Abstract
This study was designed to determine the effect of L-arginine on hypoxia inducible factor alpha (HIF-1 α) and Sonic hedgehog (Shh) levels considered to be involved in the development of ischemia/reperfusion (I/R) injury. Unilaterally nephrectomized Sprague-Dawley rats were subjected to 60 minutes of left renal ischemia followed by 45 minutes of reperfusion. Group 1 were sham-operated animals; group 2, I-R/Untreated animals; and group 3, I-R/L-Arg-treated animals. Serum creatinine, blood urea nitrogen (BUN), and kidney malondialdehyde (MDA) levels were determined as well as examining the kidneys histologically. The treatment of rats with L-Arg produced a significant reduction in the levels of BUN, creatinine, MDA, and histopathological score compared to renal I/R groups. The Shh expression in the tubulus epithelia were intensely increased in the I-R/L-Arg group when compared to that of the Sham-control and the I-R/untreated groups. Additionally, the HIF-1α expression in the tubulus epithelia and the interstitial spaces were intensely increased in the I-R/L-Arg group. These findings suggest that NO reduces the renal dysfunction associated with I/R of the kidney and may act as a trigger to induce Shh and HIF-1 activity.
INTRODUCTION
Renal ischemia-reperfusion (I-R) results in considerable alterations in cell function,Citation[1] metabolism,Citation[2] and structural regularityCitation[3] of the proximal tubule. It is an important effect of renal dysfunction in renal transplantation, surgical revascularization of the renal artery, partial nephrectomy, and treatment of suprarenal aortic aneurysms.Citation[4] The mechanisms underlying renal I-R injury are complex; though they have been subjects of several review articles, many steps are still unclear.Citation[5–7]
A number of studies have shown that hypoxiainducible factor (HIF)-1 mediates the regulation of genes that encode proteins associated with maintaining O2 homeostasis.Citation[8] HIF-1 is a heterodimer consisting of HIF-1α and HIF-1β, which are well-known as the aryl hydrocarbon nuclear translocator subunits. Of the two subunits, the expression of HIF-1α is strongly regulated by O2. The effect of intermittent hypoxia on HIF-1 and HIF-2α protein expression was studied in PC12 cells. Intermittent hypoxia increased both HIF-1 and HIF-2a expression.Citation[9] Hedgehog (Hh) proteins are morphogens that act in a wide variety of tissues throughout embryonic development.Citation[10–13] There are three mammals with highly conserved Hh genes: Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh).Citation[14] Earlier observations have suggested that Hh might also be implicated in the vascularization of certain embryonic tissues.Citation[10],Citation[15]
Nitric oxide (NO), a soluble free radical gas, is an extremely important endogenous vasodilator that has a variety of physiological and pathological effects in biological structures.Citation[16],Citation[17] NO is synthesized from L-arginine and molecular oxygen by the enzyme nitric oxide synthase (NOS)Citation[16],Citation[18] and is produced by the endothelial cells and reasons relaxation of preglomerular arteries, improving renal blood flow.Citation[19],Citation[20]
The purpose of this experimental study was to determine the role of L-arginine (a precursor of NO) on oxidative stress, renal dysfunction, and histologic alterations induced by I-R in rats. Furthermore, the effect of L-arginine on hypoxiainducible factor alpha (HIF-1a) and Sonic hedgehog (Shh) levels thought to be involved in the development of I-R was examined.
MATERIALS AND METHODS
In the present study, thirty male Sprague-Dawley rats weighing 200 to 220 g were used. All of the experimental protocols were performed according to the guidelines for the ethical treatment of experimentation animals.
Animals and Experimental Protocol
The rats were housed individually in cages and were allowed free access to standard rat chow and water before and after the experiments. The animal rooms were windowless with temperature (22±2°C) and lighting controls. The animals were fasted overnight before the experiments but were given free access to water. They were anesthetized by 100 mg/kg ketamine, and 20 mg/kg xylazine body weight, i.p. right femoral vein was cannulated for the administration of drugs and saline. The animals were randomly separated into three groups, each containing 10 rats:
Sham-control group (G1, n = 10): A sham operation was performed. The abdomen was dissected, and the right kidney was harvested and then the left renal pedicle exposed. However, renal clamps were not applied.
I-R/untreated group (G2, n = 10): Rats were subjected to the surgical procedures described below and underwent left renal ischemia for 60 minutes followed by reperfusion for 45 minutes.
I-R/L-arginine treated group (G3, n = 10): The same surgical procedure as in group 2 was performed. L-arginine (300 μg/kg/min i.v., during 20 min; Sigma Chemical Co., St. Louis, Missouri, USA) was infused for 20 min by an infusion pump.
Ischemia Reperfusion Model
The abdomen was dissected under anesthesia, the right kidney was harvested, and the left renal artery and vein were clamped with a hemostasis clip for 60 minutes. The abdomen was closed during I-R. The clip was subsequently removed to permit reperfusion. Sham-operated control rats underwent the same surgical procedure, including dissection of the renal pedicle. However, renal clamps were not applied. Blood samples (4–5 ml) were collected at 45 min after reperfusion from the abdominal aorta. Plasma was separated by centrifugation (3000 RPM for 10 minutes at room temperature). The serum samples were stored at –280°C, for the measurement of blood urea nitrogen (BUN) and creatinine. The rats were sacrificed at after reperfusion, and the kidney tissue was harvested.
Blood Biochemistry
BUN and creatinine were measured as indicators of disorders of glomeruli and endothelial cell. The activities of BUN and creatinine in plasma were determined by standard auto-analyzer methods on an Abbot Aeroset (USA).
Histopathological Evaluation
The extracted kidneys were divided into two pieces, fixed in 10% buffered formalin, and embedded in paraffin. Sections were cut at 5 μm, and coded kidney specimens were stained with hematoxylin and eosin and examined in blinded fashion. Histological changes were evaluated by quantitative measurement of tubulointerstitial injury, which was assessed by counting the number of necrotic and apoptotic cells, loss of tubular brush border, tubular dilatation, cast formation, and neutrophil infiltration. The scoring was 0 = none, 1 = 10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = 76%.Citation[21]
Immunohistochemistry
The expression of HIF-1α and Shh in the renal tissue was analyzed by use of an immunohistochemistry technique. Tissues were fixed in Bouin's solution and embedded in paraffin. Sections were cut into 4 μm thicknesses, dewaxed in xylene, and incubated for 20 minutes in 0.3% H2O2 to block endogenous peroxidase activity. Sections were then microwaved for four minutes in phosphate buffer saline (PBS), and incubated with primary antibodies (Mouse monoclonal antibody [H1alpha67-sup] to HIF-1 alpha, Santa Cruz Inc., USA; Monoclonal Anti-Sonic Hedgehog, Santa Cruz Inc., USA) overnight at room temperature. The primary antibody was visualized with diaminobenzidine (DAB) as a chromogen.
The sections were counterstained in hematoxylin and eosin, cleared with xylene, and cover-slipped. The tissue sections were examined using a light microscope interfaced with a Zeiss Color Camera. The sections analyzed by 2–3 observers blinded to the grouping of the animals using a ranking (0 = no immunoreactivity, 1 = faint, 2 = moderate, and 3 = strong immunoreactivity). At least three slides per animal were studied, and the results were presented as average (= median).
Biochemical Analyses
The other piece was washed in ice-cold 0.9% saline solution, weighted, and stored at −70°C. Homogenates of the tissues were prepared as 1.0 g/10 ml in 250 mM sucrose, 1 mM EDTA, 1 mM-dl-dithiothreitol and 15 mM Tris HCl (pH 7.4), using an all-glass Potter Elvehjem homogenizer (Selecta, Barcelona, Spain). Each homogenate was centrifuged for 20 min at 800g. The resulting supernatant fraction was used to determine enzyme activities.
Malondialdehyde Determination
Renal MDA levels were determined by Wasowiczs's methodCitation[22] based on the reaction of MDA with thiobarbituric acid at 95–100ºC. Fluorescence intensity was measured in the upper n-butanol phase by a fluorescence spectrophotometry (Hitachi, Model F-4010) adjusted excitation at 525 nm and emission at 547 nm. Arbitrary values obtained were compared with a series of standard solutions (1,1,3,3 tetramethoxypropane). Results were expressed as nanomole per gram wet tissue (nmol/g wet tissue).
Protein Assays
The protein content of homogenates was determined according to the procedure of Lowry et al.Citation[23]
Statistical Analysis
Data were entered and analyzed on an IBM compatible personal computer using SPSS version 9.0. All data were expressed as median ± standard error of mean (SEM). The significance of differences was evaluated using the Mann-Whitney U test. The level of statistical significance was accepted as p less than 0.5.
RESULTS
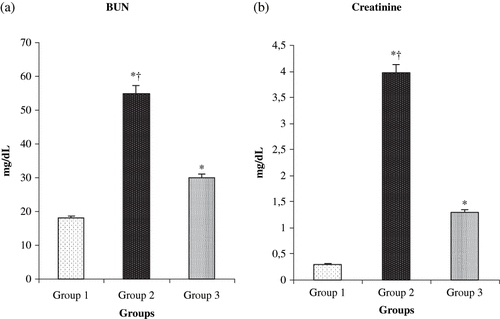
The values of BUN and creatinine measurements for the different groups are shown in and b. The treatment of rats with L-Arg produced a significant reduction in the serum levels of creatinine and BUN compared to renal I/R groups (p < 0.0001).
Figure 1. Effect of L-arg on (a) serum creatinine and (b) blood urea nitrogen (BUN) in rats exposed to renal I/R. Values expressed as mean ± SEM. *p < 0.05 as compared to group 1; †p < 0.05 as compared to group 3.
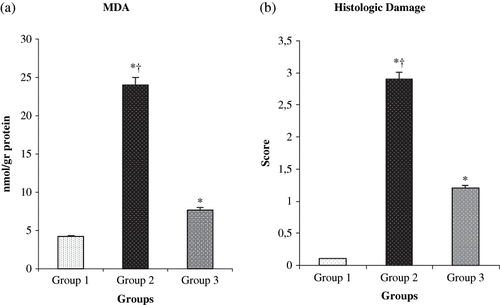
The values of MDA measurements for the different groups are shown in . MDA values decreased significantly in the L-Arg treated group when compared to group 2 (p < 0.0001). The histopathological score was found as 0.2 ± 0.1, 4.7 ± 0.5, and 1.9 ± 0.3 in group 1, 2, and 3, respectively. The histopathological score was significantly less in the group 3 compared to the group 2 rats (p < 0.0001; see ).
Figure 2. Comparative (a) MDA and (b) histologic score measurements of the three groups. *p < 0.05 compared with group 1; †p < 0.05 compared with group 3. Values are mean ± SEM. Abbreviation: MDA = malondialdehyde.
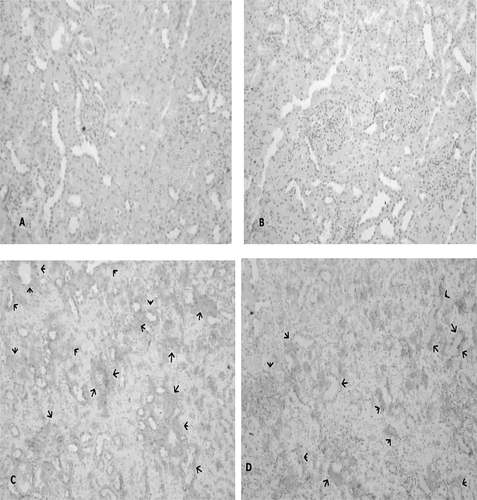
The sham-operated group did not show any morphological changes. By contrast, the kidneys of untreated ischemia rats showed tubular cell swelling, cellular vacuolization, pyknotic nuclei, medullary congestion, and moderate to severe necrosis. Treatment with L-Arg shows normal glomeruli and slight edema of the tubular cells.
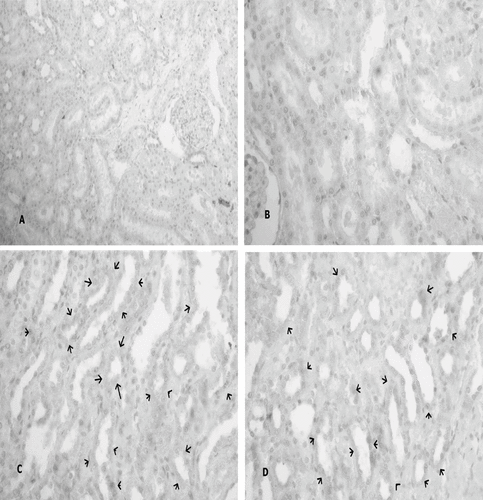
Immunoreactivity scores (IRS) in groups for Shh and HIF-1α were shown in and b. The IRS was significantly higher in the I-R/L-Arg group than in the sham and the I-R groups (p < 0.05). There were fainted Shh immunoreactive cells in the sham group rats (see ). Shh-positive immunostaining was mildly detected in the I-R group animals (see ). The Shh expression in the tubulus epithelia were intensely increased in the I-R/L-Arg group when compared to that of the sham-control and the I-R/untreated groups (see ). Additionally, the HIF-1α expression in the tubulus epithelia and the interstitial spaces were intensely increased in the I-R/L-Arg group (see ) when compared to that of the sham-control () and the I-R/untreated () groups.
Figure 3. Effects of ischemia/reperfusion and L-arg on IRS levels of renal tissue. *p < 0.05 compared with group 1; †p < 0.05 compared with group 3. Vales are mean ± SEM. Group 1 (n = 10) = sham-control group; group 2 (n = 10) = I-R untreated group; group 3 (n = 10) = I-R/L-Arg group. Abbreviation: IRS = immunoreactivity scores.
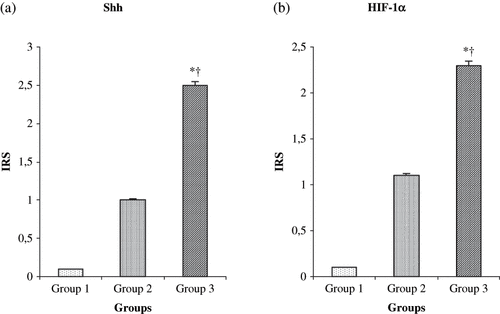
Figure 4. (A) No expression of Shh in sham-control group (Immuno-Peroxidase, × 200), and (B) Shh-positive immunostaining mildly detected in the group 2 animals (Immuno-Peroxidase, × 200). In contrast, (C) Shh expression by intensity in the tubulus epithelia from I-R/ L-Arg group rats (arrows) (Immuno-Peroxidase, × 200).
DISCUSSION
Genes induced by hypoxia play a significant role in adaptation to low oxygen supply at the cell and tissue, including the up-regulation of anaerobic metabolism, angiogenesis, and the determination of cell death/survival.Citation[24] HIF-1 is an effective transcriptional regulator of oxygen-dependent genes.Citation[25] HIFs are heterodimers, composed of a constitutive HIFβ- subunit and one of two HIFα subunits (HIF-1α and HIF-2α) that are regulated by oxygen-dependent proteolysis.Citation[26] Increased HIF-1 activity leads to the transcription of genes that are expressed in most cell types, such as those encoding glucose transporters, glycolytic enzymes, and VEGF, as well as genes that are expressed in a cell type-specific manner, such as erythropoietin, inducible nitric oxide synthase (NOS2), and insulin-like growth factor 2 (IGF-2).Citation[27] Additionally, HIF-1α is required for the normal vascularization, development, and physiologic responses to chronic hypoxia in adult mice.Citation[28],Citation[29]
Sonic hedgehog (Shh) is a morphogen known to regulate epithelial/mesenchymal interactions during the embryonic progress of limb, lung, gut, hair follicles, and bone.Citation[30–33] Pola et al.Citation[33] demonstrated that Shh induces and up-regulates two families of angiogenic growth factors, including vascular endothelial growth factor (VEGF) and angiopoietins. The Shh pathway is induced in the hind-limb ischemia model, and its inhibition with Shh-blocking antibodies decrease the angiogenic response to ischemia.Citation[34]
NO donors HIF-1α expression and HIF-1 activity in cultured cells under non-hypoxic conditions.Citation[35] Thus, an initial NO signal may act as a trigger to induce HIF-1 activity, leading to the subsequent expression of NOS2 and more production of NO, which then mediates protective effects.Citation[36] Additionally, a direct link between Shh signaling and NO functions have not been reported yet. However, it has been shown that cGMP analogs enhance the differentiating response of chick neural plate explants to Shh.Citation[37] cAMP, a well-characterized secondary messenger that acts through the cAMP-dependent protein kinase (PKA), can modulate the Hedgehog (Hh) response.Citation[37] In the present study, treatment of rats with L-Arg produced a significant reduction in the levels of BUN, creatinine, MDA, and histopathological score compared to renal I-R groups. The Sonic hedgehog (Shh) expression in the tubulus epithelia were intensely increased in the I-R/L-Arg group when compared to that of the Sham-control and the I-R/untreated groups. Additionally, the HIF-1α expression in the tubulus epithelia and the interstitial spaces were intensely increased in the I-R/L-Arg group.
These data indicate that NO not only reduces the renal dysfunction but also regulates expression of Sonic Hedgehog and Hypoxia-Inducible Factor-1α in the ischemic kidney.
REFERENCES
- Molitoris BA, Chan LK, Shapiro JI, Conger JD, Falk SA. Loss of epithelial polarity: a novel hypothesis for reduced proximal tubule Na+ transport following ischemic injury. J Membr Biol. 1989; 107(2)119–127
- Weinberg JM. The cell biology of ischemic renal injury. Kidney Int. 1991; 39(3)476–500
- Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978; 14(1)31–49
- Defraigne JO, Detry O, Pincemail J, Franssen C, Meurisse M, Lamy M, Limet R. Direct evidence of free radical production after ischaemia and reperfusion and protective effect of desferrioxamine: ESR and vitamin E studies. Eur J Vasc Surg. 1994; 8(5)537–543
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001; 132: 7–21
- Molitoris BA. Ischemic acute renal failure: exciting times at our fingertips. Curr Opin Nephrol Hypertens 1998; 7(4)405–406
- Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003; 284(4)F608–F627
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000; 88: 1474–1480
- Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol Lung Cell Mol Physiol. 2001; 281(3)L524–L528
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998; 8: 1083–1086
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 2000; 127(12)2763–2772
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996; 6(3)298–304
- Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, McMahon AP, Jessen KR, Mirsky R. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 1999; 23(4)713–724
- Zardoya R, Abouheif E, Meyer A. Evolution and orthology of hedgehog genes. Trends Genet. 1996; 12(12)496–497
- Rowitch DH, St-Jacques B, Lee SM, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999; 19(20)8954–8965
- Moncada S, Higgs A. The L-arginineynitric oxide pathway. N Engl J Med. 1993; 329(27)2002–2006
- Gros SS, Wolin MS. Nitric oxide: pathophysiologic mechanisms. Annu Rev Physiol. 1995; 57: 737–769
- Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994; 23(6 Pt 2)1121–1131
- Hanssen TN, D'Alessandro A, Southard JH. Long term cold ischemia reduces nitric oxide metabolism in reperfused rabbit kidneys. Transplant Proc. 1997; 29(8)3417–3419
- Shoskes DA, Xie Y, Gonzalez-Cadavid NF. Nitric oxide synthase activity in renal ischemia-reperfusion in the rat: implications for renal transplantation. Transplantation 1997; 63(4)495–500
- Feng L, Xiong Y, Cheng F, Zhang L, Li S, Li Y. Effect of ligustrazine on ischemia-reperfusion injury in murine kidney. Transplant Proc. 2004; 36(7)1949–1951
- Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: Importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993; 39(12)2522–2526
- Lowry OH, Rosebrough NJ, Faar AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193(1)265–275
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996; 76(3)839–885
- Baan C, van Gelder T, Peeters A, Mol W, Niesters H, Weimar WI, Jzermans J. Living kidney donors and hypoxia-inducible factor-1alpha. Transplantation 2003; 75(4)570–571
- Rosenberger C, Griethe W, Gruber G, Wiesener M, Frei U, Bachmann S, Eckardt KU. Cellular responses to hypoxia after renal segmental infarction. Kidney Int. 2003; 64(3)874–886
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996; 16(9)4604–4613
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu A Y, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998; 12(2)149–162
- Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999; 103(5)691–696
- Johnson RL, Tabin CJ. Molecular models for vertebrate limb development. Cell 1997; 90(6)979–990
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998; 8(19)1058–1068
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999; 13(19)2072–2086
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent up-regulating two families of angiogenic growth factors. Nat Med. 2001; 7(6)706–711
- Pola R, Ling LE, Aprahamian TR, Barba E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation 2003; 108(4)479–485
- Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D'Acquisto F, Addeo R, Makuuchi M, Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood 2000; 95(1)189–197
- Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000; 106(7)809–812
- Robertson CP, Gibbs SM, Roelink H. cGMP enhances the sonic hedgehog response in neural plate cells. Dev Biol. 2001; 238(1)157–167