers. A prospective study. Am J Epidemiol. 1985; 121: 645–650
- Sandler DP, Comstock GW, Helsing KJ, Shore DL. Deaths from all causes in non-smokers who lived with smokers. Am J Public Health. 1989; 79: 163–167
- Svendsen KH, Kuller LH, Martin MJ, Ockene JK. Effects of passive smoking in the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1987; 126: 783–795
- Ciruzzi M, Pramparo P, Esteban O, Rozlosnik J, Tartaglione J, Abecasis B, Cesar J, De RJ, Paterno C, Schargrodsky H. Case-control study of passive smoking at home and risk of acute myocardial infarction. Argentine FRICAS Investigators. Factores de Riesgo Coronario en America del Sur. J Am Coll Cardiol. 1998; 31: 797–803
- Nyberg F, Isaksson I, Harris JR, Pershagen G. Misclassification of smoking status and lung cancer risk from environmental tobacco smoke in never-smokers. Epidemiology. 1997; 8: 304–309
- Nyberg F, Agudo A, Boffetta P, Fortes C, Gonzalez CA, Pershagen G. A European validation study of smoking and environmental tobacco smoke exposure in nonsmoking lung cancer cases and controls. Cancer Causes Control. 1998; 9: 173–182
- Riggs JE. The “protective” influence of cigarette smoking on Alzheimer's and Parkinson's diseases. Quagmire or opportunity for neuroepidemiology?. Neurol Clin. 1996; 14: 353–358
- Keane WF, Kasiske BL, O'Donnell MP. Lipids and progressive glomerulosclerosis. A model analogous to atherosclerosis. Am J Nephrol. 1988; 8: 261–271
- Avram MM. Similarities between glomerular sclerosis and atherosclerosis in human renal biopsy specimens: a role for lipoprotein glomerulopathy. Am J Med. 1989; 87: 39N–41N
- Diamond JR. Analogous pathobiologic mechanisms in glomerulosclerosis and atherosclerosis. Kidney Int Suppl. 1991; 31: S29–S34
- Kamanna VS, Roh DD, Kirschenbaum MA. Atherogenic lipoproteins: mediators of glomerular injury. Am J Nephrol. 1993; 13: 1–5
- Grond J, van Goor H, Erkelens DW, Elema JD. Glomerular sclerotic lesions in the rat. Histochemical analysis of their macromolecular and cellular composition. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986; 51: 521–534
- McLigeyo SO. Smoking—an emerging risk factor for renal diseases. East Afr Med J. 1998; 75: 171–174
- Orth SR. Smoking—a renal risk factor. Nephron. 2000; 86: 12–26
- Auerbach O, Hammond EC, Garfinkel L. Thickening of walls of arterioles and small arteries in relation to age and smoking habits. N Engl J Med. 1968; 278: 980–984
- Black HR, Zeevi GR, Silten RM, Walker S. Effect of heavy cigarette smoking on renal and myocardial arterioles. Nephron. 1983; 34: 173–179
- Oberai B, Adams CW, High OB. Myocardial and renal arteriolar thickening in cigarette smokers. Atherosclerosis. 1984; 52: 185–190
- Tracy RE, Malcom GT, Oalmann MC, Newman WP, III, Guzman MA. Nephrosclerosis, glycohemoglobin, cholesterol, and smoking in subjects dying of coronary heart disease. Mod Pathol. 1994; 7: 301–309
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health. 1998; 24(Suppl. 1)1–51
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004; 112: 1099–1103