Abstract
Colchicine has been used to control gouty arthritis for long time; colchicine overdose, however, causes multiple organ dysfunction. To date, no investigation has revealed the site of kidney lesion or dysfunction. This investigation describes the case of a male with a history of gout who ingested a large amount of colchicine and developed renal, hematopoietic, gastrointestinal, muscular, electrolytic, and hepatic disorder. Glucosuria was noted during hospital days. Colchicine intoxication is shown to induce proximal tubule damage. Severe electrolytes imbalance was noted, including hypomagnesemia, hypophosphatemia, and hypocalcemia. After management, the renal function and serum electrolyte of the patient recovered on the sixth day of hospitalization.
INTRODUCTION
Colchicine is extracted from Colchicum autumnale, a member of the lily family. Colchicine has been used to treat gout for at least 1500 years.Citation[1] Colchicine toxic effects are generally serious and involve nausea and diarrhea. Severe toxicity involving the hepatic, hematopoietic, muscular, and renal system has also been described.Citation[2] This paper describes a male with a history of gout who ingested large quantities of colchicine and developed renal, hematopoietic, gastrointestinal, muscular, electrolytic, and hepatic disorder.
CASE REPORT
A 48-year-old Australian Caucasian male was admitted to the emergency department at 18:45 on July 30, 2005, saying that he had ingested more than 20 tablets ( >10 mg ) of colchicine on July 27, 2005, hoping to rapidly improve his symptoms of gouty arthritis. The patient reported a three-day history of oligo/anuria and diarrhea prior to admission. The patient had a medical history of gouty arthritis without regular medication and diet control. The patient had no history of smoking but occasionally drank alcohol. Initial physical examinations revealed an arterial blood pressure of 104/79 mmHg, pulse rate of 112 bpm, respiratory rate of 22 breaths per minute, and body temperature of 36.9°C with E4V5M6 Glasgow coma score. Breathing sound was clear. The abdomen exhibited low grade tenderness and abdominal cramping pain. Diarrhea was also observed, and an EKG exhibited sinus tachycardia. Blood counts revealed white blood cell, 5800/uL; red blood cell, 4.82 million/uL; hemoglobin, 16.1g/dL; and platelet, 135,000/uL. Urine analysis (see ) revealed gravity, 1.018 (1.005–1.030); pH, 5.0 (4.5–8.0); protein, 150 mg/dL; glucose, negative g/dL; RBC, 12 /uL(<20); WBC, 36 /uL; hyaline cast, 48/uL (hyaline cast 0–2/HPF); and granular cast 4.0/uL. Other laboratory tests showed serum creatinine (Cr), 6.1 mg/dL (0.4–1.4); serum blood urea nitrogen (BUN), 41mg/dL (6–21); total bilirubin, 1.1 mg/dL (0–1.3); alanine aminotransferase (ALT), 72 IU/L(0–36); and aspartate aminotransferase (AST), 220 IU/L(0–34). Electrolyte revealed serum sodium (Na), 134 meq/L (134–148); potassium (K), 3.9 meq/L (3–4.8); calcium (Ca), 7.2 mg/dL (7.9–9.9); and inorganic phosphate (P), 1.5 mg/dL (2.4–4.7). Serum myoglobin level was 3194.1 ug/L. Moreover, postprandial blood sugar was 108 mg/dL, creatinine phosphokinase was 740 IU/L (56–244), and lactate dehydrogenase (LDH) was 6076 IU/L (180–460). Finally, arterial blood gas revealed pH: 7.453, PCO2: 23.3 mmHg, PO2: 96.7 mmHg, HCO3: 15.9 meq/L, and saturation: 97.8%.
Table 1 Result of urinary analysis during hospital days
CLINICAL COURSE
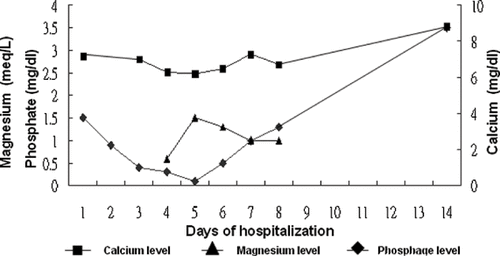
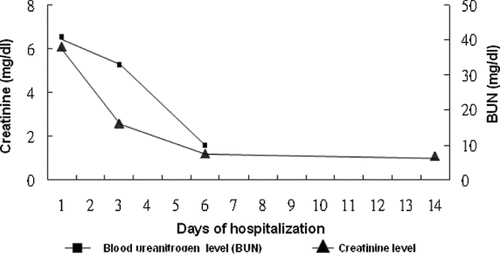
Based on the combination of high myoglobin level and renal failure, initially the patient was treated with alkalization and hydration. The long axis of the right kidney had a length of 14 cm with irregular outline while renal echo study did not reveal the left kidney. Abdominal echo study revealed a normal liver and spleen size with smooth surface and no ascites or liver tumor. Hepatitis B surface antigen and hepatitis C virus antibody were negative. Diarrhea subsided on hospital day 6. Serum creatinine and blood urea nitrogen initially elevated and recovered on hospital day 6 (see ). After discharge, at outpatient department (OPD), serum creatinine remained within the normal range. Serum potassium gradually reduced during hospitalization and was gradually corrected using intravenous potassium chloride. At our outpatient department, potassium was within the normal range. Serum calcium and phosphate initially were low and gradually decreased, reaching their lowest on hospital day 5.
Figure 1. Blood urea nitrogen (BUN) and serum creatinine level after stopping colchicine intake during the days of hospitalization.
Urinary phosphate concentration revealed less than 1 mg/dL during hospitalization. The patient received potassium phosphate (0.16 mmol/kg in 500 cc 0.45% saline over 6 hours, 3 times/day) intravenously from hospital day 4 to hospital day 5, then changed to oral phosphate solution and added intravenous calcium chloride (1 g in 100 cc 0.45% saline over 3 minutes) intermittently from hospital day 5. Following hospital day 5, calcium and phosphate levels gradually increased and were within the normal range at OPD (see ). Serum magnesium was low (0.6 meq/L; normal, 1.3–2.1) on hospital day 4. After intravenous magnesium sulfate, serum magnesium became normal (see ). Blood counts on hospital day 6 reduced to white blood cell, 4200/uL; red blood cell, 4.15 million/uL; hemoglobin, 13.4g/dL and platelet, 75,000 /uL. Initially, no glucosuria was found however on hospital days 3 and 4, increasing glucosuria was noted (see ). Owing to the stable clinical cause, the patient was discharged on hospital day 9. Following discharge, at the OPD, renal function and electrolyte returned to normal range.
DISCUSSION
Colchicine was first used in the sixth century A.D and has been used to prevent and treat gout since the eighteenth century.Citation[3] The main action through which colchicine has a high affinity for tissue containing large amounts of microtubules is involved in multiple cellular functions such as phagocytosis, mobility, and cell division.Citation[4] The phagocytic action of leukocytes in the joints of gouty patients suppressed by colchicine was also mentioned.Citation[5] Colchicine is rapidly absorbed from the gastrointestinal tract following ingestion. Therefore, the length of colchicine exposure in the gastrointestinal tract may cause gastrointestinal symptoms of toxicity. Colchicine is initially and primarily metabolized by the liver and excreted in the biliary system. Approximately 20% of unchanged colchicine is excreted by the kidney.Citation[6] Colchicine overdose is rare. The dose required for morbidity or mortality varies significantly. Some patients survived following ingesting more than 350 mg, but others died following ingesting just 7 mg.Citation[7] Colchicine overdose or intoxication may induce several systemic dysfunctions involving the gastrointestinal, nervous, skin, hematopoietic, musculoskeletal, and renal systems.Citation[2] Acute colchicine toxicity comprises three stagesCitation[7]:
Stage 1: early gastrointestinal symptoms, volume depletion, hypotension resulting from severe vomiting and diarrhea, and peripheral leukocytosis;
Stage 2: generally developing from 24 to 72 hours, involving mental status change, oliguric renal failure, hematopoietic problems, electrolyte imbalance, acid-base disturbance, and shock; and
Stage 3: involving rebound leukocytosis and alopecia.
Colchicine-induced liverCitation[3],Citation[8],Citation[9] and skeletal muscleCitation[3],Citation[8],Citation[10],Citation[11] damage and pancytopeniaCitation[3],Citation[8],Citation[9],Citation[12] have been reported in several studies. Bone marrow hypoplasia was the main pattern of bone marrow biopsy in patients with colchicine poisoning.Citation[3],Citation[12],Citation[13] “Colchicine figure” was defined as large, bizarre chromatin cells of hepatocyte, epithelial cells of the gastrointestinal tract, and bone marrow cells resembling mitotic arrest at metaphase in a microscopic study.Citation[13] The patient discussed here was also found to have abnormal liver function without hepatitis B or hepatitis C infection, in addition to rhabdomyolysis, decreased hematocrit, decreased white count, and thrombocytopenia. Another study reported alopecia due to colchicine intoxicationCitation[3]; however, the present patient did not suffer this symptom.
Skeletal muscle lesions owing to colchicine intoxication have been reported both with and without anti-hyperlipidemia medicationsCitation[3],Citation[8],Citation[10],Citation[11] The present patient did not complain of muscle ache and had no history of taking anti-hyperlipidemia medications. However, the laboratory data revealed rhabdomyolysis.
Electrolyte and acid-base imbalances such as metabolic acidosisCitation[8],Citation[14],Citation[15], hyponatremia,Citation[16] hypocalcemia,Citation[8],Citation[14] hypokalemia, hypophosphatemia,Citation[14] and hypomagnesemiaCitation[17] were reported. Unfortunately, the present patient exhibited electrolyte imbalance in the form of hypocalcemia, hypokalemia, hypophosphatemia, and hypomagnesemia. Low serum phosphate level was believed to result from severe gastrointestinal lossCitation[2] rather than renal loss. Additionally, low serum calcium level was believed to result from directly suppressing bone resorption on osteoclast.Citation[18] Respiratory alkalosis combined with metabolic acidosis was noted in the present patient; it may result from severe dehydration with tachycardia combined with hyperventilation. The present patient displayed an increased urine glucose level during hospitalization without diabetic mellitus history. Proximal tubule damage may be considered as the lesion site of colchicine intoxication. In the present patient, it is unfortunate that the urine phosphate was collected when serum phosphate was in the lowest level. Urinary phosphate excretion was low, and this investigation believes that hypophosphatemia does not impute to renal loss. Fanconi's syndrome cannot be proven in the subject patient. Serum phosphate and calcium were in the lowest level on day 5 and subsequently recovered with calcium and phosphate supplementation.
No evidence exists for the direct toxic effect of colchicine on the kidney. However, colchicines-induced renal damage to renal failure has been mentioned by several studies.Citation[3],Citation[8],Citation[16] Proteinuria was also noted by some studies.Citation[3],Citation[14],Citation[16] Oligo/anuria type renal failure is the most common form of renal failure. One previous report has attributed renal failure or renal damage to hypoxia, hypotension, and myoglobinuria caused by colchicine intoxication.Citation[9] In patients with colchicine poisoning, the renal microscopic finding was cloudy swelling only,Citation[19] without glomerular or tubular damage. Another report found that the epithelium of the collecting tubule, distal and proximal convoluted tubules, and loops of Henle showed no significant change on microscopic study, and urinary analysis revealed no glucosuria.Citation[16] However, the present patient has no history of diabetes mellitus. Moreover, the urine analysis of the present patient initially exhibited no glucose content, though some cast contents were found. Notably, the urinary glucose content gradually increased. This finding indicates that the lesion by colchicine is most likely in the proximal tubule. Acute renal failure induced by colchicine appears reversible. The renal function of the present patient recovered on day 6 following colchicine poisoning.
CONCLUSION
Colchicine overdose is associated with high mortality following rapidly progressive multiorgan failure. Care thus needs to be taken with dosage in administering this drug. Severe electrolyte imbalance should be noted, especially hypocalcemia, hypophosphatemia, hypokalemia, and hypomagnesemia. Renal failure is believed to result from colchicine directly, hypovolemic/dehydration, or rhabdomyolysis damage. Notably, this study revealed that colchicine overdose resulted in acute renal failure combining with glucosuria—revealing that proximal tubule damage my exit.
REFERENCES
- Hartung EF. History of the use of colchicum and related medicaments in gout; with suggestions for further research. Ann Rheum Dis. 1954; 13(3)190–200
- Hood RL. Colchicine poisoning. J Emerg Med. 1994; 12(2)171–177
- Naidus RM, Rodvien R, Mielke CH, Jr. Colchicine toxicity: a multisystem disease. Arch Intern Med. 1977; 137(3)394–396
- Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967; 34(2)525–533
- Seegmiller JE, Howell RR, Malawista SE. Mechanism of action of colchicine in acute gouty arthritis. J Clin Invest. 1962; 41: 1399
- Girre C, Thomas G, Scherrmann JM, Crouzette J, Fournier PE. Model-independent pharmacokinetics of colchicine after oral administration to healthy volunteers. Fundam Clin Pharmacol. 1989; 3(5)537–543
- Stapczynski JS, Rothstein RJ, Gaye WA, Niemann JT. Colchicine overdose: report of two cases and review of the literature. Ann Emerg Med. 1981; 10(7)364–369
- Milne ST, Meek PD. Fatal colchicine overdose: report of a case and review of the literature. Am J Emerg Med. 1998; 16(6)603–608
- Folpini A, Furfori P. Colchicine toxicity—clinical features and treatment. Massive overdose case report. J Toxicol Clin Toxicol. 1995; 33(1)71–77
- Caglar K, Odabasi Z, Safali M, Yenicesu M, Vural A. Colchicine-induced myopathy with myotonia in a patient with chronic renal failure. Clin Neurol Neurosurg. 2003; 105(4)274–276
- Atmaca H, Sayarlioglu H, Kulah E, Demircan N, Akpolat T. Rhabdomyolysis associated with gemfibrozil-colchicine therapy. Ann Pharmacother. 2002; 36(11)1719–1721
- Liu YK, Hymowitz R, Carroll MG. Marrow aplasia induced by colchicine. A case report. Arthritis Rheum. 1978; 21(6)731–735
- Simons RJ, Kingma DW. Fatal colchicine toxicity. Am J Med. 1989; 86(3)356–357
- Murray SS, Kramlinger KG, McMichan JC, Mohr DN. Acute toxicity after excessive ingestion of colchicine. Mayo Clin Proc. 1983; 58(8)528–532
- Stahl N, Weinberger A, Benjamin D, Pinkhas J. Fatal colchicine poisoning in a boy with familial Mediterranean fever. Am J Med Sci. 1979; 278(1)77–81
- Stemmermann GN, Hayashi T. Colchicine intoxication. A reappraisal of its pathology based on a study of three fatal cases. Hum Pathol. 1971; 2(2)321–332
- Maxwell MJ, Muthu P, Pritty PE. Accidental colchicine overdose. A case report and literature review. Emerg Med J. 2002; 19(3)265–267
- Heath DA, Palmer JS, Aurbach GD. The hypocalcemic action of colchicine. Endocrinology. 1972; 90(6)1589–1593
- Macleod JG, Phillips L. Hypersensitivity to colchicine. Ann Rheum Dis. 1947; 6: 224–229