Abstract
Introduction. GLUTs are specific membrane proteins that transport glucose down a concentration gradient. There have been few studies on their expression in the kidney. The aim of this study was to identify the expression of GLUTs 1, 3, and 4 in HGEC and their regulation under diabetic milieu. Material and Methods. An immortalized cell line of HGEC was used. Cells were cultured in medium containing 5 or 25 mM D-glucose. Western blotting and flow cytometry were used to examine the presence of GLUTs (1, 3, 4) and alterations in expression. Results. Western blotting analysis revealed that GLUT-1 levels were increased by 53% in HGEC cultured under experimental diabetes compared to cells grown in 5mM glucose. GLUT-3 levels were also increased by 15% under diabetic conditions. GLUT-4 levels were decreased by 20% in diabetes. Fluorescence Activated Cell Sorting (FACS) analysis demonstrated that cell surface expression of GLUT-1 was increased by 28% in cells grown in 25mM glucose. High glucose concentration did not affect cell surface expression of GLUT-3 and GLUT-4. Discussion. These findings suggest that depressed GLUT4 expression in glomerulus and overexpression of GLUT-1 and in a lesser extent of GLUT-3 may alter the glucose uptake in these cells. It has been suggested that the overexpression of GLUT-1 in glomerulus, being the major isoform, may lead to the initial pathologic hallmarks of diabetic nephropathy.
INTRODUCTION
In mammalian cells, glucose does not enter the cell freely, as the lipid layers that make up cell membranes are impermeable to carbohydrates. Glucose enters the cell by facilitated diffusion, a process in which specific membrane proteins passively transport glucose down a concentration gradient.Citation[1] During the last decade, two families of glucose transporters (GLUTs) have been identified: the group of sodium-linked GLUTs, which transport glucose against a glucose concentration gradient by co-transporting sodium down their electrochemical gradient, and the group of facilitated diffusion GLUTs, which mediate the uptake of glucose by most cells.Citation[2] The facilitated GLUTs comprise a family of at least 13 members, most of which function to allow glucose to diffuse down its concentration gradient across the plasma membrane.Citation[3]
The GLUT isoforms have distinct substrate specificities and kinetic properties, show different affinity for glucose, and their functional characteristics have been correlated with tissue-specific differences in glucose metabolism.Citation[4],Citation[5] However, the high-affinity GLUT isoforms are GLUT-1, GLUT-3, and GLUT-4, which have individual roles in renal glucose metabolism.Citation[6]
There is evidence that GLUTs play a significant role in diabetic nephropathy altering the cellular glucose uptake and metabolism.Citation[7] These alterations may contribute to a number of diabetic complications. The determination of the expression patterns of GLUT within the nephron is a significant step in understanding the mechanism of glucose uptake and metabolism as well as the role of GLUTs under abnormal conditions. Because the initial pathologic hallmarks of diabetic nephropathy primarily involve the cells of the renal glomerulus and specifically the loss of glomerular podocytes,Citation[8] several studies focused on the expression of glomerular GLUTs. However, there have been extremely few studies on their expression in the human renal tissue and their alterations in experimental diabetes mellitus. Initial studies of the GLUTs in the kidney of experimental animals revealed significant expression of several GLUTs,Citation[6],Citation[9] but the majority of these studies involved non-human tissues or cells. Nevertheless, this is one of the scarce studies that investigated the expression of GLUTs in human glomerular epithelial cells (HGEC). The aim of this study was to identify the expression of the high-affinity GLUTs 1, 3, and 4 in cultured HGEC and their regulation under diabetic milieu.
MATERIALS AND METHODS
Cell Line and Culture Conditions
An immortalized cell line of HGECCitation[10],Citation[11] was used. The immortalization was performed by infection with antigen T of the DNA of the virus SV40.Citation[10] Cells were cultured at 37ºC in an environment of 95% air and 5% CO2 in DMEM-Ham's F12 (1:1) medium containing 1% FCS, 15mM HEPES, 2 mM glutamine, ITS (5 μg/mL insulin, 5 μg/mL transferring and 5ng/mL sodium selenite), 50 nM dexamethasone, 100 U/mL penicillin, 100 μg/mL streptomycin, 25 μg/mL amphotericin, and either 5 or 25 mM D‐glucose (all from Biochrom Seromed, Berlin, Germany). Cells were released from their tissue culture flasks for passaging or use in experiments by treatment with 0.05% trypsin in 1mM EDTA. For experiments, cells were cultured in 75 cm2 flasks for at least three passages without exceeding the seven passages.
Western Blotting
For Western blotting analysis of conditioned media, cells (2 × 106) were cultured for 48–72 hours.
Rabbit anti-human polyclonal antiserum to Glut-1, Glut-3, and Glut-4 were obtained from Chemicon International (Temecula, California, USA). Peroxidase-conjugated goat anti-rabbit immunoglobulins were purchased from Amersham Biosciences (Uppsala, Sweden). Fluorescein-conjugated anti-rabbit antibody to immunoglobulins IgG (Fluorescein Isothiocyanate [FITC]; Cappel, ICN Pharmaceuticals, Frankfurt, Germany) was used for Fluorescence Activated Cell Sorting (FACS).
Cells were lysed in a buffer containing 1% Triton X‐100, 1mM phenylmethyl-sulfonyl fluoride, a cocktail of protease inhibitors (catalog no. P8340, Sigma) and 1mM Na2EDTA in PBS 30 min at 4ºC. Insoluble material was removed by centrifugation, and the supernatant was stored at −20ºC. Protein estimation was performed by the method of Bradford (Pierce).
Electrophoresis in the presence of SDS was performed according to the method of LaemliCitation[12] on 7.5% or 10% polyacrylamide gels under reducing or non-reducing conditions. The resolved proteins were then transferred to Hybond-ECL nitrocellulose membrane (Amersham). Blots were saturated for 2 hrs at room temperature with 5% nonfat milk in Tris-buffered saline (0.1% Tween 20) and incubated overnight at 4oC with the appropriate dilutions of polyclonal antibodies in the same buffer without Tween 20. After washing with TBS/0.1% Tween-20, membranes were incubated for 1 hour at room temperature with peroxidase (HRP)-conjugated anti-rabbit immunoglobulins as secondary antibodies. Bound antibody was detected by ECL Western blotting detection system (Amersham).
Incubations with peroxidase conjugated goat anti-rabbit immunoglobulins and the detection of bound peroxidase activity was carried out as described for the enhanced chemiluminescence blotting detection system (Amersham). Blots were stripped and reprobed with antitubulin antibody to verify protein loads, to which all quantitative data were normalized. Tubulin was used to verify protein loads to which all quantitative data were normalized.
Images of Western blots were analyzed using an image processing software (Bioprofil Vilber Loumart).
Flow Cytometry
Cells were cultured as described, released from their dishes by trypsin treatment, washed in PBS, and resuspended in FACS buffer (2%FCS, 0.02% sodium azide in PBS). Cells were incubated with GLUT-1, GLUT-3, and GLUT-4 polyclonal antibodies for 45 minutes on ice and washed with FACS buffer. Cells were subsequently incubated with anti-rabbit IgG-FITC conjugated for 45 minutes on ice, washed with FACS buffer, and fixed with 1% formaldehyde in PBS. Analysis was performed using CELL QUEST software on a FACScan (Becton Dickinson). Fluorescence was determined on a four-decade log scale and fluorescence intensity was expressed as the mean channel number of 10,000 cells. The mean of fluorescence and the percentage of positive cells were calculated in the histogram section selected by the marker (M1), in order to subtract the fluorescence of the negative controls (cells incubated only with fluorescein-conjugated anti-rabbit IgG).
Statistical Analysis
Data is presented as mean ± SD. Results from images of western blots were analyzed using Student's t-test. p < 0.05 was considered statistically significant.
RESULTS
Alterations in Expression of Gluts in Diabetic Milieu
Western Blotting
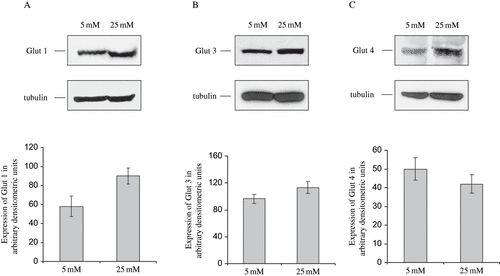
Using Western blotting, it was shown that all of the examined GLUTs are expressed in HGEC. Moreover, Western blot analysis of GLUT-1, GLUT-3, and GLUT-4 revealed that HGEC cells, cultured in 25 mM glucose expressed ∼54% (p < 0.01) more GLUT-1 and ∼13% (p < 0.05) more GLUT-3, compared to control cells cultured in the presence of 5mM glucose (see and ). In contrast, GLUT-4 levels were decreased ∼20% (p < 0.05) when cells were grown in the presence of 25mM glucose, compared to control cells (see ).
Figure 1. Western blot analysis of GLUTs expression in HGEC cultured in low and high glucose. Total protein was extracted from cells cultured in 5mM (L) and 25mM (H) D-glucose. Total protein (30 μg) was analyzed on 7.5% SDS-PAGE under non-reducing conditions and immunobloted with the appropriate dilution of polyclonal antibodies against GLUTs. Blots were stripped and reprobed with antitubulin antibody to verify protein loads, to which all quantitative data were normalized.
Flow Cytometry
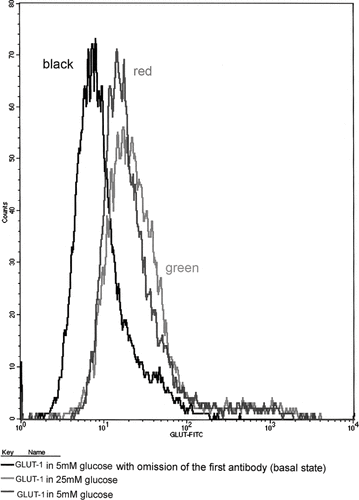
Flow cytometry was also used to determine whether glucose altered the GLUT expression on the cell surface. As shown in , exposure of cells to 25 mM glucose resulted in a significant increase of cell surface-associated GLUT-1 by ∼38% (p < 0.05). However, although an alteration in surface expression of GLUT-3 and GLUT-4 was detected in cells grown in 25 mM glucose, compared to cells grown in 5 mM glucose, the difference was marginal and not statistically significant (data not shown).
Figure 2. Flow cytometry of cell surface-associated GLUT-1 in HGEC grown in 5mM and 25mM glucose. Black line represents the expression of GLUT-1 in basal state (only with secondary antibody omitting the primary); red line represents the expression of GLUT-1 in HGEC cultured in 5mM glucose; green line in 25mM glucose.
The above data indicate that all the GLUTs examined were present in HGEC (see ). Diabetic milieu seems to increase the expression of GLUT-1 and GLUT-4, whereas under diabetic conditions the expression of GLUT-4 is decreased. Moreover, flow cytometry demonstrates that cells grown in 25mM glucose increase GLUT-1 expression.
DISCUSSION
There is evidence that T-SV 40 immortalized HGEC has been shown to be similar to their primary counterparts.Citation[10] The present study showed that all of the high-affinity GLUTs (1, 3, and 4) are expressed in HGEC. In addition, the present study demonstrated that in HGEC, the levels of GLUTs 1 and 3 are increased under a diabetic environment, whereas the levels of GLUT-4 are reduced.
Studies regarding the expression of GLUT-1 are conflicting. Initial studies did not observe GLUT-1 in glomerulus.Citation[4],Citation[13],Citation[14] However, studies using numerous methods (immunogold labeling, Western blotting) demonstrated the presence of GLUT-1 in the glomerulus of rat kidney and in rat glomerular epithelial cells.Citation[6],Citation[9],Citation[15–17]
Western blotting analysis demonstrated the presence of GLUT-3 in HGEC in the current study. Regarding the presence of GLUT-3 in the glomerulus, results from previous studies are again controversial. Haber et al. and Mantych et al. failed to detect GLUT-3 in human kidney.Citation[18],Citation[19] On the contrary, several studies were able to detect this transporter in the glomerulus.Citation[6],Citation[20]
In addition, GLUT-4 was also detected in the glomerulus. Brosius et al. demonstrated the presence of GLUT-4 in rat glomerulus and in primary cells cultures.Citation[9] Previous studies were consistent with these findings.Citation[6],Citation[9],Citation[14],Citation[21] However, Chin et al. failed to detect GLUT-4 m-RNA in adult rat kidney.Citation[14]
The discrepancies between the present findings and those of previous negative reports on the presence of GLUTs are likely to be a result of different methodologies applied and an examination of human cells, in contrast to the earlier studies performed on whole kidney tissue or cells from several experimental animals.
Recent studies demonstrated that increased glucose concentration in erythrocytes and in mesangial cells induce increased levels of GLUT-1.Citation[22–25] Studies regarding the modulations in expression of GLUT-4 in glomerulus are limited.Citation[9] The present results are compatible with reports from other tissues that express GLUT-4. In fat, glucose uptake is reduced in experimental diabetes mellitus probably due to down-regulation of GLUT-4. Hyperglycemia per se also lowers the level of GLUT-4 in rat muscleCitation[23] and in skeletal muscle cells culture.Citation[26] It has been shown that after one week of experimental diabetes, GLUT-4 m-RNA is significantly reduced in glomerulus.Citation[9] It has been also reported that the levels of GLUT-4 are reduced in diabetes mellitus in glomerulus.Citation[27] Marcus et al.Citation[27] suggested a metabolic mechanism that could contribute to the increased glomerular filtration rate and glomerular pressure seen early in diabetes.Citation[28],Citation[29] Because glucose transport is rate-limiting for glucose metabolism, the decreased expression of the so-called insulin-responsive GLUT-4 leading to glucose transport can be predicted to lead to diminished ATP generation from the glycolysis of the transported glucose. It is possible that this decrease in cell ATP might affect certain plasma membrane functions such as ATP-sensitive potassium channels leading to hyperpolarization, vasodilatation, and increased glomerular filtration. Thus, alterations of the expression of GLUT-4 may contribute to the pathogenesis of diabetic nephropathy.
Based on the increase of the levels of GLUT-1 and the reduction of the levels of GLUT-4 in diabetes, a mechanism has been proposed for the pathogenesis of diabetic nephropathyCitation[22] in target organs. Skeletal muscle cells increase their GLUT-1 expression and thus the detrimental glucose uptake, but simultaneously they are protected from hyperglycemia because levels of GLUT-4 are reduced in a proportional higher degree.Citation[1],Citation[9],Citation[15],Citation[21] On the contrary, target organs of diabetes such as the kidney and eyes do not express significant amounts of GLUT-4 and suffer from the toxic effects of diabetic milieu.
The overexpression of GLUT-1 is an important step in the pathogenesis of diabetic nephropathy because it induces increased glucose uptake, which is accompanied by increased expression of fibronectin and collagen; this leads to an increased matrix production and, therefore, to glomerulosclerosis.Citation[30]
Important findings of our study include the following conclusions:
High-affinity GLUTs 1, 3, and 4 were detected in HGEC.
The expression of GLUT-1 and GLUT-3 is increased under experimental diabetes mellitus, whereas the expression of GLUT-4 is decreased in cells grown in increased glucose concentration.
These findings suggest that depressed GLUT4 expression in the glomerulus and the overexpression of GLUT-1 and (to a lesser extent) of GLUT-3 may alter the glucose uptake in these cells. It has been suggested that the overexpression of GLUT-1 in glomerulus, being the major isoform, may lead to an increased glucose metabolic flux, extracellular matrix accumulation, glomerular basement thickening, and mesangial expansion, the four initial pathologic hallmarks of diabetic nephropathy. Strategies to prevent GLUT-1 increased expression may be the key to retard the progression of diabetic nephropathy.
ACKNOWLEDGMENTS
The authors would like to thank Dr. E.C. Tsilimpary for her priceless guidance and support.
REFERENCES
- Kahn BB. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992; 89(5)1367–1374
- Rothman DL, Shulman RG, Shulman GI. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992; 89(4)1069–1075
- Devaskar SU, Mueckler MM. The mammalian glucose transporters. Pediatr Res. 1992; 31(1)1–13
- Thorens B, Lodish HF, Brown D. Differential localization of two glucose transporter isoforms in the rat kidney. Am J Physiol. 1990; 259: C286–C294
- Thorens B, Cheng ZQ, Brown D, Lodish HF. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990; 259(6 Pt 1)C279–C285
- Heilig C, Zaloga C, Lee M, et al. Immunogold localization of high-affinity glucose transporter isoforms in normal rat kidney. Lab Invest. 1995; 73(5)674–684
- Brosius FC, Heilig CW. Glucose transporters in diabetic nephropathy. Pediatr Nephrol. 2005; 20(4)447–451
- Pagtalunan ME, Miller P, Eagle SJ, et al. Podocyte loss and progressive glomerular injury in Type II diabetes. J Clin Invest. 1997; 99: 342–348
- Brosius FC, III, Briggs JP, Marcus RG, Barac-Nieto M, Charron MJ. Insulin-responsive glucose transporter expression in renal microvessels and glomeruli. Kidney Int. 1992; 42(5)1086–1092
- Delarue F, Virone A, Hagege J, et al. Stable cell line of T-SV40 immortalized human glomerular visceral epithelial cells. Kidney Int. 1991; 40: 906–912
- Krishnamurti U, Chen Y, Michael A, et al. Integrin-mediated interactions between primary/T-sv40 immortalized human glomerular epithelial cells and type IV collagen. Lab Invest. 1996; 74(3)650–657
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680–685
- Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Localization of Na-dependent active type and erythrocyte/ HepG2 type glycose transporters in rat kidneys: immunoflourecence and immunogold study. J Histichem Cytochem. 1991; 39: 287–298
- Chin E, Zhou J, Bondy C. Anatomical and developmental patterns of facilitative glucose transporter gene expression in the rat kidney. J Clin Invest. 1993; 91: 1810–1815
- Heilig CW, Concepcion LA, Riser BL, Freytag SO, Zhu M, Cortes P. Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest. 1995; 96(4)1802–1814
- Mogyorosi A, Ziyadeh F. GLUT1 and TGF-beta: the link between hyperglycemia and diabetic nephropathy. Nephrol Dial Transplant. 1999; 14(12)2827–2829
- Dominguez JH, Camp K, Maianu L, Garvey WT. Glucose transporters of rat proximal tubule: differential expression and subcellular distribution. Am J Physiol. 1992; 262(5 Pt 2)F807–F812
- Haber R, Weinstein S, O'Boyle E, Morgello S. Tissue distribution of the human GLUT-3 glucose transporter. Endocrinology. 1993; 132(6)2538–2543
- Mantych GJ, James DE, Chung HD, Devaskar SU. Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Endocrinology. 1992; 131(3)1270–1278
- Shepherd PR, Gould GW, Colville CA, McCoid SC, Gibbs EM, Kahn BB. Distribution of GLUT3 glucose transporter protein in human tissues. Biochem Biophys Res Commun. 1992; 188(1)149–154
- Thorens B, Charron MJ, Lodish HF. Molecular physiology of glucose transporters. Diabetes Care. 1990; 13(3)209–218
- Heilig CW, Brosius FC, III, Henry DN. Glucose transporters of the glomerulus and the implications for diabetic nephropathy. Kidney Int Suppl. 1997; 60: S91–S99
- Dimitrakoudis D, Ramlal T, Rastogi S, Vranic M, Klip A. Glycaemia regulates the glucose transporter number in the plasma membrane of rat skeletal muscle. Biochem J. 1992; 284(Pt 2)341–348
- Harik SI, Behmand RA, Arafah BM. Chronic hyperglycemia increases the density of glucose transporters in human erythrocyte membranes. J Clin Endocrinol Metab. 1991; 72(4)814–818
- Knott RM, Robertson M, Muckersie E, Forrester JV. Regulation of glucose transporters (GLUT-1 and GLUT-3) in human retinal endothelial cells. Biochem J. 1996; 318(Pt 1)313–317
- Koivisto UM, Martinez-Valdez H, Bilan PJ, Burdett E, Ramlal T, Klip A. Differential regulation of the GLUT-1 and GLUT-4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J Biol Chem. 1991; 266(4)2615–2621
- Marcus RG, England R, Nguyen K, Charron MJ, Briggs JP, Brosius FC, III. Altered renal expression of the insulin-responsive glucose transporter GLUT4 in experimental diabetes mellitus. Am J Physiol. 1994; 267(5 Pt 2)F816–F824
- Hofstetter TH. Diabetic nephropathy: metabolic versus hemodynamic considerations. Diabetes Care. 1992; 15: 1205–1215
- Hofstetter TH, Rennke HG, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981; 19: 410–415
- Gnudi L, Viberti G, Raij L, et al. Glut-1 overexpression”. Link between hemodynamic and metabolic factors in glomerular injury?. Hypertension. 2003; 42: 19–24